Abstract
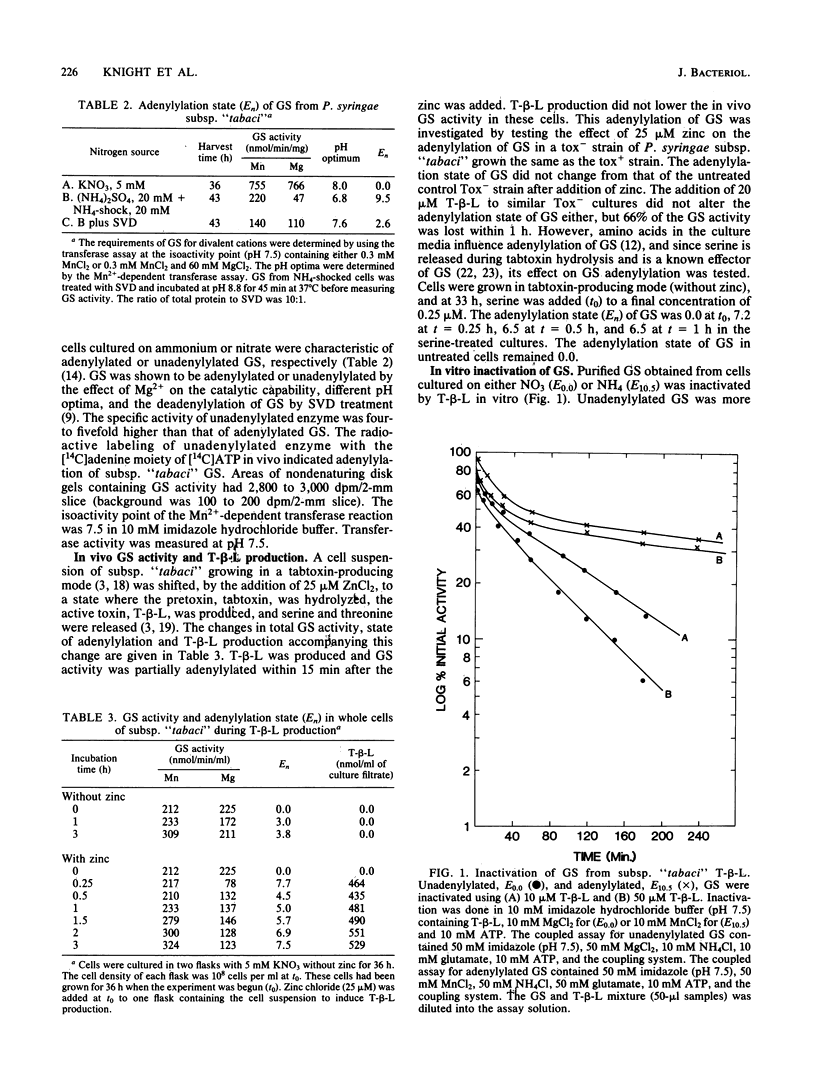
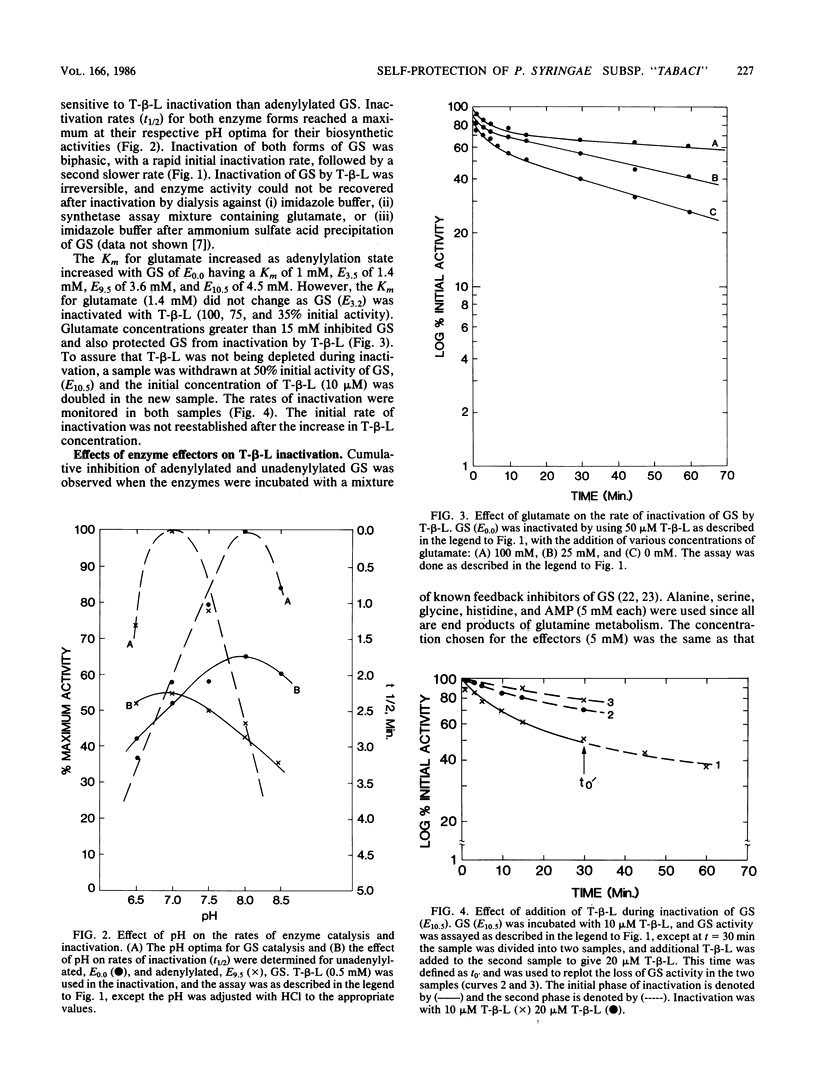
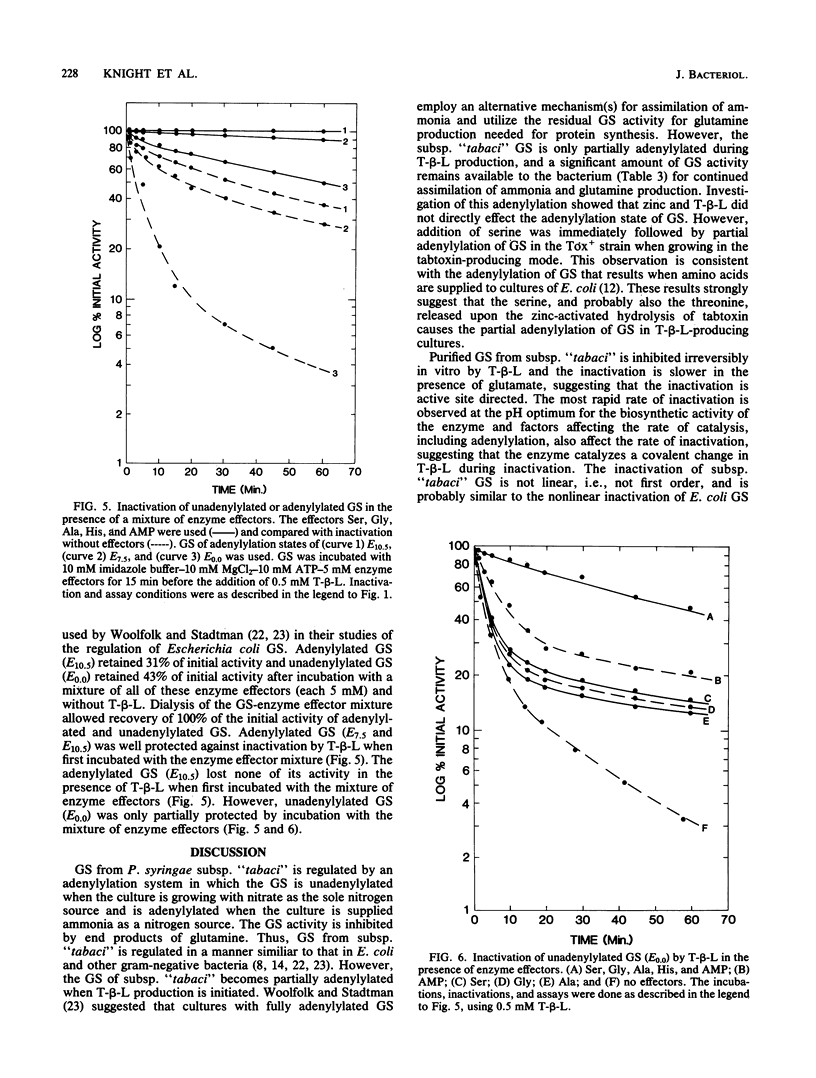
Selected pathovars of Pseudomonas syringae produce an extracellular phytotoxin, tabtoxinine-beta-lactam, that irreversibly inhibits its known physiological target, glutamine synthetase (GS). Pseudomonas syringae subsp. "tabaci" retains significant amounts of glutamine synthetase activity during toxin production in culture. As part of our investigation of the self-protection mechanism(s) used by these pathovars, we have determined that GS becomes adenylylated after toxin production is initiated and that the serine released from the zinc-activated hydrolysis of tabtoxin is a factor in the initiation of this adenylylation. The adenylylation state of this GS was estimated to range from E5.0-7.5. The irreversible inactivation by tabtoxinine-beta-lactam of unadenylylated and adenylylated glutamine synthetase purified from P. syringae subsp. "tabaci" was investigated. Adenylylated GS was inactivated by tabtoxinine-beta-lactam at a slower rate than was unadenylylated enzyme. Adenylylated GS (E7.5-10.5) was significantly protected from this inactivation in the presence of the enzyme effectors, AMP, Ala, Gly, His, and Ser. Thus, the combination of the adenylylation of GS after toxin production is initiated and the presence of the enzyme effectors in vivo could provide part of the self-protection mechanism used by subsp. "tabaci".
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender R. A., Janssen K. A., Resnick A. D., Blumenberg M., Foor F., Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Frantz T. A., Peterson D. M., Durbin R. D. Sources of ammonium in oat leaves treated with tabtoxin or methionine sulfoximine. Plant Physiol. 1982 Feb;69(2):345–348. doi: 10.1104/pp.69.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston-Unkefer P. L., Macy P. A., Durbin R. D. Inactivation of Glutamine Synthetase by Tabtoxinine-beta-lactam : Effects of Substrates and pH. Plant Physiol. 1984 Sep;76(1):71–74. doi: 10.1104/pp.76.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi M. R., Ginsburg A. Reactivation of glutamine synthetase from Escherichia coli after auto-inactivation with L-methionine-S-sulfoximine, ATP, and Mn2+. J Biol Chem. 1982 Apr 25;257(8):4271–4278. [PubMed] [Google Scholar]
- Meyer J. M., Stadtman E. R. Glutamine synthetase of pseudomonads: some biochemical and physicochemical properties. J Bacteriol. 1981 May;146(2):705–712. doi: 10.1128/jb.146.2.705-712.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S. S., Tam L. Q. Mode of Action of the Toxin from Pseudomonas phaseolicola: I. Toxin Specificity, Chlorosis, and Ornithine Accumulation. Plant Physiol. 1972 May;49(5):803–807. doi: 10.1104/pp.49.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. G., Chock P. B., Wedler F. C., Sugiyama Y. Subunit interaction in unadenylylated glutamine synthetase from Escherichia coli. Evidence from methionine sulfoximine inhibition studies. J Biol Chem. 1981 Jan 25;256(2):644–648. [PubMed] [Google Scholar]
- Shrake A., Whitley E. J., Jr, Ginsburg A. Conformational differences between unadenylylated and adenylylated glutamine synthetase from Escherichia coli on binding L-methionine sulfoximine. J Biol Chem. 1980 Jan 25;255(2):581–589. [PubMed] [Google Scholar]
- Stadtman E. R., Smyrniotis P. Z., Davis J. N., Wittenberger M. E. Enzymic procedures for determining the average state of adenylylation of Escherichia coli glutamine synthetase. Anal Biochem. 1979 May;95(1):275–285. doi: 10.1016/0003-2697(79)90217-3. [DOI] [PubMed] [Google Scholar]
- Thomas M. D., Langston-Unkefer P. J., Uchytil T. F., Durbin R. D. Inhibition of Glutamine Synthetase from Pea by Tabtoxinine-beta-lactam. Plant Physiol. 1983 Apr;71(4):912–915. doi: 10.1104/pp.71.4.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wedler F. C., Sugiyama Y., Fisher K. E. Catalytic cooperativity and subunit interactions in Escherichia coli glutamine synthetase: binding and kinetics with methionine sulfoximine and related inhibitors. Biochemistry. 1982 Apr 27;21(9):2168–2177. doi: 10.1021/bi00538a028. [DOI] [PubMed] [Google Scholar]
- Woolfolk C. A., Shapiro B., Stadtman E. R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]
- Woolfolk C. A., Stadtman E. R. Regulation of glutamine synthetase. 3. Cumulative feedback inhibition of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1967 Mar 20;118(3):736–755. doi: 10.1016/0003-9861(67)90412-2. [DOI] [PubMed] [Google Scholar]



