Abstract
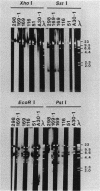
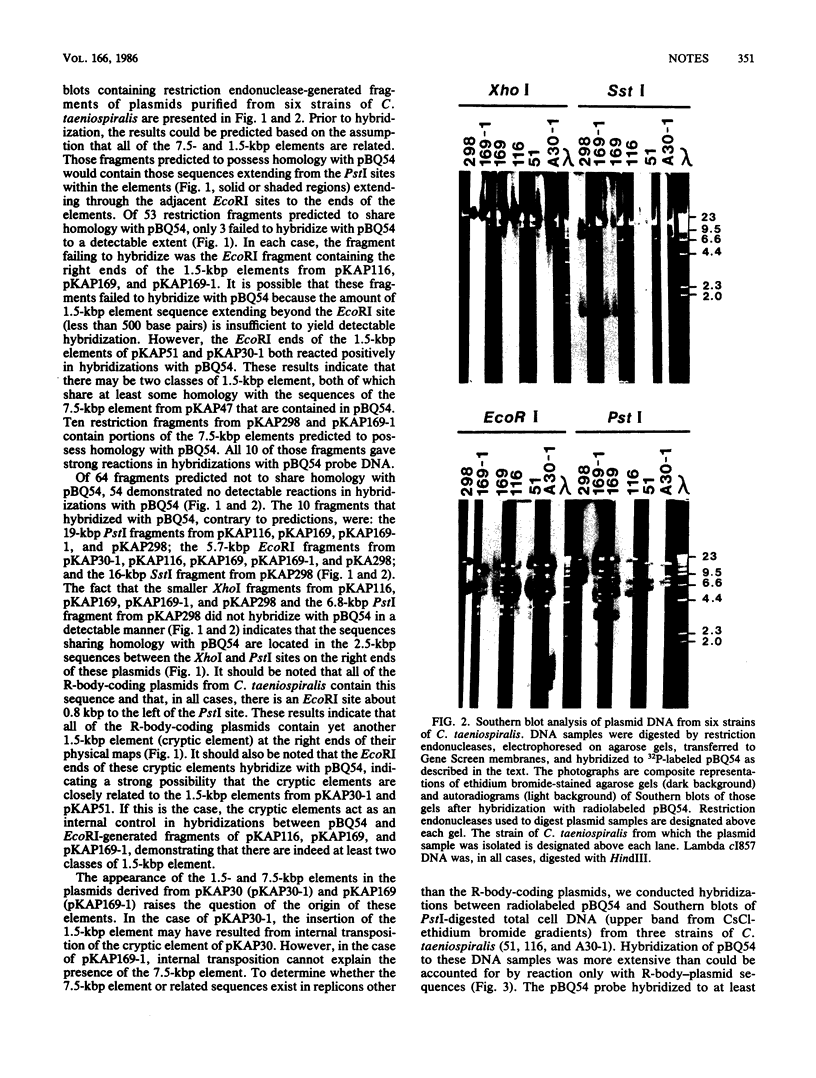
We report that the 1.5- and 7.5-kilobase-pair (kbp) transposonlike sequences present in the R-body-coding plasmids of Caedibacter taeniospiralis share homology. The R-body-coding plasmids of two new strains of C. taeniospiralis, derived from strains 169 and A30, carry the 7.5- and 1.5-kbp elements, respectively, inserted at new positions. Sequences homologous to the 7.5-kbp sequence from C. taeniospiralis 47 were detected in the chromosomes of three other strains of C. taeniospiralis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dilts J. A. Covalently closed, circular DNA in kappa endosymbionts of Paramecium. Genet Res. 1976 Apr;27(2):161–170. doi: 10.1017/s0016672300016360. [DOI] [PubMed] [Google Scholar]
- Hänni C., Meyer J., Iida S., Arber W. Occurrence and properties of composite transposon Tn2672: evolution of multiple drug resistance transposons. J Bacteriol. 1982 Jun;150(3):1266–1273. doi: 10.1128/jb.150.3.1266-1273.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush R. L., Burbach J. A. Cloning and expression of DNA sequences associated with the killer trait of Paramecium tetraurelia stock 47. Proc Natl Acad Sci U S A. 1983 Jan;80(1):250–254. doi: 10.1073/pnas.80.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush R. L., Dilts J. A., Maser R. L. Physical map of a plasmid from Caedibacter taeniospiralis 51. J Bacteriol. 1982 Nov;152(2):939–942. doi: 10.1128/jb.152.2.939-942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush R. L. Phylogenetic relationships of bacterial endosymbionts of Paramecium aurelia: polynucleotide sequence relationships of 51 kappa and its mutants. J Bacteriol. 1977 Feb;129(2):895–900. doi: 10.1128/jb.129.2.895-900.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush R. L. Plasmids from bacterial endosymbionts of hump-killer paramecia. Plasmid. 1983 May;9(3):298–306. doi: 10.1016/0147-619x(83)90007-0. [DOI] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]