Abstract
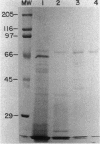
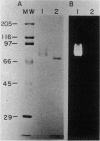
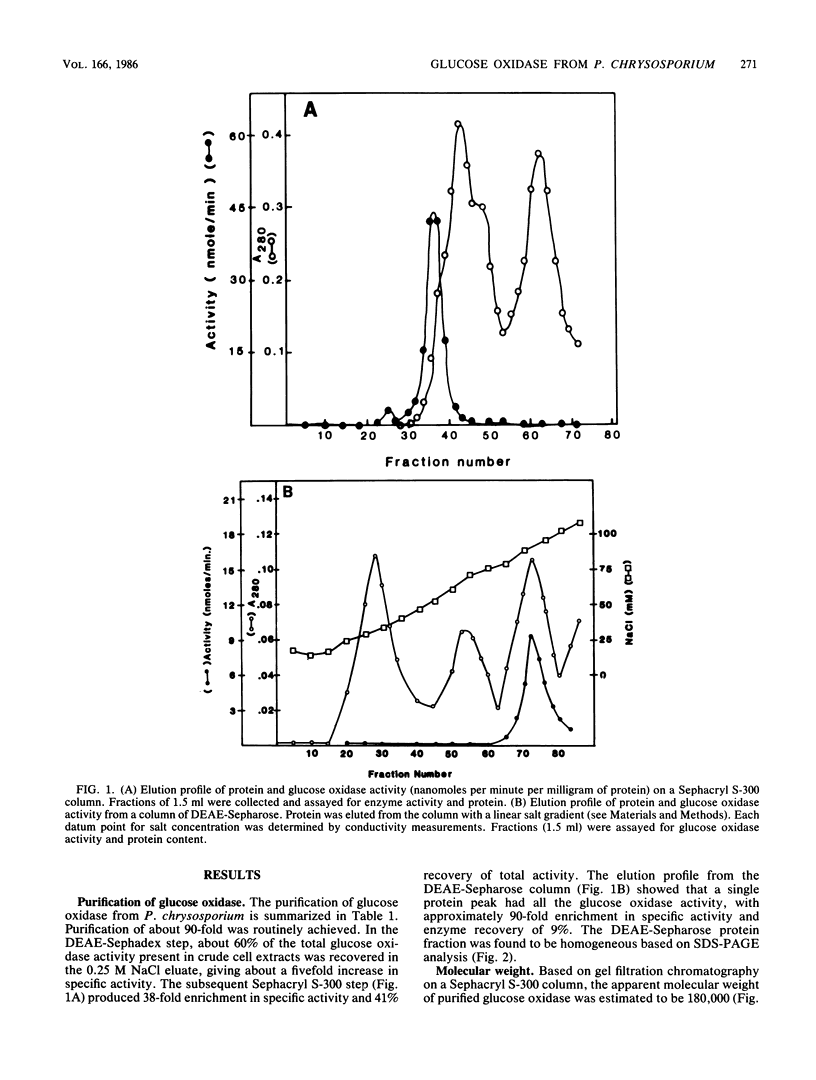
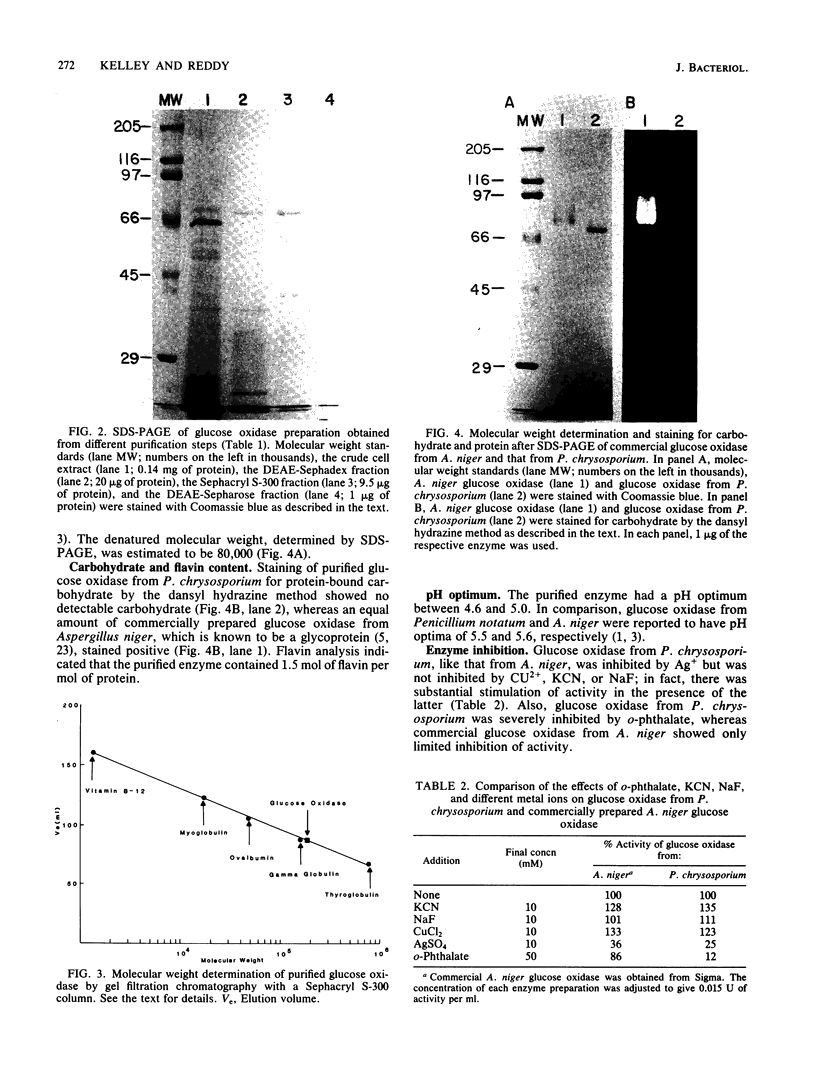
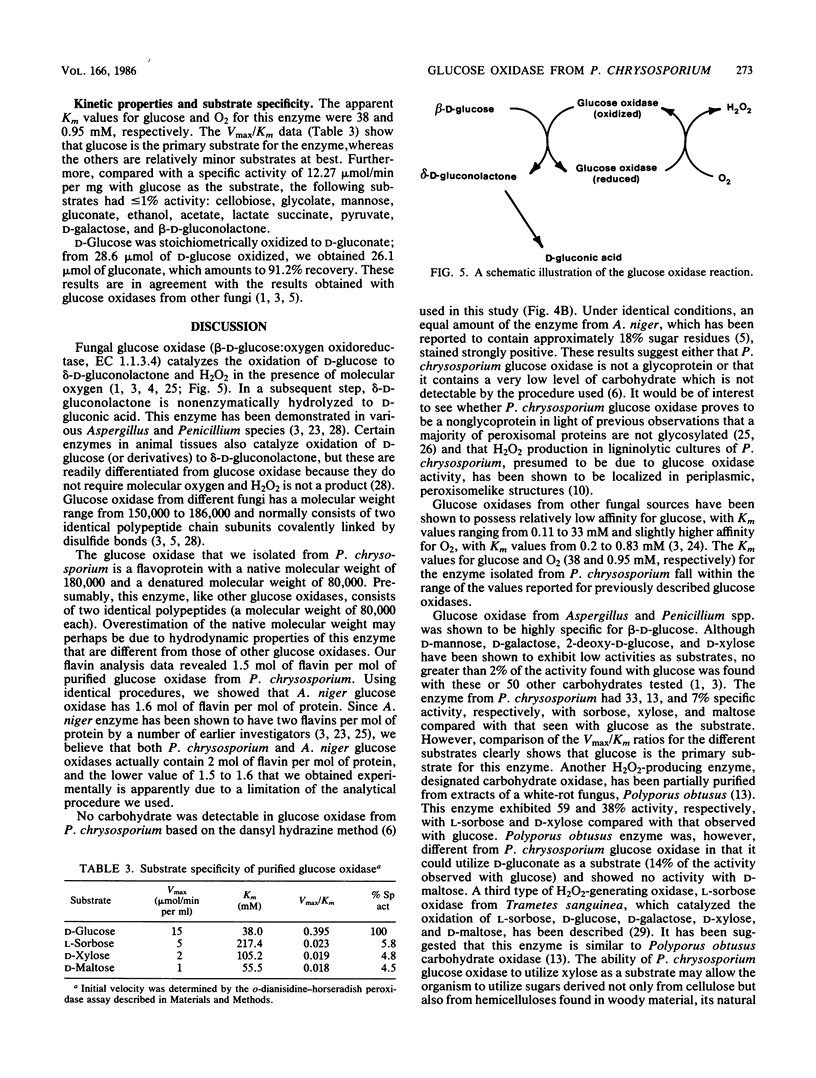
Glucose oxidase, an important source of hydrogen peroxide in lignin-degrading cultures of Phanerochaete chrysosporium, was purified to electrophoretic homogeneity by a combination of ion-exchange and molecular sieve chromatography. The enzyme is a flavoprotein with an apparent native molecular weight of 180,000 and a denatured molecular weight of 80,000. This enzyme does not appear to be a glycoprotein. It gives optimal activity with D-glucose, which is stoichiometrically oxidized to D-gluconate. The enzyme has a relatively broad pH optimum of 4 to 5. It is inhibited by Ag+ (10 mM) and o-phthalate (100 mM), but not by Cu2+, NaF, or KCN (each 10 mM).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS E. C., Jr, MAST R. L., FREE A. H. Specificity of glucose oxidase. Arch Biochem Biophys. 1960 Dec;91:230–234. doi: 10.1016/0003-9861(60)90495-1. [DOI] [PubMed] [Google Scholar]
- Andersson L. A., Renganathan V., Chiu A. A., Loehr T. M., Gold M. H. Spectral characterization of diarylpropane oxygenase, a novel peroxide-dependent, lignin-degrading heme enzyme. J Biol Chem. 1985 May 25;260(10):6080–6087. [PubMed] [Google Scholar]
- CERLETTI P., STROM R., GIORDANO M. G. Flavine peptides in tissues: the prosthetic group of succinic dehydrogenase. Arch Biochem Biophys. 1963 Jun;101:423–428. doi: 10.1016/0003-9861(63)90497-1. [DOI] [PubMed] [Google Scholar]
- Eckhardt A. E., Hayes C. E., Goldstein I. J. A sensitive fluorescent method for the detection of glycoproteins in polyacrylamide gels. Anal Biochem. 1976 May 21;73(1):192–197. doi: 10.1016/0003-2697(76)90154-8. [DOI] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Relationship Between Lignin Degradation and Production of Reduced Oxygen Species by Phanerochaete chrysosporium. Appl Environ Microbiol. 1983 Nov;46(5):1140–1145. doi: 10.1128/aem.46.5.1140-1145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney L. J., Reddy C. A., Pankratz H. S. Ultrastructural Localization of Hydrogen Peroxide Production in Ligninolytic Phanerochaete chrysosporium Cells. Appl Environ Microbiol. 1982 Sep;44(3):732–736. doi: 10.1128/aem.44.3.732-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. V., Gould J. M. Fatty acyl-coenzyme A oxidase activity and H2O2 production in Phanerochaete chrysosporium mycelia. Biochem Biophys Res Commun. 1984 Jan 30;118(2):437–443. doi: 10.1016/0006-291x(84)91322-6. [DOI] [PubMed] [Google Scholar]
- Janssen F. W., Ruelius H. W. Carbohydrate oxidase, a novel enzyme from Polyporus obtusus. II. Specificity and characterization of reaction products. Biochim Biophys Acta. 1968 Nov 19;167(3):501–510. doi: 10.1016/0005-2744(68)90040-5. [DOI] [PubMed] [Google Scholar]
- Kelley R. L., Reddy C. A. Ethylene production from alpha-oxo-gamma-methylthiobutyric acid is a sensitive measure of ligninolytic activity by Phanerochaete chrysosporium. Biochem J. 1982 Aug 15;206(2):423–425. doi: 10.1042/bj2060423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Tien M., Kalyanaraman B., Kirk T. K. The ligninase of Phanerochaete chrysosporium generates cation radicals from methoxybenzenes. J Biol Chem. 1985 Mar 10;260(5):2609–2612. [PubMed] [Google Scholar]
- Kutsuki H., Gold M. H. Generation of hydroxyl radical and its involvement in lignin degradation by Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1982 Nov 30;109(2):320–327. doi: 10.1016/0006-291x(82)91723-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- SWOBODA B. E., MASSEY V. PURIFICATION AND PROPERTIES OF THE GLUCOSE OXIDASE FROM ASPERGILLUS NIGER. J Biol Chem. 1965 May;240:2209–2215. [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamada Y., Lizuka K., Aida K., Uemura T. Enzymatic studies on the oxidation of sugar and sugar alcohol. 3. Purification and properties of L-sorbose oxidase from Trametes sanguinea. J Biochem. 1967 Aug;62(2):223–229. doi: 10.1093/oxfordjournals.jbchem.a128652. [DOI] [PubMed] [Google Scholar]