Abstract
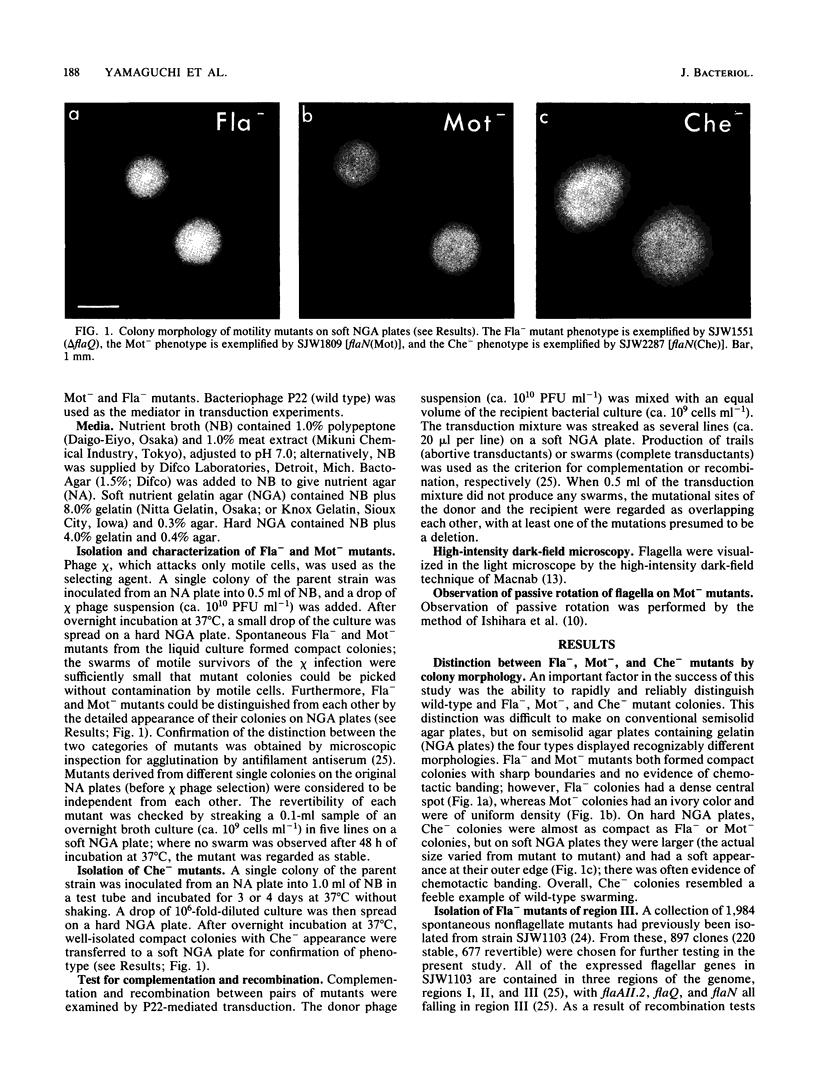
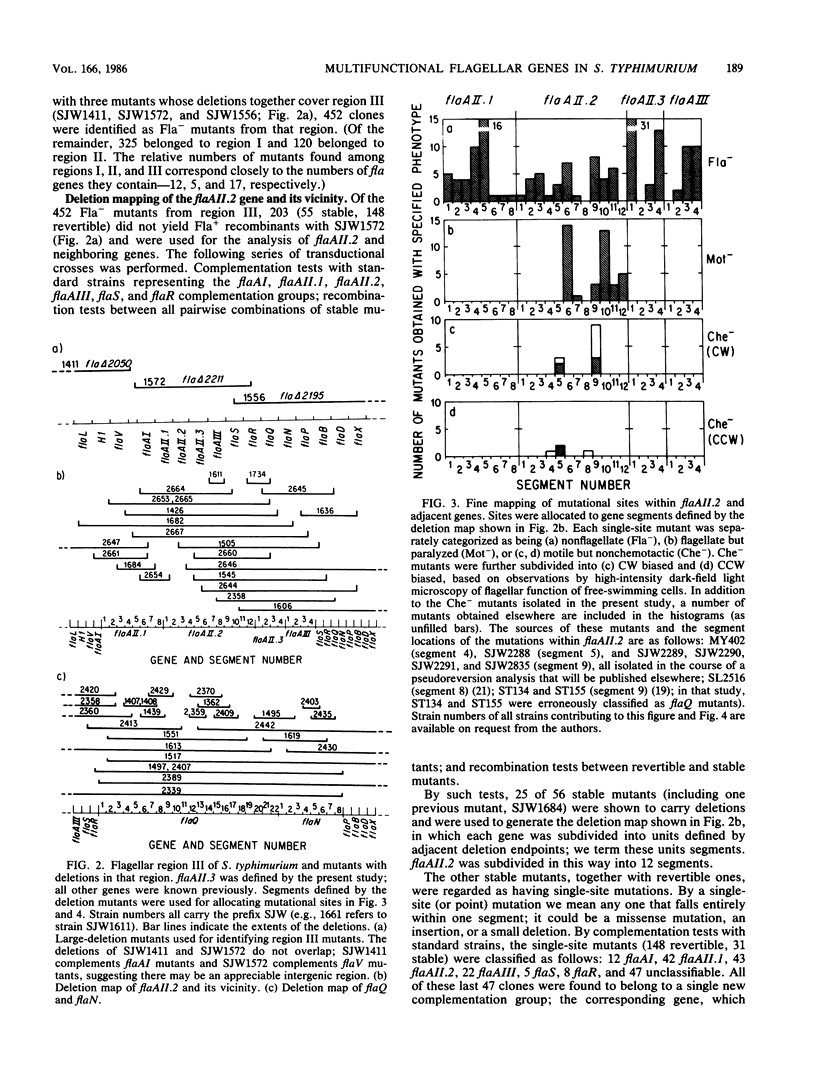
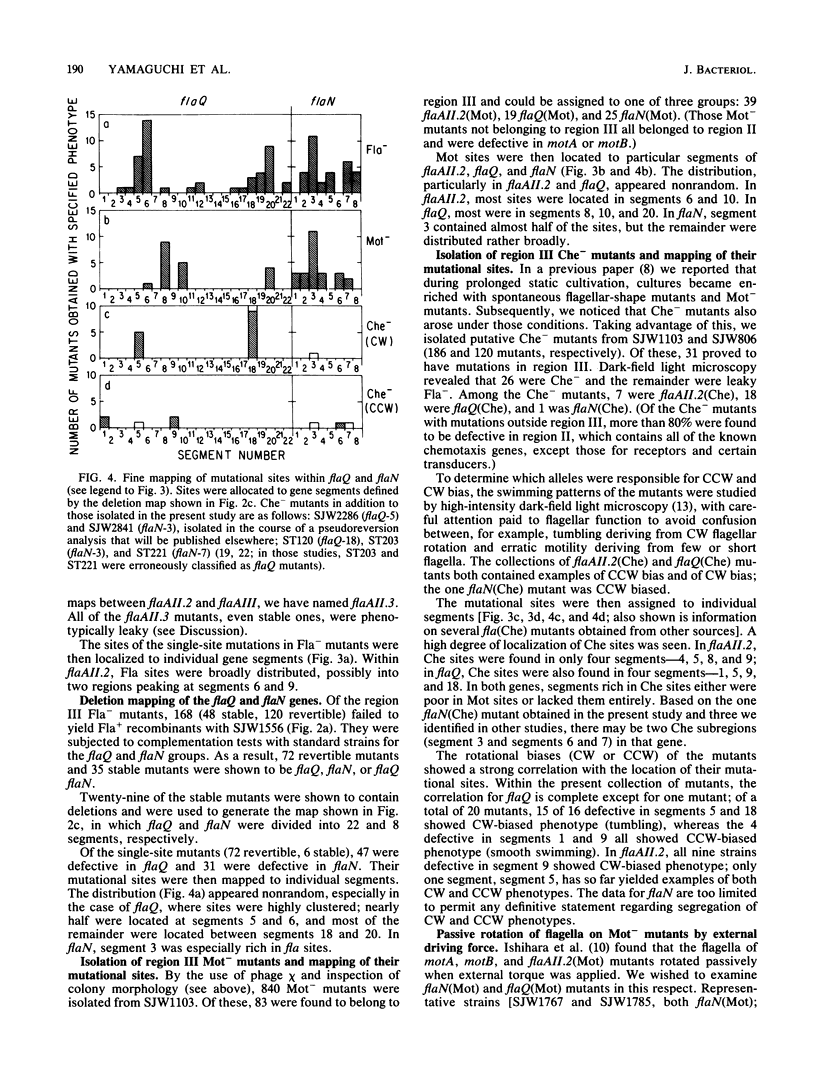
Three flagellar genes of Salmonella typhimurium (flaAII.2, flaQ, and flaN) were found to be multifunctional, each being associated with four distinct mutant phenotypes: nonflagellate (Fla-), paralyzed (Mot-), nonchemotactic (Che-) with clockwise motor bias, and nonchemotactic (Che-) with counterclockwise motor bias. The distribution of Fla, Mot, and Che mutational sites within each gene was examined. Fla sites were fairly broadly distributed, whereas Mot and Che sites were more narrowly defined. Local subregions rich in sites of one type were not generally rich in sites of another type. Among Che sites, there was little overlap between those corresponding to a clockwise bias and those corresponding to a counterclockwise bias. Our results suggest that within the corresponding gene products there are specialized subregions for flagellar structure, motor rotation, and control of the sense of rotation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa S. I., Dean G. E., Jones C. J., Macnab R. M., Yamaguchi S. Purification and characterization of the flagellar hook-basal body complex of Salmonella typhimurium. J Bacteriol. 1985 Mar;161(3):836–849. doi: 10.1128/jb.161.3.836-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswad D., Koshland D. E., Jr Isolation, characterization and complementation of Salmonella typhimurium chemotaxis mutants. J Mol Biol. 1975 Sep 15;97(2):225–235. doi: 10.1016/s0022-2836(75)80036-2. [DOI] [PubMed] [Google Scholar]
- Bartlett D. H., Matsumura P. Identification of Escherichia coli region III flagellar gene products and description of two new flagellar genes. J Bacteriol. 1984 Nov;160(2):577–585. doi: 10.1128/jb.160.2.577-585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg D. O., Koshland D. E., Jr Identification of a bacterial sensing protein and effects of its elevated expression. J Bacteriol. 1985 Apr;162(1):398–405. doi: 10.1128/jb.162.1.398-405.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A. L., Stocker B. A. Salmonella typhimurium mutants generally defective in chemotaxis. J Bacteriol. 1976 Dec;128(3):754–765. doi: 10.1128/jb.128.3.754-765.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco A. L., Parkinson J. S., Koshland D. E., Jr Functional homology of chemotaxis genes in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1979 Jul;139(1):107–114. doi: 10.1128/jb.139.1.107-114.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean G. E., Aizawa S. I., Macnab R. M. flaAII (motC, cheV) of Salmonella typhimurium is a structural gene involved in energization and switching of the flagellar motor. J Bacteriol. 1983 Apr;154(1):84–91. doi: 10.1128/jb.154.1.84-91.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M. Genetic studies of paralyzed mutant in Salmonella. I. Genetic fine structure of the mot loci in Salmonella typhimurium. Genetics. 1966 Sep;54(3):715–726. doi: 10.1093/genetics/54.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H., Yamaguchi S., Taira T., Iino T. A simple method for the isolation of flagellar shape mutants in Salmonella. J Gen Microbiol. 1981 Jul;125(1):213–216. doi: 10.1099/00221287-125-1-213. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L., Harayama S. Sensory transduction in bacterial chemotaxis. Int Rev Cytol. 1983;81:33–70. doi: 10.1016/s0074-7696(08)62334-7. [DOI] [PubMed] [Google Scholar]
- Ishihara A., Yamaguchi S., Hotani H. Passive rotation of flagella on paralyzed Salmonella typhimurium (mot) mutants by external rotatory driving force. J Bacteriol. 1981 Feb;145(2):1082–1084. doi: 10.1128/jb.145.2.1082-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Macnab R. M., DeFranco A. L., Koshland D. E., Jr Inversion of a behavioral response in bacterial chemotaxis: explanation at the molecular level. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4150–4154. doi: 10.1073/pnas.75.9.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsukake K., Iino T., Komeda Y., Yamaguchi S. Functional homology of fla genes between Salmonella typhimurium and Escherichia coli. Mol Gen Genet. 1980 Apr;178(1):59–67. doi: 10.1007/BF00267213. [DOI] [PubMed] [Google Scholar]
- MEYNELL E. W. A phage, phi chi, which attacks motile bacteria. J Gen Microbiol. 1961 Jun;25:253–290. doi: 10.1099/00221287-25-2-253. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Aizawa S. Bacterial motility and the bacterial flagellar motor. Annu Rev Biophys Bioeng. 1984;13:51–83. doi: 10.1146/annurev.bb.13.060184.000411. [DOI] [PubMed] [Google Scholar]
- Macnab R. M. Examination of bacterial flagellation by dark-field microscopy. J Clin Microbiol. 1976 Sep;4(3):258–265. doi: 10.1128/jcm.4.3.258-265.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W. Bacterial chemotaxis: biochemistry of behavior in a single cell. Crit Rev Microbiol. 1985;12(2):95–130. doi: 10.3109/10408418509104426. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S., Parker S. R., Talbert P. B., Houts S. E. Interactions between chemotaxis genes and flagellar genes in Escherichia coli. J Bacteriol. 1983 Jul;155(1):265–274. doi: 10.1128/jb.155.1.265-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravid S., Eisenbach M. Direction of flagellar rotation in bacterial cell envelopes. J Bacteriol. 1984 Apr;158(1):222–230. doi: 10.1128/jb.158.1.222-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubik B. A., Koshland D. E., Jr Potentiation, desensitization, and inversion of response in bacterial sensing of chemical stimuli. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2820–2824. doi: 10.1073/pnas.75.6.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Iino T., Horiguchi T., Yamaguchi S. Incomplete flagellar structures in nonflagellate mutants of Salmonella typhimurium. J Bacteriol. 1978 Feb;133(2):904–915. doi: 10.1128/jb.133.2.904-915.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrick H. M., Taylor B. L., Koshland D. E., Jr Chemotactic mechanism of Salmonella typhimurium: preliminary mapping and characterization of mutants. J Bacteriol. 1977 Apr;130(1):223–231. doi: 10.1128/jb.130.1.223-231.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Fujita H., Sugata K., Taira T., Iino T. Genetic analysis of H2, the structural gene for phase-2 flagellin in Salmonella. J Gen Microbiol. 1984 Feb;130(2):255–265. doi: 10.1099/00221287-130-2-255. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Fujita H., Taira T., Kutsukake K., Homma M., Iino T. Genetic analysis of three additional fla genes in Salmonella typhimurium. J Gen Microbiol. 1984 Dec;130(12):3339–3342. doi: 10.1099/00221287-130-12-3339. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Iino T., Horiguchi T., Ota K. Genetic analysis of fla and mot cistrons closely linked to H1 in Salmonella abortusequi and its derivatives. J Gen Microbiol. 1972 Apr;70(1):59–75. doi: 10.1099/00221287-70-1-59. [DOI] [PubMed] [Google Scholar]




