Abstract
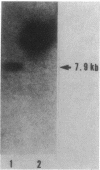
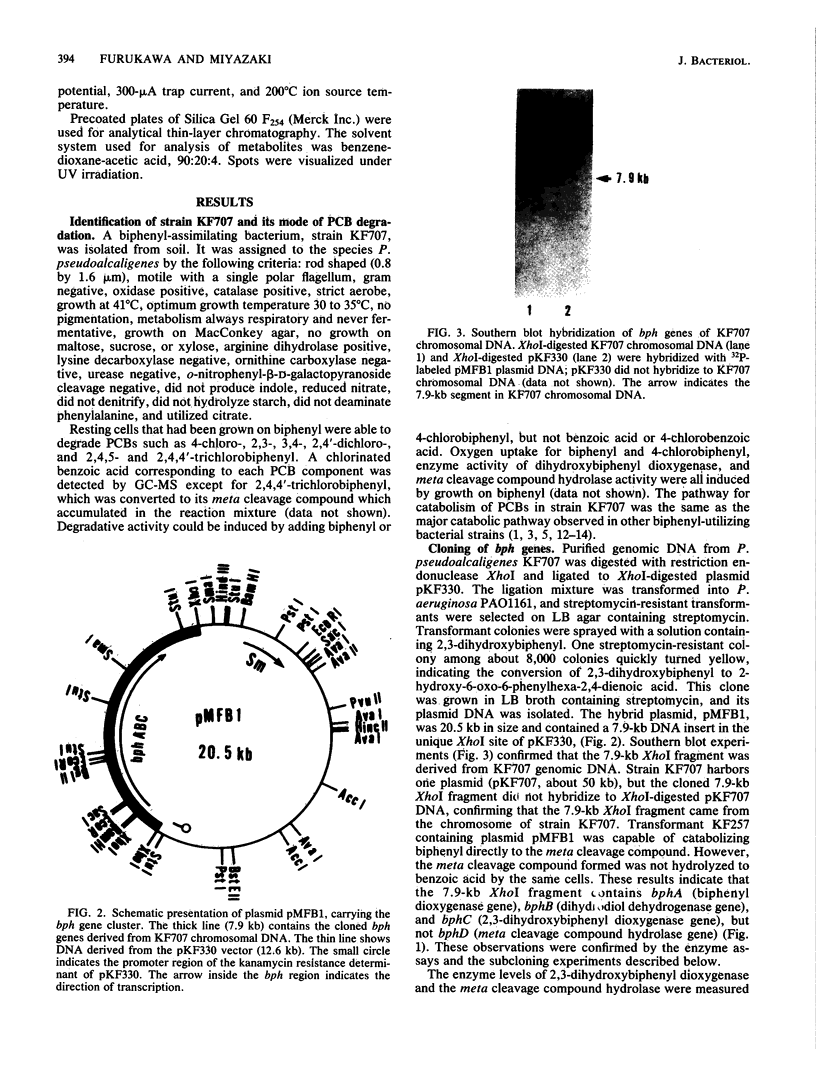
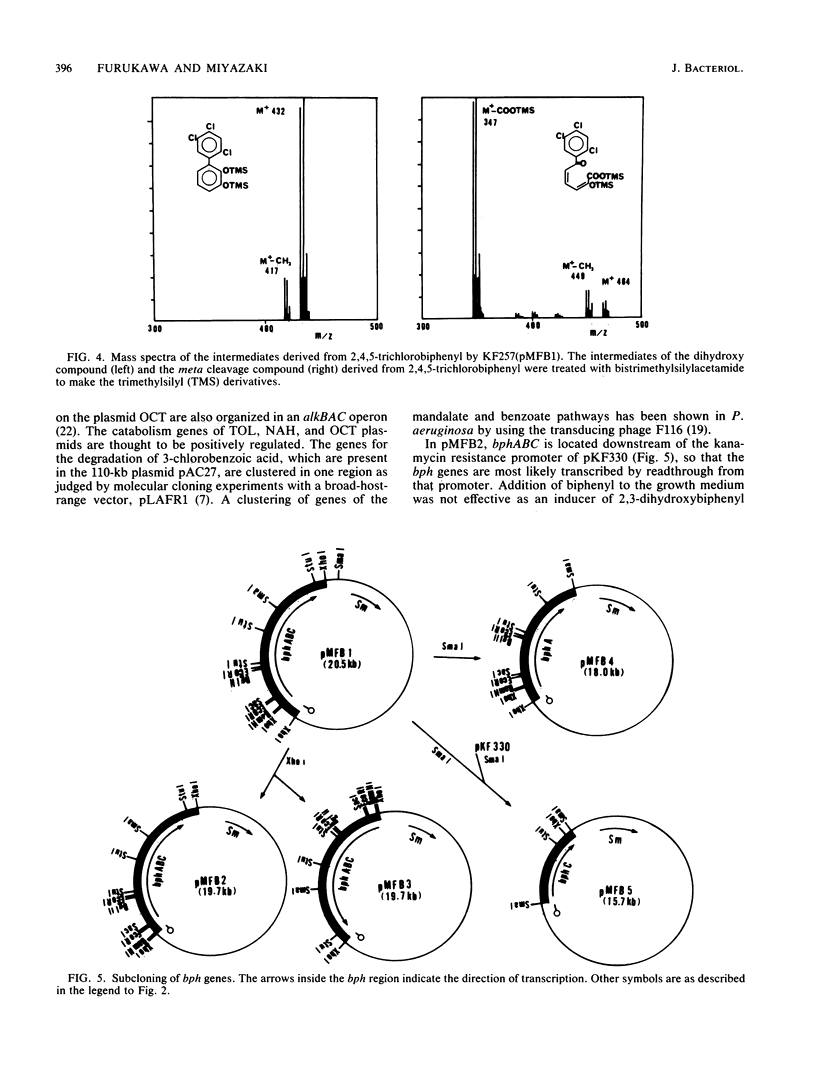
A gene cluster encoding biphenyl- and chlorobiphenyl-degrading enzymes was cloned from a soil pseudomonad into Pseudomonas aeruginosa PAO1161. Chromosomal DNA from polychlorinated biphenyl-degrading Pseudomonas pseudoalcaligenes KF707 was digested with restriction endonuclease XhoI and cloned into the unique XhoI site of broad-host-range plasmid pKF330. Of 8,000 transformants tested, only 1, containing the chimeric plasmid pMFB1, rendered the host cell able to convert biphenyls and chlorobiphenyls to ring meta cleavage compounds via dihydrodiols and dihydroxy compounds. The chimeric plasmid contained a 7.9-kilobase XhoI insert. Subcloning experiments revealed that the genes bphA (encoding biphenyl dioxygenase), bphB (encoding dihydrodiol dehydrogenase), and bphC (encoding 2,3-dihydroxybiphenyl dioxygenase) were coded for by the 7.9-kilobase fragment. The gene order was bphA-bphB-bphC. The hydrolase activity, which converted the intermediate meta cleavage compounds to the final product, chlorobenzoic acids, and was encoded by a putative bphD gene, was missing from the cloned 7.9-kilobase fragment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M., Focht D. D. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can J Microbiol. 1973 Jan;19(1):47–52. doi: 10.1139/m73-007. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M., Timmis K. N. Host: vector systems for gene cloning in Pseudomonas. Curr Top Microbiol Immunol. 1982;96:47–67. doi: 10.1007/978-3-642-68315-2_4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelani D., Sorlini C., Treccani V. The metabolism of biphenyl by Pseudomonas putida. Experientia. 1971 Oct 15;27(10):1173–1174. doi: 10.1007/BF02286908. [DOI] [PubMed] [Google Scholar]
- Chatterjee D. K., Chakrabarty A. M. Genetic rearrangements in plasmids specifying total degradation of chlorinated benzoic acids. Mol Gen Genet. 1982;188(2):279–285. doi: 10.1007/BF00332688. [DOI] [PubMed] [Google Scholar]
- Chatterjee D. K., Chakrabarty A. M. Restriction mapping of a chlorobenzoate degradative plasmid and molecular cloning of the degradative genes. Gene. 1984 Feb;27(2):173–181. doi: 10.1016/0378-1119(84)90138-0. [DOI] [PubMed] [Google Scholar]
- Franklin F. C., Bagdasarian M., Bagdasarian M. M., Timmis K. N. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Chakrabarty A. M. Involvement of plasmids in total degradation of chlorinated biphenyls. Appl Environ Microbiol. 1982 Sep;44(3):619–626. doi: 10.1128/aem.44.3.619-626.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Simon J. R., Chakrabarty A. M. Common induction and regulation of biphenyl, xylene/toluene, and salicylate catabolism in Pseudomonas paucimobilis. J Bacteriol. 1983 Jun;154(3):1356–1362. doi: 10.1128/jb.154.3.1356-1362.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Tomizuka N., Kamibayashi A. Effect of chlorine substitution on the bacterial metabolism of various polychlorinated biphenyls. Appl Environ Microbiol. 1979 Aug;38(2):301–310. doi: 10.1128/aem.38.2.301-310.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Tonomura K., Kamibayashi A. Effect of chlorine substitution on the biodegradability of polychlorinated biphenyls. Appl Environ Microbiol. 1978 Feb;35(2):223–227. doi: 10.1128/aem.35.2.223-227.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grund A. D., Gunsalus I. C. Cloning of genes for naphthalene metabolism in Pseudomonas putida. J Bacteriol. 1983 Oct;156(1):89–94. doi: 10.1128/jb.156.1.89-94.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Nakazawa A., Nakazawa T. Molecular cloning of regulatory gene xylR and operator-promoter regions of the xylABC and xylDEGF operons of the TOL plasmid. J Bacteriol. 1983 Sep;155(3):1192–1199. doi: 10.1128/jb.155.3.1192-1199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. B., Hegeman G. D. Genetic control of the beta-ketoadipate pathway in Pseudomonas aeruginosa. J Bacteriol. 1968 Nov;96(5):1488–1499. doi: 10.1128/jb.96.5.1488-1499.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuna M., Someda K., Morita K., Yamanouchi Y., Kurimoto T., Kawamura Y., Matsumura H. [Ischemic cerebral symptoms after subarachnoid hemorrhage due to aneurysmal rupture (author's transl)]. No Shinkei Geka. 1978 Jun;6(6):543–548. [PubMed] [Google Scholar]
- Lehrbach P. R., Jeenes D. J., Broda P. Characterization by molecular cloning of insertion mutants in TOL catabolic functions. Plasmid. 1983 Mar;9(2):112–125. doi: 10.1016/0147-619x(83)90014-8. [DOI] [PubMed] [Google Scholar]
- Owen D. J., Eggink G., Hauer B., Kok M., McBeth D. L., Yang Y. L., Shapiro J. A. Physical structure, genetic content and expression of the alkBAC operon. Mol Gen Genet. 1984;197(3):373–383. doi: 10.1007/BF00329932. [DOI] [PubMed] [Google Scholar]
- Reineke W., Jeenes D. J., Williams P. A., Knackmuss H. J. TOL plasmid pWW0 in constructed halobenzoate-degrading Pseudomonas strains: prevention of meta pathway. J Bacteriol. 1982 Apr;150(1):195–201. doi: 10.1128/jb.150.1.195-201.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields M. S., Hooper S. W., Sayler G. S. Plasmid-mediated mineralization of 4-chlorobiphenyl. J Bacteriol. 1985 Sep;163(3):882–889. doi: 10.1128/jb.163.3.882-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weightman A. J., Don R. H., Lehrbach P. R., Timmis K. N. The identification and cloning of genes encoding haloaromatic catabolic enzymes and the construction of hybrid pathways for substrate mineralization. Basic Life Sci. 1984;28:47–80. doi: 10.1007/978-1-4684-4715-6_4. [DOI] [PubMed] [Google Scholar]
- Worsey M. J., Franklin F. C., Williams P. A. Regulation of the degradative pathway enzymes coded for by the TOL plasmid (pWWO) from Pseudomonas putida mt-2. J Bacteriol. 1978 Jun;134(3):757–764. doi: 10.1128/jb.134.3.757-764.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen K. M., Gunsalus I. C. Plasmid gene organization: naphthalene/salicylate oxidation. Proc Natl Acad Sci U S A. 1982 Feb;79(3):874–878. doi: 10.1073/pnas.79.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]