Abstract
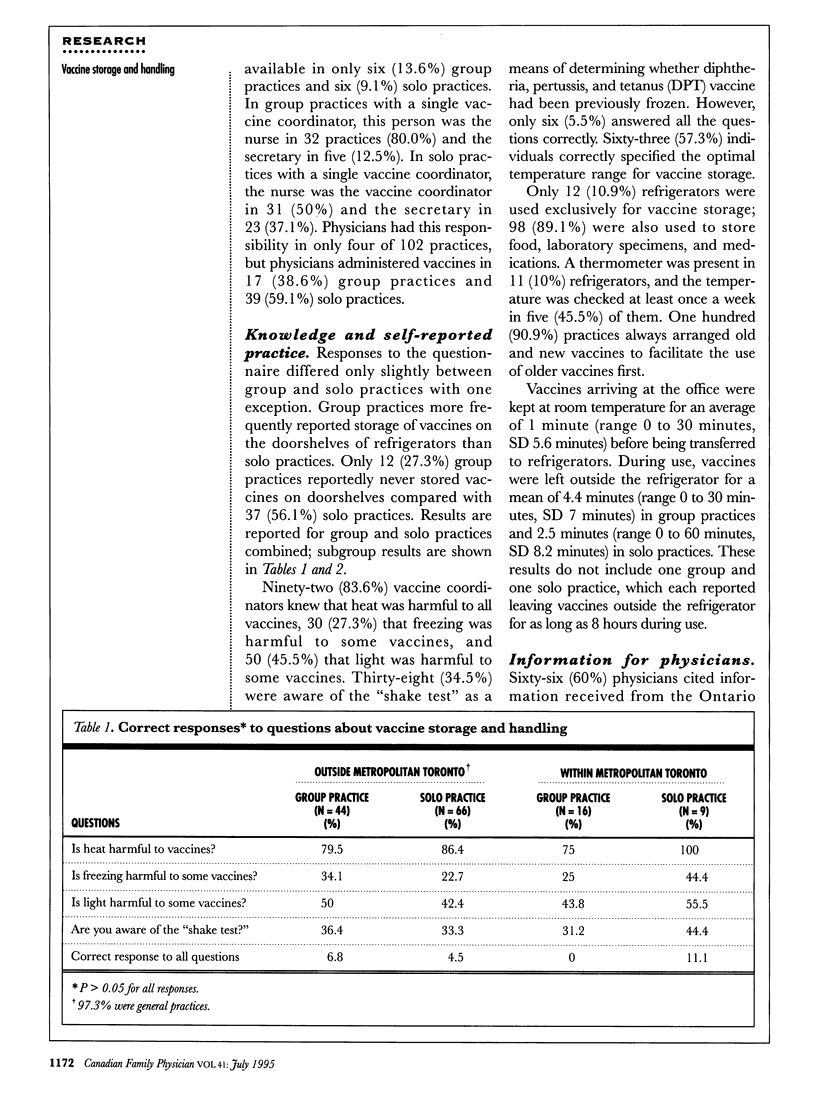
OBJECTIVE: To assess the knowledge and practice of vaccine storage and handling in primary care physicians' offices. DESIGN: A cross-sectional study was conducted from August to December 1992. Staff responsible for vaccine storage were interviewed about their knowledge and practices of vaccine handling and storage. Refrigerators were inspected to document refrigerator temperature and vaccine storage conditions. SETTING: General and pediatric practices in 12 regions of Ontario. PARTICIPANTS: Practices outside metropolitan Toronto were selected by choosing every 10th physician who ordered vaccine from the local health department in 1992. Practices chosen in metropolitan Toronto were a random selection of physicians affiliated with a Toronto teaching hospital. Eighteen pediatric and 138 general practices were approached to participate; 12 pediatric and 123 general practices participated in the study. The overall response rate was 86.5%. MAIN OUTCOME MEASURES: Survey responses and temperature and storage conditions of refrigerators upon inspection. RESULTS: Fewer than seven (6%) practices answered all questions related to vaccine storage and handling correctly, and only 11 (10%) refrigerators had thermometers. One-third of refrigerators had temperatures outside the recommended range of 2 degrees C to 8 degrees C. Older refrigerators were more likely to have inappropriate temperatures than newer ones. CONCLUSIONS: Knowledge and practice of vaccine storage and handling are often inadequate in primary care physicians' offices.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishai D. M., Bhatt S., Miller L. T., Hayden G. F. Vaccine storage practices in pediatric offices. Pediatrics. 1992 Feb;89(2):193–196. [PubMed] [Google Scholar]
- Elbert L. B., Terletskay E. N., Timofeev A. V., Mironova L. L., Khapchaev U. K., Amosenko F. A., Svitkin Y. V., Alpatova G. A., Krutyanskaya G. L. Inactivated vaccine against tick-borne encephalitis (TBE) derived from heteroploid continuous monkey cell line. Vaccine. 1989 Oct;7(5):477–477. doi: 10.1016/0264-410x(89)90183-7. [DOI] [PubMed] [Google Scholar]
- Krugman R. D., Meyer B. C., Enterline J. C., Parkman P. D., Witte J. J., Meyer H. M., Jr Impotency of live-virus vaccines as a result of improper handling in clinical practice. J Pediatr. 1974 Oct;85(4):512–514. doi: 10.1016/s0022-3476(74)80455-5. [DOI] [PubMed] [Google Scholar]
- Miller L. G., Loomis J. H., Jr Advice of manufacturers about effects of temperature on biologicals. Am J Hosp Pharm. 1985 Apr;42(4):843–848. [PubMed] [Google Scholar]
- Subramanyam K. Vaccine distribution: an operations research study. Rev Infect Dis. 1989 May-Jun;11 (Suppl 3):S623–S628. doi: 10.1093/clinids/11.supplement_3.s623. [DOI] [PubMed] [Google Scholar]
- Thakker Y., Woods S. Storage of vaccines in the community: weak link in the cold chain? BMJ. 1992 Mar 21;304(6829):756–758. doi: 10.1136/bmj.304.6829.756. [DOI] [PMC free article] [PubMed] [Google Scholar]



