Abstract
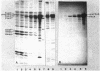
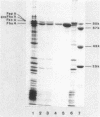
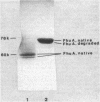
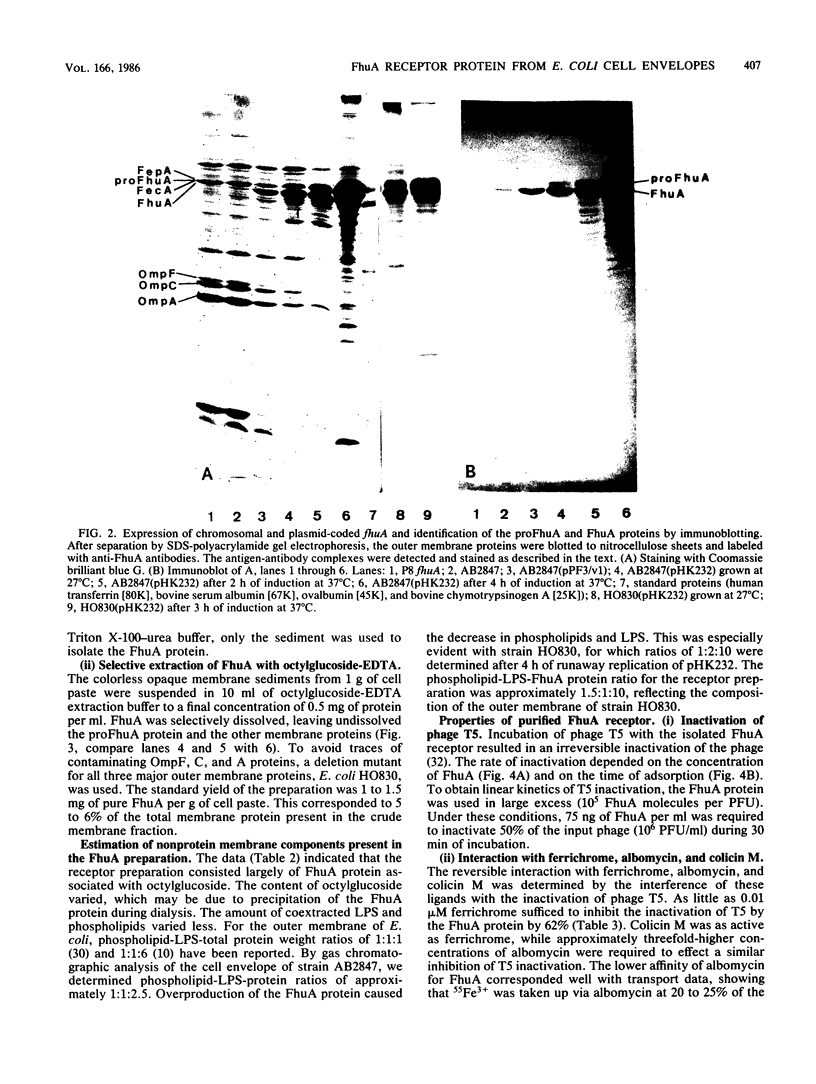
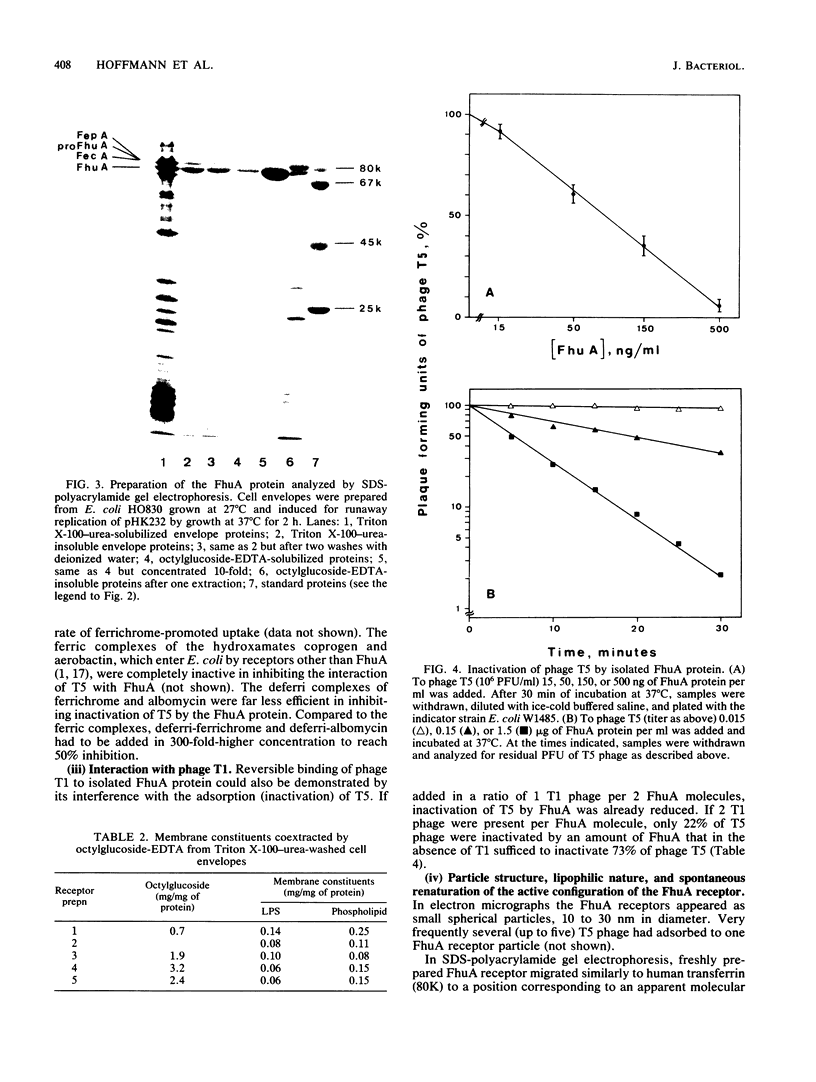
A rapid and simple method for purification of the FhuA receptor protein from cell envelopes of a FhuA-overproducing strain of Escherichia coli K-12 was developed. The overproduction of FhuA was programmed by the thermoamplifiable plasmid pHK232, which carried the fhuACD genes of pLC19-19 of the Clarke and Carbon collection. At low temperature (27 degrees C), pHK232 specified the overproduction of FhuA to levels comparable to those of major outer membrane proteins OmpF, OmpC, and OmpA. The amount of these proteins in the outer membrane was reduced along with overproduction of FhuA. Upon runaway replication of pHK232 at 37 degrees C, the precursor of the FhuA protein, proFhuA, was also accumulated in the cell envelope in amounts similar to FhuA. For extraction of the FhuA protein, crude cell envelopes were washed with 2% Triton X-100-6 M urea to remove less tightly bound proteins. Then FhuA but not proFhuA was solubilized by treating Triton X-100-urea-washed membranes with 1% octylglucoside-1 mM EDTA. This procedure yielded FhuA protein free from other membrane proteins. The amount of lipopolysaccharide and phospholipids was low and ranged from 5 to 15% and 10 to 25% of the weight of the FhuA protein, respectively. As shown by direct inactivation and by competition assays, the isolated FhuA protein retained receptor activity for ferrichrome, albomycin, colicin M, and phages T5 and T1.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bindereif A., Braun V., Hantke K. The cloacin receptor of ColV-bearing Escherichia coli is part of the Fe3+-aerobactin transport system. J Bacteriol. 1982 Jun;150(3):1472–1475. doi: 10.1128/jb.150.3.1472-1475.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Schaller K., Wolff H. A common receptor protein for phage T5 and colicin M in the outer membrane of Escherichia coli B. Biochim Biophys Acta. 1973 Sep 27;323(1):87–97. doi: 10.1016/0005-2736(73)90433-1. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., DuBow M. S. Molecular cloning of the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol. 1983 Dec;156(3):1315–1321. doi: 10.1128/jb.156.3.1315-1321.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W. The ferrichrome-iron receptor of Escherichia coli K-12. Antigenicity of the fhuA protein. Biochim Biophys Acta. 1982 Jul 16;717(1):154–162. doi: 10.1016/0304-4165(82)90393-2. [DOI] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group B. J Bacteriol. 1975 Jul;123(1):96–101. doi: 10.1128/jb.123.1.96-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leij L., Witholt B. Structural heterogeneity of the cytoplasmic and outer membranes of Escherichia coli. Biochim Biophys Acta. 1977 Nov 15;471(1):92–104. doi: 10.1016/0005-2736(77)90396-0. [DOI] [PubMed] [Google Scholar]
- Fecker L., Braun V. Cloning and expression of the fhu genes involved in iron(III)-hydroxamate uptake by Escherichia coli. J Bacteriol. 1983 Dec;156(3):1301–1314. doi: 10.1128/jb.156.3.1301-1314.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. W., Braun V. Nature of the energy requirement for the irreversible adsorption of bacteriophages T1 and phi80 to Escherichia coli. J Bacteriol. 1976 Feb;125(2):409–415. doi: 10.1128/jb.125.2.409-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Functional interaction of the tonA/tonB receptor system in Escherichia coli. J Bacteriol. 1978 Jul;135(1):190–197. doi: 10.1128/jb.135.1.190-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Identification of an iron uptake system specific for coprogen and rhodotorulic acid in Escherichia coli K12. Mol Gen Genet. 1983;191(2):301–306. doi: 10.1007/BF00334830. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., Heller K., Coulton J. W., Braun V. Genetic control of hydroxamate-mediated iron uptake in Escherichia coli. J Bacteriol. 1980 Jul;143(1):256–264. doi: 10.1128/jb.143.1.256-264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Biosynthesis of branched-chain fatty acids. IV. Factors affecting relative abundance of fatty acids produced by Bacillus subtilis. Can J Microbiol. 1966 Jun;12(3):501–514. doi: 10.1139/m66-073. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Menichi B., Buu A. Integration of the overproduced bacteriophage T5 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1983 Apr;154(1):130–138. doi: 10.1128/jb.154.1.130-138.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plastow G. S., Pratt J. M., Holland I. B. The ferrichrome receptor protein (tonA) of Escherichia coli is synthesised as a precursor in vitro. FEBS Lett. 1981 Aug 31;131(2):262–264. doi: 10.1016/0014-5793(81)80380-8. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Characterization of group B colicin-resistant mutants of Escherichia coli K-12: colicin resistance and the role of enterochelin. J Bacteriol. 1976 Jul;127(1):218–228. doi: 10.1128/jb.127.1.218-228.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Schwarz H., Sonntag I., Henning U. Mutational change of membrane architecture. Mutants of Escherichia coli K12 missing major proteins of the outer cell envelope membrane. Biochim Biophys Acta. 1976 Oct 19;448(3):474–491. doi: 10.1016/0005-2736(76)90301-1. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Nishimura A., Nishimura Y., Yamada M., Yasuda S., Suzuki H., Hirota Y. Synthetic ColE1 Plasmids carrying genes for penicillin-binding proteins in Escherichia coli. Plasmid. 1981 Jul;6(1):86–98. doi: 10.1016/0147-619x(81)90056-1. [DOI] [PubMed] [Google Scholar]
- Tosi F., Labedan B., Legault-Démare J. Analysis of the coliphage T5 DNA ejection process with free and liposome-associated TonA protein. J Virol. 1984 Apr;50(1):213–219. doi: 10.1128/jvi.50.1.213-219.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Uhlin B. E., Nordström K. A runaway-replication mutant of plasmid R1drd-19: temperature-dependent loss of copy number control. Mol Gen Genet. 1978 Oct 4;165(2):167–179. doi: 10.1007/BF00269904. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., KELLENBERGER E. The E. coli B-receptor for the phage T5. II. Electron microscopic studies. Biochim Biophys Acta. 1955 May;17(1):1–9. doi: 10.1016/0006-3002(55)90314-0. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Takagi T. Overproduction of Escherichia coli replication proteins by the use of runaway-replication plasmids. J Bacteriol. 1983 Jun;154(3):1153–1161. doi: 10.1128/jb.154.3.1153-1161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]