Abstract
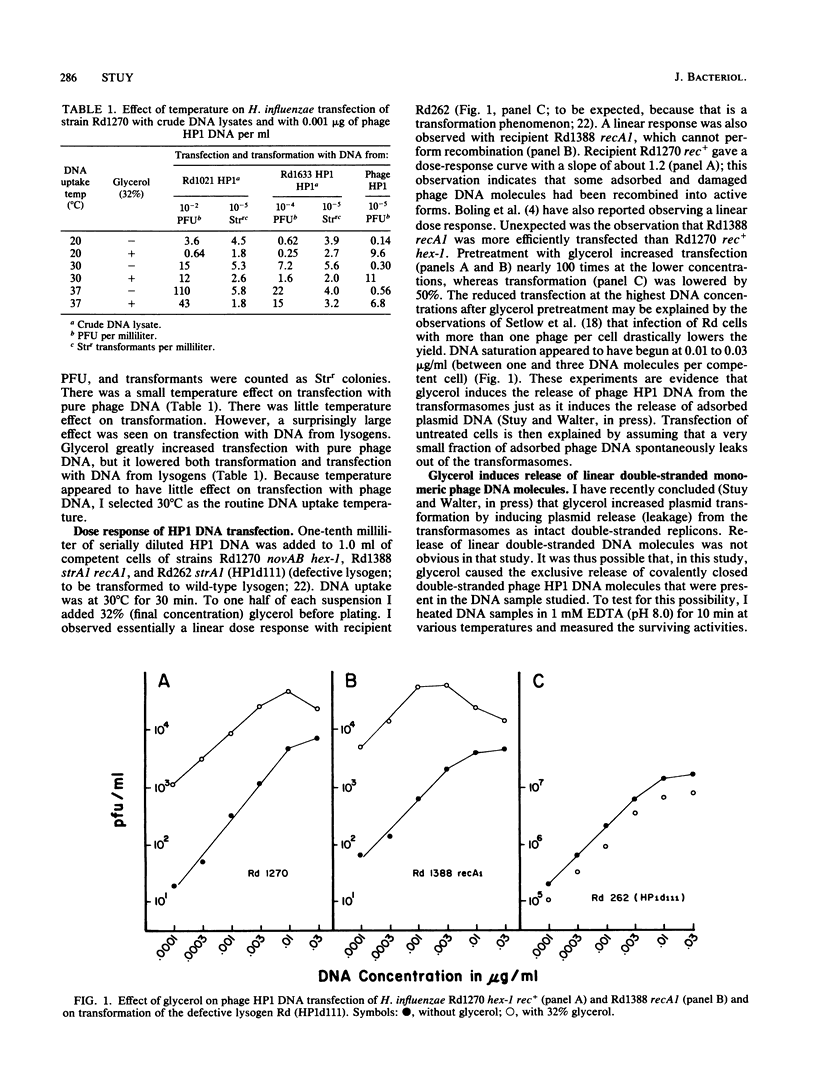
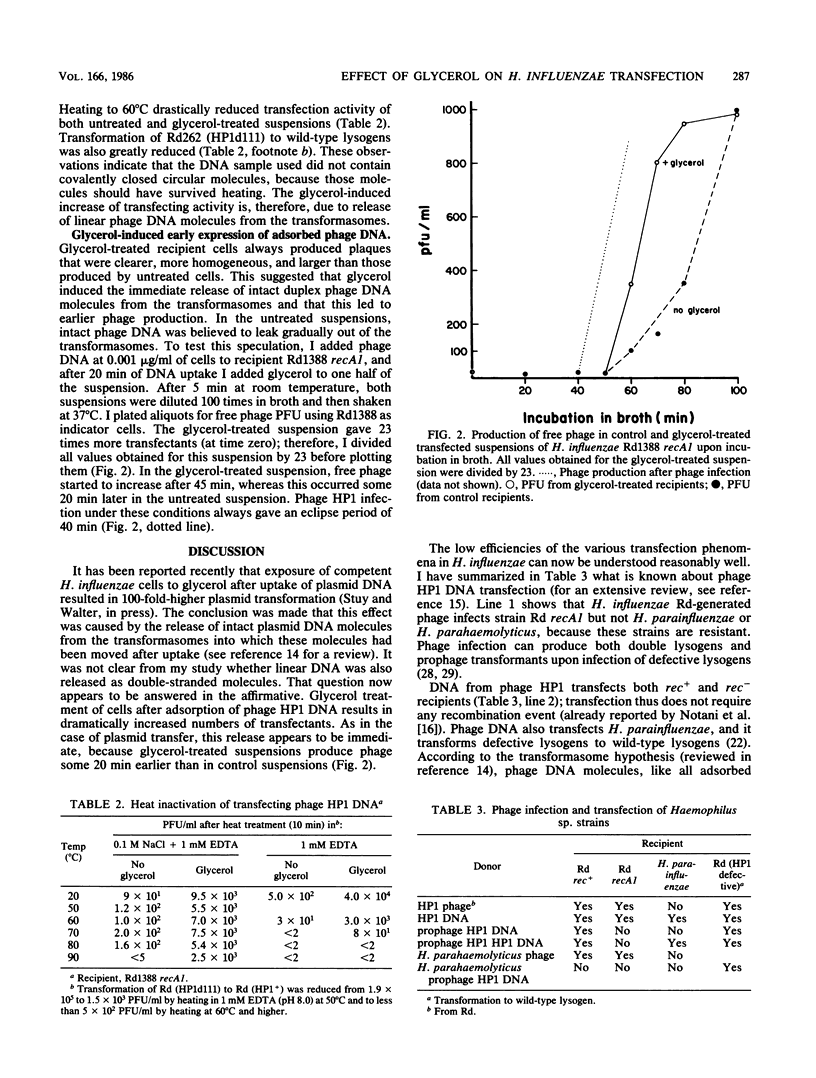
Competent Haemophilus influenzae bacteria were exposed to purified phage HP1 DNA and then plated for transfectants (PFU). When 32% (final concentration) glycerol was added before plating, between 10- and 100-fold more transfectants were observed. Glycerol had no significant effect on transfection with DNA from single or tandem double lysogens. It also had little effect on transformation with chromosomal DNA or on transformation of defective HP1 lysogens with phage HP1 DNA. It was concluded that glycerol induced the release of adsorbed linear double-stranded DNA into the interior of the cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barany F., Kahn M. E. Comparison of transformation mechanisms of Haemophilus parainfluenzae and Haemophilus influenzae. J Bacteriol. 1985 Jan;161(1):72–79. doi: 10.1128/jb.161.1.72-79.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barany F., Kahn M. E., Smith H. O. Directional transport and integration of donor DNA in Haemophilus influenzae transformation. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7274–7278. doi: 10.1073/pnas.80.23.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart B. J., Cox S. H. Radiation-sensitive and radiation-resistant mutants of Haemophilus influenzae. J Bacteriol. 1968 Jul;96(1):280–282. doi: 10.1128/jb.96.1.280-282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K., Allison D. P. Bacteriophage of Haemophilus influenzae. I. Differences between infection by whole phage, extracted phage DNA and prophage DNA extracted from lysogenic cells. J Mol Biol. 1972 Feb 14;63(3):335–348. doi: 10.1016/0022-2836(72)90431-7. [DOI] [PubMed] [Google Scholar]
- Deich R. A., Hoyer L. C. Generation and release of DNA-binding vesicles by Haemophilus influenzae during induction and loss of competence. J Bacteriol. 1982 Nov;152(2):855–864. doi: 10.1128/jb.152.2.855-864.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice W. P., Waldman A. S., Benjamin R. C., Huang P. C., Scocca J. J. Nucleotide sequence and properties of the cohesive DNA termini from bacteriophage HP1c1 of Haemophilus influenzae Rd. Gene. 1984 Nov;31(1-3):197–203. doi: 10.1016/0378-1119(84)90210-5. [DOI] [PubMed] [Google Scholar]
- Flock J. I. Transfection with replicating DNA from the temperate Bacillus bacteriophage phi 105 and with T4-ligase treated phi105 DNA: the importance in transfection of being longer than genome-length. Mol Gen Genet. 1978 Jul 6;163(1):7–15. doi: 10.1007/BF00268958. [DOI] [PubMed] [Google Scholar]
- Goodgal S. H. DNA uptake in Haemophilus transformation. Annu Rev Genet. 1982;16:169–192. doi: 10.1146/annurev.ge.16.120182.001125. [DOI] [PubMed] [Google Scholar]
- HARM W., RUPERT C. S. INFECTION OF TRANSFORMABLE CELLS OF HAEMOPHILUS INFLUENZAE BY BACTERIOPHAGE AND BACTERIOPHAGE DNA. Z Vererbungsl. 1963 Dec 30;94:336–348. doi: 10.1007/BF00897593. [DOI] [PubMed] [Google Scholar]
- Hoffmann J. F., Stuy J. H. Prophage recombination in transformation -negative mutants of Haemophilus influenzae. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1388–1393. doi: 10.1016/s0006-291x(72)80129-3. [DOI] [PubMed] [Google Scholar]
- Kahn M. E., Barany F., Smith H. O. Transformasomes: specialized membranous structures that protect DNA during Haemophilus transformation. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6927–6931. doi: 10.1073/pnas.80.22.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. E., Maul G., Goodgal S. H. Possible mechanism for donor DNA binding and transport in Haemophilus. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6370–6374. doi: 10.1073/pnas.79.20.6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. E., Smith H. O. Transformation in Haemophilus: a problem in membrane biology. J Membr Biol. 1984;81(2):89–103. doi: 10.1007/BF01868974. [DOI] [PubMed] [Google Scholar]
- Kahn M., Concino M., Gromkova R., Goodgal S. DNA binding activity of vesicles produced by competence deficient mutants of Haemophilus. Biochem Biophys Res Commun. 1979 Apr 13;87(3):764–772. doi: 10.1016/0006-291x(79)92024-2. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., Allison D. P. Intracellular events during infection by Haemophilus influenzae phage and transfection by its DNA. J Mol Biol. 1973 Apr 25;75(4):581–599. doi: 10.1016/0022-2836(73)90293-3. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K. Mechanism of bacterial transformation and transfection. Prog Nucleic Acid Res Mol Biol. 1974;14(0):39–100. doi: 10.1016/s0079-6603(08)60205-6. [DOI] [PubMed] [Google Scholar]
- STUY J. H. Transformability of Haemophilus influenzae. J Gen Microbiol. 1962 Nov;29:537–549. doi: 10.1099/00221287-29-3-537. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E. Bacteriophage of Haemophilus influenzae. II. Repair of ultraviolet-irradiated phage DNA and the capacity of irradiated cells to make phage. J Mol Biol. 1972 Feb 14;63(3):349–362. doi: 10.1016/0022-2836(72)90432-9. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachura I., Mckinley F. W., Leidy G., Alexander H. E. Incomplete bacteriophage-like particles in ultraviolet-irradiated haemophilus. J Bacteriol. 1969 May;98(2):818–820. doi: 10.1128/jb.98.2.818-820.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Fate of transforming DNA in the Haemophilus influenzae transformation system. J Mol Biol. 1965 Sep;13(2):554–570. doi: 10.1016/s0022-2836(65)80117-6. [DOI] [PubMed] [Google Scholar]
- Stuy J. H. Fate of transforming bacteriophage HP1 deoxyribonucleic acid in Haemophilus influenzae lysogens. J Bacteriol. 1975 Jun;122(3):1038–1044. doi: 10.1128/jb.122.3.1038-1044.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H., Hoffmann J. F. Influence of transformability on the formation of superinfection double lysogens in Haemophilus influenzae. J Virol. 1971 Jan;7(1):127–136. doi: 10.1128/jvi.7.1.127-136.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Mechanism of Haemophilus influenzae transfection by single and double prophage deoxyribonucleic acid. J Bacteriol. 1980 Dec;144(3):1003–1008. doi: 10.1128/jb.144.3.1003-1008.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Mechanism of additive genetic transformation in Haemophilus influenzae. J Bacteriol. 1980 Dec;144(3):999–1002. doi: 10.1128/jb.144.3.999-1002.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Origin and direction of Haemophilus bacteriophage HP1 DNA replication. J Virol. 1974 Mar;13(3):757–759. doi: 10.1128/jvi.13.3.757-759.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Prophage mapping by transformation. Virology. 1969 Aug;38(4):567–572. doi: 10.1016/0042-6822(69)90177-9. [DOI] [PubMed] [Google Scholar]
- Stuy J. H. Restriction enzymes do not play a significant role in Haemophilus homospecific or heterospecific transformation. J Bacteriol. 1976 Oct;128(1):212–220. doi: 10.1128/jb.128.1.212-220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H., Walter R. B. Addition, deletion, and substitution of long nonhomologous deoxyribonucleic acid segments by genetic transformation of Haemophilus influenzae. J Bacteriol. 1981 Nov;148(2):565–571. doi: 10.1128/jb.148.2.565-571.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


