Abstract
Celiac disease is a common severe intestinal disease resulting from intolerance to dietary wheat gluten and related proteins. The large majority of patients expresses the HLA-DQ2 and/or DQ8 molecules, and gluten-specific HLA-DQ-restricted T cells have been found at the site of the lesion in the gut. The nature of peptides that are recognized by such T cells, however, has been unclear so far. We now report the identification of a gliadin-derived epitope that dominantly is recognized by intestinal gluten-specific HLA-DQ8-restricted T cells. The characterization of such epitopes is a key step toward the development of strategies to interfere in mechanisms involved in the pathogenesis of celiac disease.
In 1953, it was first recognized that ingestion of gluten, a common dietary protein present in wheat, causes celiac disease (CD) in sensitive individuals (1). Gluten is a complex mixture of glutamine- and proline-rich glutenin and gliadin molecules, the latter of which are thought to be responsible for disease induction (2, 3). Ingestion of gluten by sensitive individuals induces a T cell infiltrate, crypt cell hyperplasia, and villous atrophy in the small intestine, resulting in diarrhea and malabsorption. The hypothesis that CD is a T cell-mediated immunological disease has been supported by the observation that the large majority of patients expresses the HLA-DQ2 [DQ(α1*0501,β1*02)] and/or -DQ8 [DQ(α1*0301,β1*0302)] molecules (4). Furthermore, HLA-DQ-restricted, gluten-specific T cells have been isolated from the small intestine of CD patients (5, 6). The identification of the putative disease-inducing, gluten-derived T cell stimulatory epitopes, however, has been hampered by the vast heterogeneity and peculiar nature of the gluten proteins. We now report the identification of a gliadin peptide that is recognized by T cell clones from small intestine biopsies of two unrelated CD patients.
METHODS
Antigens and Peptides.
A pepsin/trypsin digest of gluten was prepared as follows: 1 g of gluten (Fluka) was solubilized in 10 ml of 1 M acetic acid and boiled for 10 min. Pepsin (10 mg) (Sigma P-6887, purified, activity 3,200–4,500 units/mg) was added first, and the mixture was incubated for 4 h at 37°C. Subsequently, the pH was adjusted to 7.8 with NaOH, followed by the addition of 10 mg of trypsin (Sigma T-8253, type III, activity 10,000–13,000 benzoyl l-arginine ethyl ester units/mg). Then, the mixture was incubated for another 4 h at 25°C. Finally, 10 mg of trypsin inhibitor (Sigma) was used to stop further trypsin activity, followed by dialysis of the mixture against a large volume of water. Protein concentration was determined by using a bicinchoninic acid protein assay (Pierce). Synthetic peptides were made as described (8).
Generation of Gluten-Specific T Cells.
A small intestinal biopsy was taken from patient S., an adult Dutch CD patient on a gluten-free diet for several years. The patient gave informed consent for the study, which was approved by the hospital ethics committee. The patient was typed serologically to be HLA-DR3/4, DQ2/8, thus carrying both CD-associated DQ dimers. After treatment of the biopsy with 1 mM DTT (10 min) and 0.75 mM EDTA (4 h) in calcium/magnesium-free Hanks’ balanced salt solution (GIBCO) at 37°C under mild agitation, the biopsy was put into culture with autologous interleukin 4/granulocyte/macrophage-colony stimulating factor-cultured monocytes as a source of highly endocytic antigen-presenting cells (19). The antigen-presenting cells had been cultured in the presence of 250 μg/ml enzyme-treated gluten preparation for 48 h before the biopsy was taken. After 5 and 10 days of coculture, 20 units/ml r-interleukin 2 (Eurocetus, Amsterdam, The Netherlands) was added to the culture medium [RPMI medium 1640 (GIBCO)]. Subsequently, the cells were restimulated on an 8-day cycle with 20 units/ml r-interleukin 2 and 1 μg/ml phytohemagglutinin (Murex Diagnostics, Dartford, U.K.), antigen and autologous monocyte-enriched irradiated PBMCs as antigen-presenting cells. TCCs were established by limiting dilution (0.3 cell/well) in 150 μl of culture medium in 96-well, round-bottomed plates (Falcon) by using 105 allogeneic PBMCs (3,000 rad), 1 μg/ml phytohemagglutinin, and 20 units/ml r-interleukin 2. This procedure resulted in the establishment of several HLA-DQ-restricted TCCs with a CD3+, CD4+, T cell antigen receptor α/β+ phenotype. Proliferation assays were performed in duplicate or triplicate in 150 μl of culture medium in 96-well, flat-bottomed plates (Falcon) by using 104 T cells stimulated with 105 PBMCs (3,000 rad) either in the absence or presence of antigen at several concentrations. After 48 h, cultures were pulsed with 0.5 μCi 3H-thymidine and harvested 18 h thereafter. The generation of gluten-specific TCCs from the Norwegian patient Å.W. has been described (6).
HPLC Purification of the Pepsin/Trypsin Digest of Gluten.
The enzymatic digest of gluten was purified in three successive rounds of HPLC as follows: first, ±3 mg of gluten was purified on an analytical HPLC system (Jasco, Easton, MD), using a gradient of 2% B/min (column Vydac 218TP1010, buffer B: acetonitrile, containing 0.1% trifluoric acid). Subsequently, bioactive fractions were separated further by micro-rp-HPLC (SMART, Pharmacia) using a gradient of 0.25% B/min [column C2/C18 sc 2.1/10 (Pharmacia), buffer B: acetonitrile, containing 0.1% trifluoric acid]. Finally, bioactive fractions were subfractionated by micro-rp-HPLC, using a gradient of 0.25% B/min (buffer B: acetonitrile, 0.1% heptafluorobutyric acid).
Mass Spectrometry.
HPLC fractions were monitored for their peptide content by matrix-assisted laser desorption ionization time-of-flight mass spectrometry on a Lasermat (Finnigan-MAT, San Jose, CA) as described (8). Electrospray ionization mass spectrometry was performed on a hybrid quadrupole time-of-flight mass spectrometer, the Q-TOF (Micromass, Manchester, U.K.). The final bioactive fraction was introduced either by off-line nanoelectrospray using type A needles (Micromass) or by flow injection analysis using the on-line nanoflow electrospray interface (capillary tip 20-μm internal diameter × 90-μm outer diameter) with an approximate flow rate of 200 nl/min. In the latter case, 0.5- to 1.0-μl injections were done with a dedicated micro/nano HPLC autosampler, the FAMOS (LC Packings, Amsterdam, The Netherlands). MS/MS analyses were performed on the most abundant peptide peaks present in the bioactive fraction. Precursors (the triple and quadruple protonated molecules in case of the 35-mer) were selected with the quadrupole, and fragments were collected with high efficiency with the orthogonal time-of-flight mass spectrometer. The collision gas applied was argon (pressure 4 × 10−5 mbar), and the collision voltage was ≈30 V.
Database Searching.
The program PeptideSearch was used for sequence elucidation. This program has been developed to identify sequences in databases using mass spectral information. This program is available on request from the authors of the program (20).
RESULTS
Isolation of HLA-DQ-Restricted Gluten-Specific T Cells.
Gluten-specific T cell clones (TCCs) were isolated from a small intestine biopsy of the Dutch CD patient S. The reactivity of the TCCs was investigated by using a panel of HLA-typed peripheral blood mononuclear cells (PBMCs) in the presence of a gluten digest. On the basis of HLA-restriction, the TCCs could be divided into two categories: restricted either by HLA-DQ2 (represented by TCC S11 in Table 1) or by HLA-DQ8 (represented by TCC S2 in Table 1). In line with this, we observed that the gluten-specific proliferative response of these TCCs could be blocked by an HLA-DQ-specific mAb but not by an HLA-DR-specific mAb (Table 1).
Table 1.
TCC S11 is restricted via HLA-DQ2; TCC S2 is restricted via HLA-DQ8
| TCC | Proliferative response, cpm × 10−3
|
|||||
|---|---|---|---|---|---|---|
| TCC S2 | TCC S11 | |||||
| APC HLA-DQ type | 2/8 | 1/8 | 2/2 | 2/8 | 1/8 | 2/2 |
| −Gluten | 0.1 | 0.1 | 0.1 | 0.1 | 0.4 | 0.1 |
| +Gluten | 7.8 | 7.2 | 0.1 | 8.1 | 0.4 | 7.3 |
| +Anti-DR | 8.5 | 6.1 | 1.2 | 7.5 | 0.3 | 7.5 |
| +Anti-DQ | 0.1 | 0.1 | 0.1 | 0.7 | 0.2 | 0.7 |
| +Gliadin peptide | 36.0 | 30.0 | 0.7 | 0.2 | nd | nd |
T cell responses of two types of gluten-specific TCCs derived from patient S. T cells (104) of the indicated TCCs were incubated with 105 irradiated (3,000 rad) HLA-DQ2/8-, DQ1/8-, or DQ2/2-positive PBMCs as antigen-presenting cells either in the absence of gluten, in the presence of 0.7 mg/ml pepsin/trypsin digest of gluten, or in the presence of 10 μg/ml the gliadin peptide identified in this report (gliadin 198-222). For blocking of the T cell responses, the anti-DR and anti-DQ-specific mAbs B8.11.2 and SPV-L3 were used, respectively. Values shown are mean cpm (×10−3) of duplicate cultures. Values considered positive are displayed in bold. nd, not done.
Identification of an HLA-DQ8-Restricted, Gliadin-Derived T Cell Epitope.
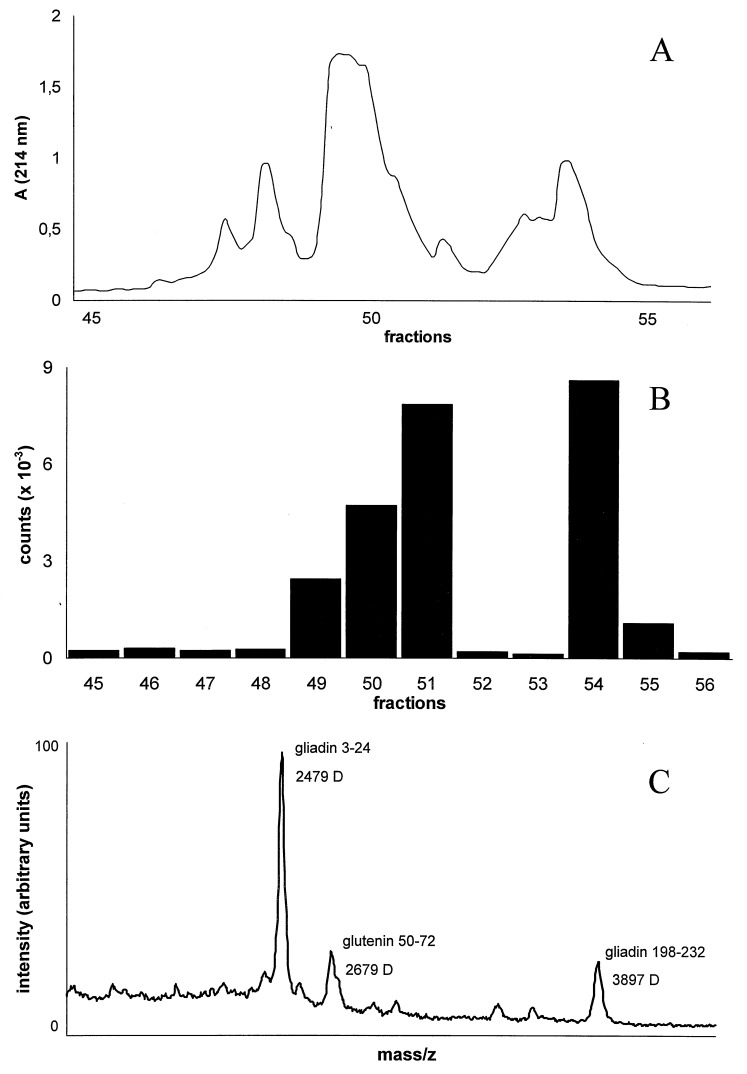
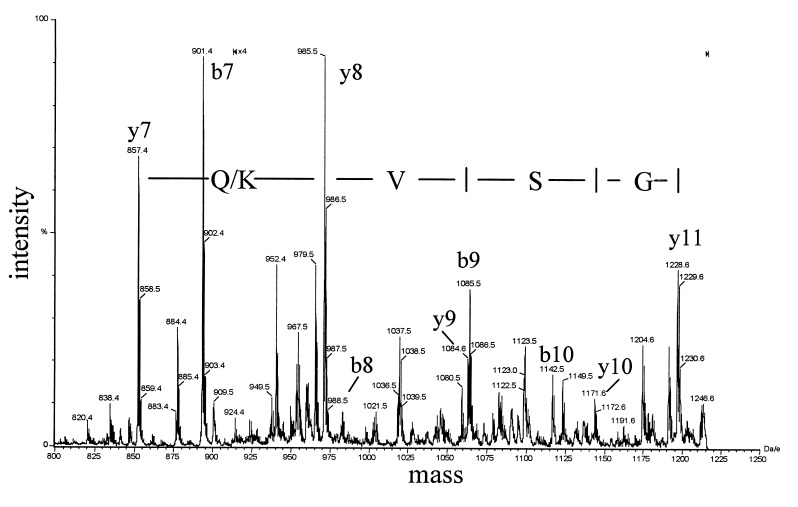
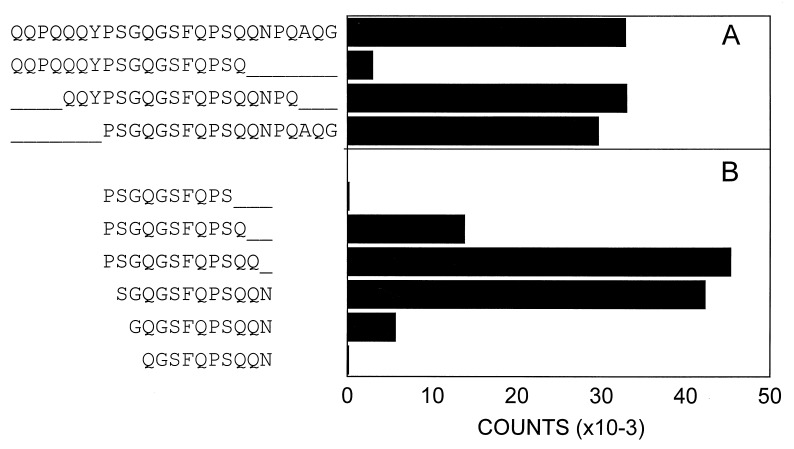
To identify the gluten-derived epitopes that are recognized by the TCCs of patient S., a pepsin/trypsin digest of gluten was purified in consecutive rounds of HPLC. After each purification step, the peptide content of the fractions was monitored by time-of-flight mass spectrometry, and the fractions were screened for their bioactivity in a standard T cell proliferation assay. After three rounds of HPLC purification (Fig. 1A), one of the fractions (54) that strongly stimulated TCC S2 (DQ8-restricted) (Fig. 1B) was found to contain three dominant peptides with masses of 2,479, 2,679, and 3,897-Da (Fig. 1C). To obtain amino acid sequence information, these peptides were subjected individually to tandem mass spectral analyses using a hybrid quadrupole–time-of-flight mass spectrometer. Such an analysis yields a peptide fragmentation pattern that contains information on the nature and order of the amino acids in the peptide. As an example, part of the fragmentation pattern of the 3,897 Da fragment is shown in Fig. 2. The interpretation of this pattern yielded the partial amino acid sequence Q/K-V-S. This sequence is preceded by a peptide fragment of 857.5 Da, with unknown amino acid composition. On the basis of this information and the actual mass of the complete peptide (3,897 Da), a database search was carried out that identified gliadin (residues 198–232) as the source protein (gda09, SwissProt P18573). The source proteins for the other two peptides were identified in a similar fashion. The peptide of 2,679 Da was found to represent a glutenin sequence (residues 50–72), whereas the 2,479-Da peptide was derived from gliadin (residues 3–24) (Fig. 1C). Synthetic versions of the identified peptides showed that only the peptide corresponding to the 3,897-Da peak stimulated TCC S2 (data not shown). The stimulating capacity was found in a peptide corresponding to the first 25 aa of this gliadin fragment (residues 198–222). Next, we tested a set of overlapping peptides, which indicated that the epitope is located in the central region of the gliadin 198–222 peptide (Fig. 3A). Additional truncation analysis revealed that the minimal epitope comprises residues 206–216 (sequence: SGQGSFQPSQQ) (Fig. 3B). A sequence homology search with this peptide resulted in the identification of five highly similar gliadin sequences. Only one of these peptides (sequence: SSQGSFQPSQQN) was found to stimulate TCC S2 (not shown). Next, we tested a panel of HLA-DQ8-restricted, gluten-specific TCCs derived from the small intestinal mucosa of a Norwegian CD patient (CD282). Six of eight HLA-DQ8-restricted TCCs derived from this patient recognized the gliadin 198–222 peptide (three representative examples are shown in Table 2).
Figure 1.
HPLC purification of the gluten digest combined with a T cell bioassay and mass spectral analysis. (A) rp-HPLC profile of the pepsin/trypsin digest of gluten after three rounds of purification. (B) Stimulatory capacity of the collected rp-HPLC fractions shown in A. T cells (104) of TCC S2 were stimulated with 105 PBMCs (3,000 rad) from an HLA-DQ2/DQ8-positive donor in the presence of 2 μl of each rp-HPLC fraction (total fraction size: 100 μl). Values show mean cpm (×10−3) of duplicate cultures. SD is <10%. (C) Matrix-associated laser desorption ionization mass spectrometry profile (Lasermat) of the bioactive fraction 54 (see A and B).
Figure 2.
Partial product ion mass spectrum of the 35-mer gliadin peptide (residues 198–232). The spectrum displays two series of peaks, the so-called b-ions and y-ions, that allow the sequence to be elucidated. A part of the y-ion series consists of the masses 857.5, 985.5, 1,084.5, and 1,171.6, which correspond to the partial sequence Glutamine/Lysine (Q/K),Valine (V), Serine (S). This information, together with the total mass of the 35-mer peptide (3,897 Da), was put in the program peptidesearch. This led to several hits, but only one gliadine-related peptide was found, gda09 198–232. In a similar fashion, the peptide sequences of the 2,479- and 2,679-Da peaks (Fig. 1C) were elucidated. These peaks were found to represent gliadin residues 3–24 (sequence VPVPQLQPQNPSQQQPQEQVPL) and glutenin residues 50–72 (sequence SQQQQPPFSQQQPPFSQQELPIL).
Figure 3.
Truncation analysis of gliadin peptide 198–222. T cells (104) (TCC S2) were stimulated with 105 PBMCs (3,000 rad) from an HLA-DQ2/DQ8-positive donor in the presence of 7.5 μg/ml the indicated peptide sequences. Amino acids are shown as single-letter codes. (A) Results of a set of 18-mer peptides covering the gliadin 198–222 sequence. (B) Results of a set of truncated variants of the central portion of gliadin 198–222. Values show mean cpm (×10−3) of truplicate cultures. SD is <10%.
Table 2.
Proliferative T cell responses of HLA-DQ8-restricted, gluten-specific TCCs from the Norwegian patient CD282
| TCC | Proliferative response, cpm × 10−3
|
||
|---|---|---|---|
| 1.12 | 2.2 | 2.35 | |
| −Gliadin | 0.9 | 1.7 | 0.7 |
| +Gliadin | 45.0 | 12.9 | 164.0 |
| +Gliadin 198-222 | 1.2 | 42.0 | 233.6 |
T cells (5 × 104) of the indicated TCCs were incubated with 5 × 104 irradiated (10,000 rad) EBV-BLCLs from an HLA-DQ8+ individual as antigen-presenting cells, either in the absence of gliadin, in the presence of 1 mg/ml a pepsin/trypsin digest of gliadin or 10 μg/ml of the gliadin 198-222 peptide. Values shown are mean cpm (×10−3) of duplicate cultures. Values considered positive are displayed in bold. In total, eight clones were tested, six of which responded to both pepsin/trypsin digest of gliadin and to the gliadin 198-222 peptide.
DISCUSSION
This report describes the identification of a gliadin-derived peptide that is recognized by HLA-DQ-restricted TCCs isolated from small intestine biopsies of two CD patients. The identification of such epitopes has been complicated by the large heterogeneity and complexity of gluten molecules. The nature of the present epitope could be elucidated only by the application of tandem mass spectrometry. The peptide is located in the carboxyl-terminal region of gliadin and represents a unique sequence that is found in only one of the many gliadin variants (gda09). The minimal peptide necessary to stimulate the TCCs is 11 aa long. This sequence is found in only two gliadin variants (gda04 and gda09). The minimal peptide contains the amino acid sequence PSQQ at its carboxyl terminus, representing one of the repetitive motifs within gliadin that has been suggested to be responsible for toxicity in CD patients (2).
In previous studies, two gliadin peptides were found to stimulate TCCs isolated from the peripheral blood of patients (9, 10). There is no indication, however, that these peptides are also stimulatory for T cells in the gut. Of interest, these peptides are located in the amino-terminal region of gliadin, whereas our current T cell epitope resides in the carboxyl terminal region. Although several studies reported that the disease-promoting activity of gliadin is located in the first 55 amino-terminal residues of the molecule (11, 12), there is also evidence that gliadin fragments in the carboxyl terminal region of gliadin can be toxic for CD patients (11, 13, 14). Peptide challenges with a peptide corresponding to residues 202–220 of gliadin, which displays high homology with the current HLA-DQ8- restricted T cell epitope, induced significant morphometric changes in the jejunal mucosa of one of four CD patients (15). Another in vivo study showed histological abnormalities in the small intestine of two CD patients on infusion of 100 mg of a very similar gliadin peptide (16). Unfortunately, in both studies, the outcome of the peptide challenges was not correlated to the HLA type of the individuals studied. With the current knowledge, one can conceive that these peptides were effective in the relatively small group of patients expressing the HLA-DQ8 molecule, and that the histological changes have occurred because of T cell recognition of these peptides. This possibility will be the subject of further studies.
In a recent study, tissue transglutaminase has been identified as the target for the antiendomysium antibodies that are characteristic of CD (7). Although tissue transglutaminase may be an important ancillary target antigen, it is unlikely that its recognition by antiendomysium antibodies plays a primary role in the disease process (21). The current epitope is derived from the disease-triggering antigen gliadin. This peptide is recognized by DQ8-restricted TCCs from two unrelated patients, which suggests that the epitope could be a common, perhaps disease-related antigen. It is clear, however, that other intestinal gluten-derived T cell stimulatory peptides exist. First of all, we observed that the DQ2-restricted, gluten-specific TCCs did not respond to the gliadin 198–222 peptide (Table 1). Moreover, not all DQ8-restricted TCCs recognized the gliadin 198–222 peptide (Table 2). Finally, it has been reported that a set of HLA-DQ-restricted, gluten-specific TCCs isolated from the small intestinal mucosa of five CD patients displayed a heterogeneous pattern of reactivity against three purified gliadins (17). The identification of these additional gluten-derived epitopes and their immunodominance will be the subject of additional studies.
The identified 35-mer gliadin fragment (residues 198–232) results most likely from pepsin digestion of the gliadin molecule because it contains pepsin-cleavage sites on both ends of the peptide. This idea implies that the fragment can be generated in the stomach, allowing direct binding to cell surface-expressed HLA-DQ molecules in the small intestine without a need for uptake and intracellular processing by professional antigen-presenting cells. Consequently, also nonprofessional antigen-presenting cells might stimulate gluten-specific T cells. In support of this possibility, we found that interferon γ-cultured intestinal epithelial cells can indeed activate the gliadin-specific T cell clone S2 in the presence of the gliadin peptide (not shown). It has been shown that gluten-specific T cells from CD patients produce relatively large amounts of interferon γ (ref. 18 and unpublished results), which indicates that intestinal epithelial cells could play an important role in perpetuation of the disease by the continuous activation of gluten-specific T cells in the gut during inflammation. The identification of the gluten-derived, T cell-stimulatory peptides is a key step toward the development of strategies to prevent T cell recognition of these peptides, thereby interfering with the production of cytokines and tissue destruction. Furthermore, this knowledge raises the possibility of future manipulation of the wheat genome to produce wheat products that are tolerated by gluten-sensitive individuals.
Acknowledgments
We thank Drs. E. Zanelli, F. A. W. Verreck, M. C. A. A. Tan, M. J. D. van Tol, S. Schoenberger, and B. O. Roep for critically reading the manuscript. This study was supported financially by the Dutch Organization for Scientific Research (NWO) Project 900-509-171, the European Community Project B104-CT95-0263, and the Research Council of Norway.
ABBREVIATIONS
- CD
celiac disease
- TCCs
T cell clones
- PBMCs
peripheral blood mononuclear cells
References
- 1.Van de Kamer J H, Weijers H A, Dicke W K. Acta Paediatr Scand. 1953;42:223–231. doi: 10.1111/j.1651-2227.1953.tb05586.x. [DOI] [PubMed] [Google Scholar]
- 2.Marsh M N. Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 3.Trier J S. N Engl J Med. 1991;325:1709–1719. doi: 10.1056/NEJM199112123252406. [DOI] [PubMed] [Google Scholar]
- 4.Sollid L M, Thorsby E. Gastroenterology. 1993;105:910–922. doi: 10.1016/0016-5085(93)90912-v. [DOI] [PubMed] [Google Scholar]
- 5.Lundin K E A, Scott H, Hansen T, Paulsen G, Halstensen T S, Fausa O, Thorsby E, Sollid L M. J Exp Med. 1993;178:187–196. doi: 10.1084/jem.178.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundin K E A, Scott H, Fausa O, Thorsby E, Sollid L M. Hum Immunol. 1994;41:285–291. doi: 10.1016/0198-8859(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 7.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken E O, Schuppan D. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 8.van de Wal Y, Kooy Y M C, Drijfhout J W, Amons R, Koning F. Immunogenetics. 1996;44:246–253. doi: 10.1007/BF02602553. [DOI] [PubMed] [Google Scholar]
- 9.Gjertsen H A, Lundin K E A, Sollid L M, Eriksen J A, Thorsby E. Hum Immunol. 1994;39:243–252. doi: 10.1016/0198-8859(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 10.Franco A, Appella E, Kagnoff M F, Chowers Y, Sakaguchi K, Grey H M, Sette A. Clin Immunol Immunopathol. 1994;71:75–81. doi: 10.1006/clin.1994.1054. [DOI] [PubMed] [Google Scholar]
- 11.de Ritis G, Auricchio S, Jones H W, Lew E J, Bernardin J F, Kasarda D D. Gastroenterology. 1988;94:41–49. doi: 10.1016/0016-5085(88)90607-5. [DOI] [PubMed] [Google Scholar]
- 12.Wieser H, Belitz H D, Idar D, Ashkenzai A. Z Lebensm Unters Forsch. 1986;182:115–117. doi: 10.1007/BF01454241. [DOI] [PubMed] [Google Scholar]
- 13.Kagnoff M F, Austin R K, Hubert J J, Bernardin J E, Kasarda D D. J Exp Med. 1984;160:1544–1557. doi: 10.1084/jem.160.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karagiannis J A, Priddle J D, Jewell D P. Lancet. 1987;1:884–886. doi: 10.1016/s0140-6736(87)92859-5. [DOI] [PubMed] [Google Scholar]
- 15.Sturgess R, Day P, Ellis H J, Lundin K E, Gjertsen H A, Kontakou M, Ciclitira P J. Lancet. 1994;343:758–761. doi: 10.1016/s0140-6736(94)91837-6. [DOI] [PubMed] [Google Scholar]
- 16.Mantzaris G, Jewell D P. Scand J Gastroenterol. 1991;26:392–398. doi: 10.3109/00365529108996500. [DOI] [PubMed] [Google Scholar]
- 17.Lundin K E A, Sollid L M, Anthonson D, Norén O, Molberg Ø, Thorsby E, Sjöström H. Gastroenterology. 1997;112:752–759. doi: 10.1053/gast.1997.v112.pm9041236. [DOI] [PubMed] [Google Scholar]
- 18.Nilsen E M, Lundin K E, Krajci P, Scott H, Sollid L M, Brandtzaeg P. Gut. 1995;37:766–776. doi: 10.1136/gut.37.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallusto F, Lanzavecchia A. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann M, Wilm M. Anal Chem. 1994;66:4390–4399. doi: 10.1021/ac00096a002. [DOI] [PubMed] [Google Scholar]
- 21.Marsh M N. Nat Med. 1997;3:725–726. doi: 10.1038/nm0797-725. [DOI] [PubMed] [Google Scholar]