Abstract
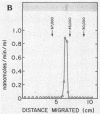
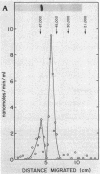
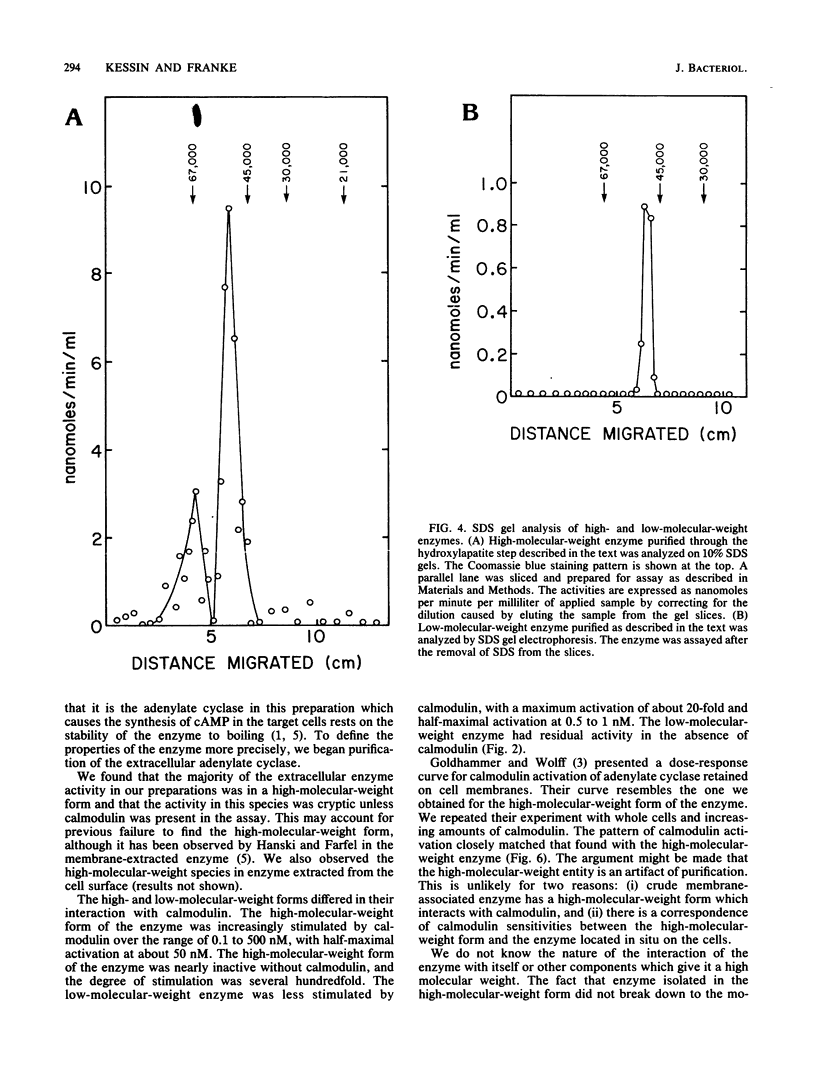
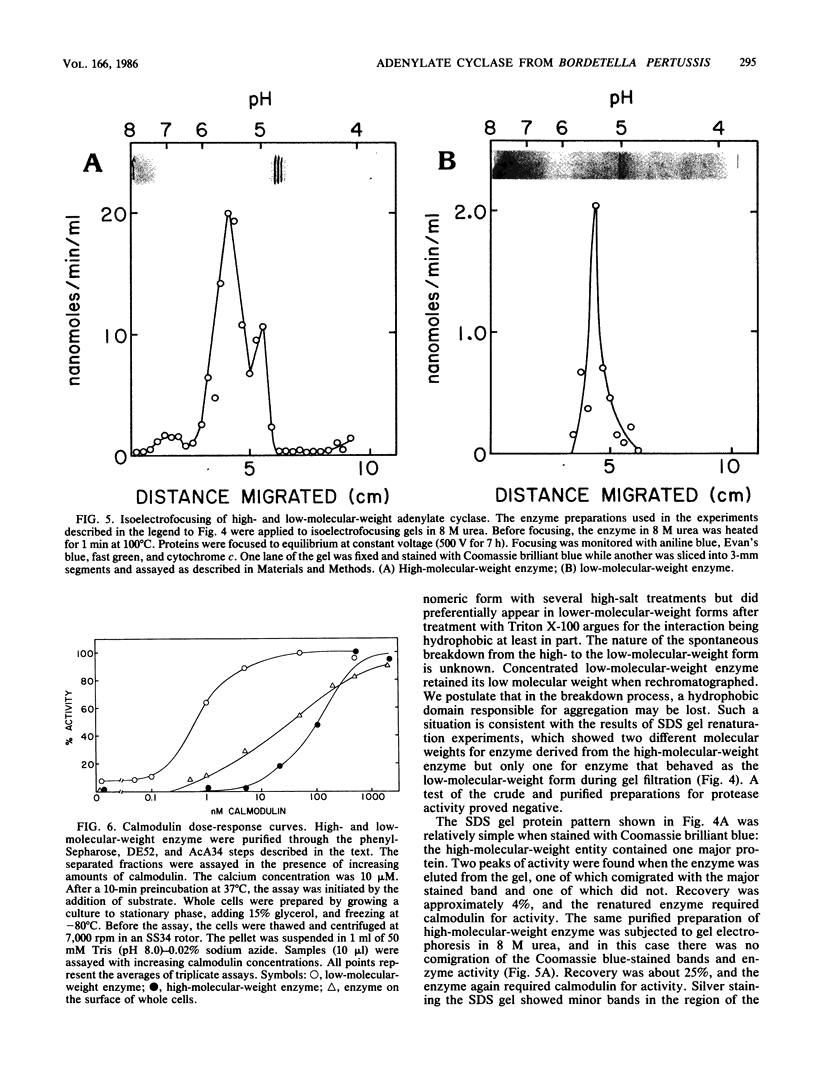
The extracellular adenylate cyclase of Bordetella pertussis was partially purified and found to contain high- and low-molecular-weight species. The high-molecular-weight form had a variable molecular weight with a peak at about 700,000. The smaller species had a molecular weight of 60 to 70,000 as determined by gel filtration. The low-molecular-weight form could be derived from the high-molecular-weight species. The high-molecular-weight complex purified from the cellular supernatant was highly stimulated by calmodulin, while the low-molecular-weight enzyme was much less stimulated. Active enzyme could be recovered from sodium dodecyl sulfate (SDS) gels at positions corresponding to molecular weights of about 50,000 and 65,000. Active low-molecular-weight enzyme recovered from SDS gels migrated with a molecular weight of about 50,000, which coincides with a coomassie blue-stained band. However, when both high- and low-molecular weight preparations were analyzed in 8 M urea isoelectrofocusing gels, the enzyme activity recovered did not comigrate with stained protein bands. The enzyme recovered from denaturing isoelectrofocusing or SDS gels was activated by calmodulin, indicating a direct interaction of calmodulin and enzyme. The high-molecular-weight form of the enzyme showed increasing activity with calmodulin concentrations ranging from 0.1 to 500 nM, while the low-molecular-weight form was fully activated by calmodulin at 20 nM. Adenylate cyclase on the surface of living cells was activated by calmodulin in a manner which resembled that found for the high-molecular-weight form.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Franke J., Kessin R. H. The cyclic nucleotide phosphodiesterase inhibitory protein of Dictyostelium discoideum. Purification and characterization. J Biol Chem. 1981 Jul 25;256(14):7628–7637. [PubMed] [Google Scholar]
- Goldhammer A., Wolff J. Assay of calmodulin with Bordetella pertussis adenylate cyclase. Anal Biochem. 1982 Jul 15;124(1):45–52. doi: 10.1016/0003-2697(82)90217-2. [DOI] [PubMed] [Google Scholar]
- Greenlee D. V., Andreasen T. J., Storm D. R. Calcium-independent stimulation of Bordetella pertussis adenylate cyclase by calmodulin. Biochemistry. 1982 May 25;21(11):2759–2764. doi: 10.1021/bi00540a028. [DOI] [PubMed] [Google Scholar]
- Hanski E., Farfel Z. Bordetella pertussis invasive adenylate cyclase. Partial resolution and properties of its cellular penetration. J Biol Chem. 1985 May 10;260(9):5526–5532. [PubMed] [Google Scholar]
- Hewlett E. L., Urban M. A., Manclark C. R., Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E., Wolff J. Soluble adenylate cyclase from the culture medium of Bordetella pertussis: purification and characterization. J Bacteriol. 1976 Aug;127(2):890–898. doi: 10.1128/jb.127.2.890-898.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilhoffer M. C., Cook G. H., Wolff J. Calcium-independent activation of adenylate cyclase by calmodulin. Eur J Biochem. 1983 Jun 1;133(1):11–15. doi: 10.1111/j.1432-1033.1983.tb07423.x. [DOI] [PubMed] [Google Scholar]
- Krishna G., Birnbaumer L. On the assay of adenyl cyclase. Anal Biochem. 1970 Jun;35(2):393–397. doi: 10.1016/0003-2697(70)90200-9. [DOI] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leppla S. H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Shattuck R. L., Oldenburg D. J., Storm D. R. Purification and characterization of a calmodulin-sensitive adenylate cyclase from Bordetella pertussis. Biochemistry. 1985 Nov 5;24(23):6356–6362. doi: 10.1021/bi00344a006. [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984 Aug;150(2):219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- Wolff J., Cook G. H. Activation of thyroid membrane adenylate cyclase by purine nucleotides. J Biol Chem. 1973 Jan 10;248(1):350–355. [PubMed] [Google Scholar]
- Wolff J., Cook G. H. Amphiphile-mediated activation of soluble adenylate cyclase of Bordetella pertussis. Arch Biochem Biophys. 1982 May;215(2):524–531. doi: 10.1016/0003-9861(82)90111-4. [DOI] [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Berkowitz S. A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]