Abstract
Generation of a T cell-mediated antitumor response depends on T cell receptor engagement by major histocompatibility complex/antigen as well as CD28 ligation by B7. CTLA-4 is a second B7 receptor expressed by T cells upon activation that, unlike CD28, appears to deliver an inhibitory signal to T cells. Recently, we and others demonstrated that administration of an anti-CTLA-4 antibody was sufficient to promote regression of several murine tumors. However, certain tumors, such as the SM1 mammary carcinoma, remain refractory to this type of immunotherapy. In the present study, we report that the combination of both CTLA-4 blockade and a vaccine consisting of granulocyte–macrophage colony-stimulating factor-expressing SM1 cells resulted in regression of parental SM1 tumors, despite the ineffectiveness of either treatment alone. This synergistic therapy resulted in long-lasting immunity to SM1 and depended on both CD4+ and CD8+ T cells. Interestingly, synergy was not observed between CTLA-4 and a B7-expressing SM1 vaccine. Given that granulocyte–macrophage colony-stimulating factor promotes differentiation and activation of dendritic cells as well as enhances cross-priming of T cells to tumor-derived antigens and that SM1 is major histocompatibility complex class II-negative, our findings suggest that CTLA-4 blockade acts at the level of a host-derived antigen-presenting cell. In addition, these results also support the idea that the most effective and synergistic vaccine strategy targets treatments that enhance T cell priming at the level of host-derived antigen-presenting cells.
It is well established that effective T cell activation requires both an antigen-specific signal through the T cell antigen receptor and an antigen-independent costimulatory signal mediated through the interaction of CD28 with B7 on the antigen-presenting cell (APC) (as reviewed in ref. 1). Generation of an effective antitumor T cell response has these same requirements. Accordingly, the poor immunogenicity of many tumors may be due to a general lack of B7 expression. Consistent with this possibility, we and others demonstrated that conferring B7 expression to tumors of a variety of tissue origins was, in many cases, sufficient to promote tumor rejection by a CD8+ T cell-dependent mechanism (2–4).
Another approach taken to enhance the antitumor immune response has been to bypass the need for direct costimulation by conferring cytokine expression to tumors. Cytokine-expressing tumor cells used as vaccines may have paracrine effects on T cells or APCs. Interleukin-2 (IL-2) (5, 6), IL-4 (7, 8), and interferon-γ (IFN-γ) (9, 10) are T cell-derived cytokines that were demonstrated to promote tumor rejection in a T cell-dependent mechanism, presumably by augmenting T cell (IL-2, IL-4, IFN-γ) or APC (IFN-γ) activation. Granulocyte–macrophage colony-stimulating factor (GM-CSF) is another T cell-derived cytokine that was demonstrated to enhance the immunogenicity of tumors (11, 12). GM-CSF is a pleiotropic cytokine that can promote the differentiation and activation of macrophages and dendritic cells, a population of powerful APCs (13–15). In tumor model systems where neither B7 nor cytokine expression resulted in tumor rejection, it has been demonstrated that coexpression of both may be sufficient to enhance tumor immunogenicity (16, 17).
Recently, a different approach to promoting tumor rejection was described. CTLA-4 is a second T cell receptor for B7 that plays an inhibitory role in regulation of T cell responses. Several studies have demonstrated that in vitro, soluble anti-CTLA-4 can enhance T cell responses whereas crosslinking CTLA-4 results in block of cell cycle progression, diminished cytokine expression, and decreased proliferation (18–21). The observation that CTLA-4 null mice suffer a fatal lymphoproliferative disorder supports the idea that CTLA-4 functions as a negative regulator of T cell responses. Using an antibody directed against CTLA-4, we and others demonstrated that CTLA-4 blockade enhanced rejection of B7-transfected tumors and, more strikingly, induced rejection of unmodified tumor cells and immunity to rechallenge in a T cell-dependent mechanism (22–24) (D.R.L. and A.A.H., unpublished data). We interpreted these data as confirming the idea that CTLA-4 delivers an inhibitory signal and that blockade of CTLA-4-mediated signals in vivo enhances T cell activation.
In most of the immunotherapeutic approaches studied previously, rejection of or protection against tumor challenge depended on the tumor’s inherent immunogenicity. Weakly immunogenic or nonimmunogenic tumors were not rejected when genetically modified to express B7. In our studies as well, the susceptibility of tumors to CTLA-4 blockade seems to correlate with their inherent immunogenicity (D.R.L. et al., unpublished data). Recently, we described a weakly immunogenic mammary carcinoma (SM1) that was not rejected when transfected with B7; SM1 tumors were rejected only when they coexpressed B7 and IFN-γ (17). These findings supported those of others and suggested that even weakly immunogenic tumors can be rejected when the immune response is enhanced sufficiently by combining immunomodulatory agents (16, 25, 26).
In the present study, we describe the rejection of SM1 tumors by using both CTLA-4 blockade and a GM-CSF-expressing tumor vaccine (GMSM1). SM1 was shown to grow progressively in mice treated with anti-CTLA-4 or the GMSM1 vaccine alone. Anti-CTLA-4 treatment also was ineffective against B7–1-expressing SM1 tumors. In contrast, mice implanted with an SM1 tumor and treated with a GM-CSF-expressing vaccine followed by anti-CTLA-4 rejected the SM1 tumors and were immune to subsequent SM1 rechallenge. Not surprisingly, rejection depended on both CD4+ and CD8+ T cells. The finding that CTLA-4 blockade synergizes with a GM-CSF-expressing but not a B7-expressing vaccine suggests that CTLA-4 blockade may enhance tumor immunogenicity by blocking the interaction between B7 on host APCs-derived B7 and CTLA-4 on tumor-specific T cells.
MATERIALS AND METHODS
Cell Lines.
The BALB/C-derived mammary carcinoma SM1 was derived in the laboratory of Satyabrata Nandi (University of California at Berkeley) (27). Briefly, a pre-neoplastic, BALB/C-derived mammary cell line was mutagenized with methylnitrosourea and injected into a cleared fat pad of syngeneic mice. Palpable tumors from the mammary tissue were excised and put into tissue culture after enzymatic release from extracellular matrices. Tumor lines serially passaged through syngeneic mice were selected for malignant phenotype. SM1 was selected as a line that consistently caused tumors at low inocula (tumorigenic at or below 2,000 cells) and stained positive for vimentin protein expression by immunohistochemistry. It then was cultured in MEM (University of California at San Francisco Cell Culture Facility) supplemented with 10% fetal calf serum/1× MEM nonessential amino acids/l-glutamine/MEM vitamins (BioWhittaker). Cells were lifted from tissue culture dishes by using either 5 mM EDTA in calcium/magnesium-free saline or 0.25% trypsin solution (BioWhittaker).
B7–1-expressing lines were prepared as described previously (3) by using an electroporation method. Briefly, cells in logarithmic growth phase were released from tissue culture dishes by trypsinization and washed in electroporation buffer (250 mM sucrose/1 mM magnesium chloride in 2 mM PBS, pH 7.4). DNA (100 μg) was added and 107 cells were electroporated by using electrodes with a 2-mm gap and a setting of 5 pulses of 99 msec at 550 V. Cells were cultured in selective medium [0.5 mg/ml G418 (Gibco)] added 48 hr after electroporation. Clones were produced by limiting dilution and screened for B7 expression by flow cytometric analysis by using CTLA-4 Ig (a chimeric molecule consisting of the extracellular domain of CTLA-4 and the Fc domain of human IgG1) followed by a fluorescein isothiocyanate-conjugated goat anti-human antibody (Caltag, South San Francisco, CA).
To obtain GM-CSF-expressing lines, cells were infected with a retrovirus containing the mouse IFN-γ or GM-CSF gene driven by the Moloney murine leukemia virus long terminal repeat, using the ψCRIP producer line (gift from Somatix, Alameda, CA). Retrovirus-containing supernatants were added to SM1 cultures overnight in the presence of 8 mg/ml polybrene (Sigma). Clones were generated by limiting dilution and supernatants were tested for cytokine expression by ELISA (PharMingen).
Animal Procedures.
All animal procedures were performed according National Institutes of Health guidelines under protocols approved by the University of California Animal Care and Use Committee. SM1 cells propagated in culture were harvested with trypsin (BioWhittaker), washed three times in balanced salt solution, and resuspended in saline as described. The minimum tumorigenic dose for SM1 is 2 × 103 cells. Mice were injected s.c. into a shaved area on the back with 100 μl of tumor cell suspensions. Tumor growth was monitored by measuring bisecting diameters with a caliper. When the tumor area exceeded 250 mm2, mice were euthanized and a value of 250 mm2 was entered for each euthanized mouse. This value was used to calculate mean tumor area until all mice from a given group were euthanized.
In vaccination studies, cell suspensions were irradiated with 12,000 rad by using a 137Cs-source irradiator. Vaccines were delivered to animals on the contralateral side from the live tumor challenge at the times indicated (generally days 0, 3, and 6).
Antibody Treatment in Vivo.
Anti-CTLA-4 was prepared as described previously (19). Briefly, antibody-containing supernatants from the hybridoma 9H10 were bound to a Protein G-Sepharose column (Gibco) and eluted using 25 mM diethylamine. The eluate was dialyzed against isotonic saline, and antibody concentration was quantitated by UV spectrophotometry. Mice were injected with 100 μg of anti-CTLA-4 at the indicated times (generally, days 4, 7, and 10 subsequent to tumor challenge).
For lymphocyte-depletion experiments, mice were injected with anti-CD4 (GK 1.5, 400 μg), anti-CD8 (2.43, 600 μg), a combination of both anti-CD4 and anti-CD8, or control antibody (purified rat IgG, 600 μg, Sigma) three times before tumor injection (days −6, −5, and −4) as well as once every 10 days subsequent to tumor inoculation. Lymphocyte depletion was confirmed using non-cross-reactive antibodies (CD4: clone CTCD4, Caltag; CD8: CT-CD8β, Caltag) before tumor injection by testing peripheral blood or lymph node cells (from control animals) for the appropriate lymphocyte populations.
RESULTS
Transduction of SM1 with GM-CSF or IFN-γ Enhances Its Immunogenicity.
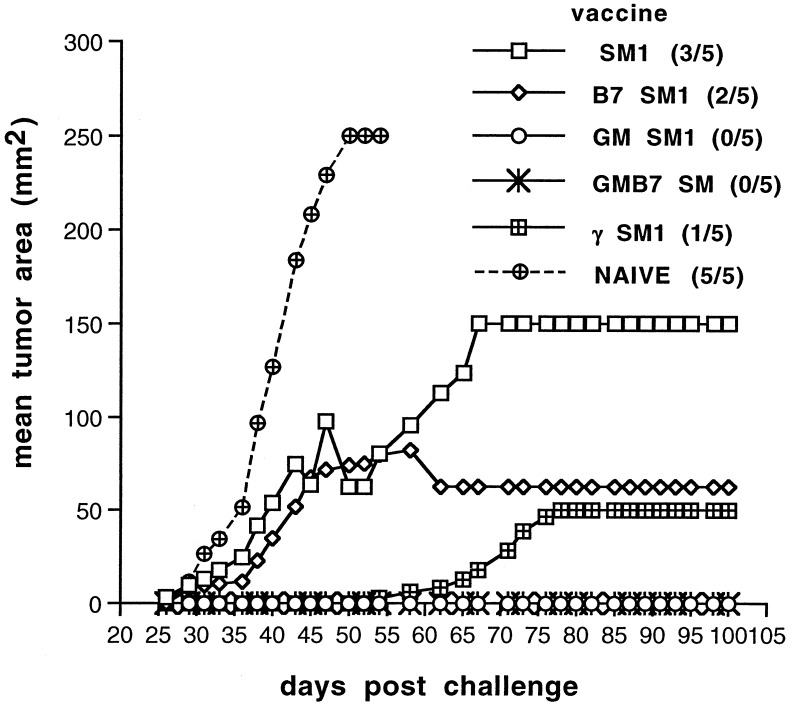
We reported recently that the SM1 mammary carcinoma grows progressively, even after transduction, to express B7–1 (17). This suggested that SM1 is not strongly immunogenic. To test this directly, syngeneic mice were vaccinated s.c. with irradiated SM1 cells or the genetically modified derivative lines. Mice were rechallenged with the unmodified (parental) tumor 4–5 weeks after immunization and tumor growth was monitored (Fig. 1). In three experiments, approximately half (8/15) of mice vaccinated with the parental SM1 tumor were immune to rechallenge. Consistent with our previous observations, B7 expression conferred little enhancement of immunity (9/15). In contrast, expression of IFN-γ or GM-CSF significantly enhanced immunogenicity. All mice vaccinated with GMSM1, GMB7SM1, or γB7SM1 rejected a subsequent challenge with the parental SM1 tumor at an inoculum approximately 100 times the minimum tumorigenic dose. These findings were consistent with the idea that SM1 is inherently weakly immunogenic but its immunogenicity can be enhanced by transduction with genes encoding immunostimulatory cytokines such as GM-CSF or IFN-γ.
Figure 1.
SM1 is a weakly immunogenic tumor. Mice were vaccinated s.c. with 1 × 106 irradiated cells of the indicated cell line. Thirty days later, mice were rechallenged with 2 × 105 live SM1 cells and tumor growth was monitored. Incidence of SM1 tumors is indicated in parentheses.
SM1 or Its B7–1-Expressing Derivative Is Not Rejected as a Consequence of Anti-CTLA-4 Treatment.
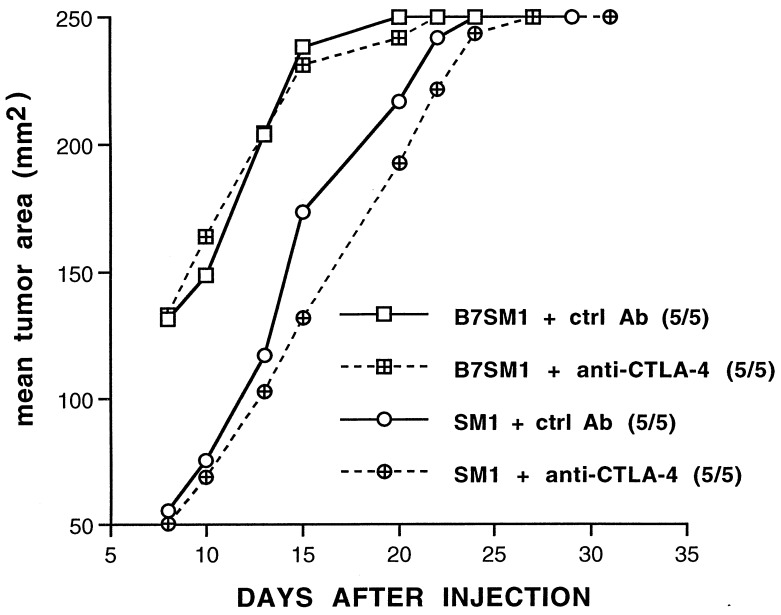
We demonstrated previously that treatment with anti-CTLA-4 can enhance rejection of a B7+ colorectal carcinoma (22) as well as promote the rejection of a B7− colorectal carcinoma, fibrosarcoma, and prostate carcinoma (23). To extend these findings, we tested the effectiveness of CTLA-4 blockade on the growth of SM1 and or SM1 tumors transduced to express B7–1 (B7SM1). Mice were implanted s.c. with SM1 cells and treated with anti-CTLA-4 or a control antibody 4, 7, and 10 days after tumor challenge, and tumor growth was monitored. As shown in Fig. 2, administration of anti-CTLA-4 had no significant effect on SM1 tumor growth when mice were challenged with 2 × 105 SM1 cells. Similarly, CTLA-4 blockade had no effect on B7SM1 growth when using the same-sized tumor inoculum (Fig. 2). When mice were challenged with a smaller tumor inoculum (2 × 104 cells), no significant decrease in the tumorigenicity of SM1 or B7SM1 was observed, although we did detect delayed growth of SM1 tumors (Fig. 3a). These findings are consistent with others from our laboratory using a variety of murine tumor models and suggest that anti-CTLA-4 treatment alone is not an effective treatment for poorly immunogenic tumors (D.R.L. et al., unpublished data).
Figure 2.
SM1 is not rejected as a consequence of anti-CTLA-4 treatment. Mice were implanted s.c. with SM1 tumors (circles) or B7SM1 tumors (squares) and treated i.p. with 100 μg of either control antibody (solid lines) or anti-CTLA-4 (dashed lines) 4, 7, and 10 days later. Tumor growth was monitored, and incidence is indicated in parentheses.
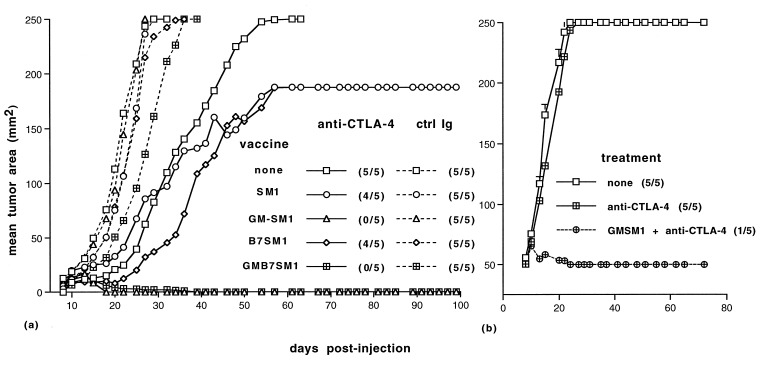
Figure 3.
GM-CSF and anti-CTLA-4 synergize in treatment of SM1 tumors. On day 0, mice were implanted with 2 × 104 (a) or 2 × 105 SM1 (b) cells. (a) On days 0, 3, and 6, mice were injected s.c. on the contralateral flank with the 1 × 106 irradiated cells of the indicated vaccine. On days 4, 7, and 10, mice were injected i.p. with either control antibodies (dashed lines) or anti-CTLA-4 (solid lines). (b) Mice were treated with a combination of anti-CTLA-4 and an irradiated GMSM1 vaccine or anti-CTLA-4 alone as described in a. Growth of the parental SM1 tumor was monitored, and incidence is indicated in parentheses.
GM-CSF Expression and CTLA-4 Blockade Synergize in Treatment of SM1 Tumors.
Previous studies suggested that GM-CSF is capable of enhancing antitumor immunity (11, 12, 28). As described above, GMSM1 was effective at providing immunity against rechallenge with the parental SM1 tumor. We next tested whether this vaccination strategy alone, or in combination with CTLA-4 blockade, would promote tumor regression in mice implanted with SM1 cells. Treatment with a vaccine consisting of irradiated GM-CSF-expressing SM1 cells (GMSM1) alone was not effective at promoting regression of SM1. As described above, treatment with anti-CTLA-4 resulted in delayed SM1 growth, but rarely promoted rejection (Figs. 3a and 4). Treatment with anti-CTLA-4 and a vaccine consisting of either SM1 or B7SM1 was not significantly more effective than anti-CTLA-4 treatment alone.
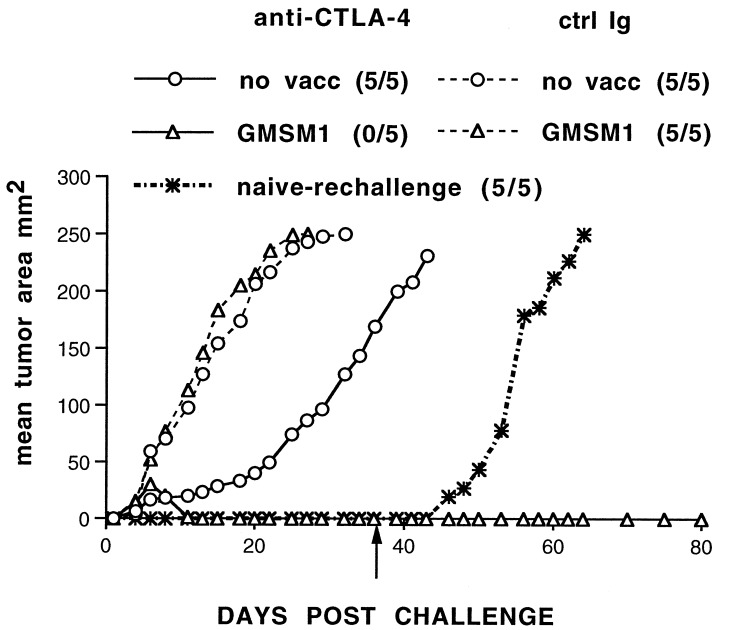
Figure 4.
Rejection as a consequence of anti-CTLA-4 treatment and a GM-CSF-expressing vaccine results in immunity to rechallenge. Mice were treated as in Fig. 3a. Six weeks after initial challenge with SM1, mice were rechallenged (arrow) on a separate, shaved area of the back with 2 × 105 SM1 cells. Tumor growth was monitored, and incidence is indicated in parentheses.
In contrast, treatment with both an irradiated GMSM1 vaccine and anti-CTLA-4 resulted in regression of the SM1 tumor in a significant fraction of animals (Fig. 3a). In addition, an SM1 line transduced to express both B7 and GM-CSF was equally as effective at promoting regression of SM1 tumors when used in combination with anti-CTLA-4 (Fig. 3a). In six separate experiments, progression of SM1 tumors after GMSM1 vaccination and anti-CTLA-4 treatment was profoundly inhibited and tumor incidence was less than 20% (7/40). We also observed regression of SM1 tumors in mice given a 10-fold-larger SM1 challenge (2 × 105 cells) and using a similar treatment protocol (5/10 mice tested, Fig. 3b). Together, these data suggest that CTLA-4 blockade enhances the potency of the GM-CSF-expressing vaccine.
To determine whether this treatment regimen was a result of induction of a transient effector mechanism or longer-lasting immunity, mice that rejected SM1 tumors were rechallenged with the parental tumors 30 days after regression of the initial tumor challenge. As demonstrated in Fig. 4, treatment with a GM-CSF-expressing vaccine and anti-CTLA-4 resulted in immunity to rechallenge with SM1. In two experiments, 100% of mice (10 mice) rejected rechallenge with a large dose of SM1 (2 × 105 cells). These data confirm that rejection of SM1 after GMSM1 vaccination and CTLA-4 blockade is accompanied by immunity to SM1 tumors.
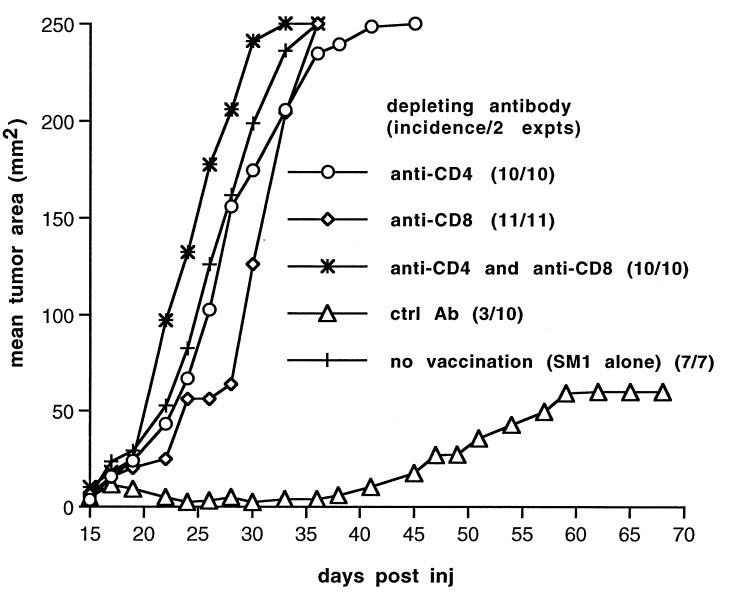
Both CD4+ and CD8+ T Cells Are Required for Regression of SM1 Tumors.
To identify the population of lymphocytes involved in rejection of SM1, mice were injected with ascitic fluid containing depleting antibodies directed against CD4 and/or CD8. After confirmation of depletion, mice were implanted with SM1 cells and treated with the GMSM1 vaccine and anti-CTLA-4 as described above. Not surprisingly, SM1 tumors grew in mice depleted of both CD4+ and CD8+ cells, despite a treatment regimen that was effective in mice previously administered a control rat IgG suspension (Fig. 5). Depletion of CD8+ cells also resulted in tumor outgrowth, consistent with the idea CD8+ cytotoxic T lymphocytes are the effector population mediating antitumor cytotoxicity. In addition, SM1 tumors also grew in mice depleted of CD4+ cells alone. Given that SM1 does not express class II MHC, these data imply that GM-CSF expression by the vaccine recruits and activates host-derived APCs that present class II-restricted antigens to CD4+ T cells and that this cross-priming may provide T cell help necessary for elimination of SM1 tumors. Accordingly, CTLA-4 blockade may block inhibitory interactions between these APCs and antitumor T cells.
Figure 5.
Both CD4+ and CD8+ cells are required for regression of SM1 tumors. Six days before SM1 tumor challenge and initiation of treatment, mice were depleted of the indicated cell population as described in Materials and Methods. Mice were implanted with SM1 tumors and treated as described in Fig. 3a, and tumor growth was monitored. In contrast to mice that were mock-depleted (triangles), all mice depleted of either CD4+ or CD8+ cells (or both populations) grew tumors.
DISCUSSION
We and others have shown that administration of anti-CTLA-4 can be sufficient to promote regression of unmodified tumors, presumably by blocking inhibitory signals provided by CTLA-4/B7 interactions (22–24). In the present study, we demonstrate that although CTLA-4 blockade was not effective against a weakly immunogenic mammary carcinoma, SM1, the combination of CTLA-4 blockade with a GM-CSF-expressing tumor vaccine promoted regression of SM1. This treatment strategy depended on both CD4+ and CD8+ T cells and induced protective immunity to rechallenge with the parental SM1 tumor.
Previously, we showed that conferring B7 expression was not effective at promoting rejection of SM1 (17). Similarly, the present study demonstrates that treatment with anti-CTLA-4 did not significantly reduce tumorigenicity of SM1, although it did slow tumor growth at lower inocula. We also demonstrated that CTLA-4 blockade does not synergize with B7 expression by SM1 cells, as administration of anti-CTLA-4 to mice implanted with B7SM1 tumors was ineffective at enhancing rejection. Vaccination using B7SM1 and anti-CTLA-4 was similarly ineffective at promoting regression of SM1 tumors. These findings are consistent with other results from our laboratory suggesting that in poorly immunogenic tumors where B7 expression has no effect on tumor rejection, CTLA-4 blockade and B7 expression by tumors do not act synergistically to enhance antitumor immunity. These findings also may be reflective of the idea that B7+ tumors may directly stimulate T cells whereas CTLA-4 blockade may act by enhancing T cell priming by host-derived APCs.
The most effective treatment strategy we observed in this study was CTLA-4 blockade in combination with a GM-CSF-expressing tumor cell vaccine. By itself, GM-CSF elicited immunity as a protective vaccine, consistent with the findings of others (11), but was ineffective at treating SM1 tumors. However, in combination with anti-CTLA-4, it was a powerful treatment for recently established SM1 tumors. Treatment of the fast-growing SM1 tumors was most effective at a dose of SM1 that is at least 10 times the minimum tumorigenic dose, suggesting that there is a threshold of treatment efficacy that is dependent on the initial tumor burden. Recently, we have extended these findings to the BL6 variant of the B16 melanoma where neither GM-CSF-expressing tumor nor anti-CTLA-4 treatment alone are effective, but the combination results in regression of tumors in about 2/3 of the mice, again with a strong dependence on initial tumor inoculum (data not shown).
Although the detailed mechanism of rejection in this system remains to be established, our studies demonstrate that both CD4+ and CD8+ cells are required. Because SM1 does not express MHC class II, even after exposure to IFN-γ (17), the requirement for CD4+ cells suggests that class II-restricted, CD4+ T cells are primed by host APCs. This idea is consistent with previous reports that tumor-derived GM-CSF enhances host presentation of tumor antigens and permits cross-priming to occur (29). GM-CSF is known to promote growth and activation of dendritic cells and render them more potent APCs (15, 30, 31). Accordingly, CTLA-4 blockade in this treatment regimen may block inhibitory interactions between host APCs (potentially GM-CSF-stimulated dendritic cells) and T cells, and facilitate costimulatory interactions between APC and T cells, thereby enhancing priming of T cells to promote immunity to and rejection of SM1.
Our previous findings suggested that both IFN-γ and B7 enhanced immunogenicity of SM1 by directly enhancing T cell activation (17). They also suggested that if “cotherapies” were to act synergistically, they both needed to activate the same “arm” of T cell activation (i.e., enhancement of T cell activation by the tumor as APC or antigen presentation by host-derived APC). Consistent with this idea, the data in the current study demonstrated that CTLA-4 blockade did not synergize with B7 expression by the tumor but it did synergize with tumor-derived GM-CSF expression. Accordingly, both tumor-derived GM-CSF expression and CTLA-4 blockade presumably enhance T cell activation at the level of host-derived APC function and therefore result in successful T cell priming. In contrast, by enhancing two different mechanisms of T cell priming (enhancing APC function of a tumor by conferring B7 expression and enhancing host APC function by CTLA-4 blockade), efficient T cell activation and, therefore, tumor clearance could not take place.
The findings presented in this report have important implications for immunotherapy in humans. Our data suggest that it is important to consider whether two therapies will act cooperatively when developing an immunotherapeutic strategy. Moreover, they also suggest that CTLA-4 blockade may be an important adjuvant for therapies in which a GM-CSF-expressing vaccine alone is inefficient. Along these lines, we currently are testing this approach to immunotherapy using two model systems of primary tumor development (27, 32). These model systems will assist in refining these and other approaches to cancer immunotherapy as well as in dissecting the mechanisms involved in T cell activation in the antitumor immune response.
Acknowledgments
We appreciate the technical assistance of Stan Grell, Jennifer Villasenor, and Kaichi Sung and the gift of the SM1 mammary line from Drs. Satyabrata Nandi and Raphael Guzman. This work was supported in part by the Howard Hughes Medical Institute (J.P.A.) and the National Institutes of Health [CA 40041 and 09179 (J.P.A.) and K08 CA 71613 (D.R.L.)]. A.A.H. is a fellow of the Department of Defense Breast Cancer Research Program.
ABBREVIATIONS
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- MHC
major histocompatibility complex
- APC
antigen-presenting cell
- IFN-γ
interferon-γ
- GMSM1
GM-CSF-expressing SM1 tumor
- B7SM1
B7–1-expressing SM1 tumor
References
- 1.Allison J P. Curr Opin Immunol. 1994;6:414–419. doi: 10.1016/0952-7915(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Ashe S, Brady W A, Hellstrom I, Hellstrom K E, Ledbetter J A, McGowan P, Linsley P S. Cell. 1992;71:1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 3.Townsend S, Allison J P. Science. 1993;259:368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 4.Townsend S E, Su F W, Atherton J M, Allison J P. Cancer Res. 1994;54:6477–6483. [PubMed] [Google Scholar]
- 5.Gansbacher B, Zier K, Daniels B, Cronin K, Bannerji R, Gilboa E. J Exp Med. 1990;172:1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fearon E, Pardoll D, Itaya T, Golumbek P, Levitsky H, Simons J, Karasuyama H, Vogelstein B, Frost P. Cell. 1990;60:397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- 7.Golumbek P, Lazanby H, Levitsky H, Jaffee L, Karasuyama H, Baker M, Pardoll D M. Science. 1991;254:713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- 8.Tepper R, Pattengale P, Leder P. Cell. 1989;57:503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- 9.Gansbacher B, Bannerji R, Daniels B, Zier K, Cronin K, Gilboa E. Cancer Res. 1990;50:7820–7825. [PubMed] [Google Scholar]
- 10.Watanabe Y, Kuribayashi K, Miyatake S. Proc Natl Acad Sci USA. 1989;86:9456–9462. doi: 10.1073/pnas.86.23.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R C. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levitsky H I, Montgomery J, Ahmadzadeh M, Staveley-O’Carroll K, Guarnieri F, Longo D L, Kwak L W. J Immunol. 1996;156:3858–3865. [PubMed] [Google Scholar]
- 13.Morrissey P J, Bressler L, Park L S, Alpert A, Gillis S. J Immunol. 1987;139:1113–1119. [PubMed] [Google Scholar]
- 14.Smith P D, Lamerson C L, Wong H L, Wahl L M, Wahl S M. J Immunol. 1990;144:3829–3834. [PubMed] [Google Scholar]
- 15.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. Nature (London) 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 16.Sumimoto H, Tani K, Nakazaki Y, Tanabe T, Hibino H, Hamada H, Azuma M, Asano S. Int J Cancer. 1997;73:556–561. doi: 10.1002/(sici)1097-0215(19971114)73:4<556::aid-ijc17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz A A, Townsend S E, Yu T F-Y, Atherton J, Allison J P. Int J Cancer. 1998;77:107–113. doi: 10.1002/(sici)1097-0215(19980703)77:1<107::aid-ijc17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Krummel M F, Allison J P. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krummel M F, Allison J P. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walunas T L, Lenschow D J, Bakker C Y, Linsley P S, Freeman G J, Green J M, Thompson C B, Bluestone J A. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 21.Walunas T L, Bakker C Y, Bluestone J A. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leach D R, Krummel M F, Allison J P. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 23.Kwon E D, Hurwitz A A, Foster B A, Madias C, Feldhaus A, Greenberg N M, Burg M B, Allison J P. Proc Natl Acad Sci USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y F, Zou J P, Mu J, Wijesuriya R, Ono S, Walunas T, Bluestone J, Fujiwara H, Hamaoka T. Cancer Res. 1997;57:4036–4041. [PubMed] [Google Scholar]
- 25.Zitvogel L, Robbins P D, Storkus W J, Clarke M R, Maeurer M J, Campbell R L, Davis C G, Tahara H, Schreiber R D, Lotze M T. Eur J Immunol. 1996;26:1335–1341. doi: 10.1002/eji.1830260624. [DOI] [PubMed] [Google Scholar]
- 26.Coughlin C M, Wysocka M, Kurzawa H L, Lee W M, Trinchieri G, Eck S L. Cancer Res. 1995;55:4980–4987. [PubMed] [Google Scholar]
- 27.Guzman R C, Osborn R C, Swanson S M, Sakthivel R, Hwang S I, Miyamoto S, Nandi S. Cancer Res. 1992;52:5732–5737. [PubMed] [Google Scholar]
- 28.Levitsky H, Lazenby A, Hayashi R J, Pardoll D M. J Exp Med. 1994;179:1215–1224. doi: 10.1084/jem.179.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang A Y C, Golumbek P, Ahmadzedeh M, Jaffee E, Pardoll D, Levitsky H. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 30.Heufler C, Koch F, Schuler G. J Exp Med. 1988;167:700–705. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto F, Lanzavecchia A. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenberg N M, DeMayo F J, Sheppard P C, Barrios R, Lebovitz R, Finegold M, Angelopolou R, Dodd J G, Duckworth M L, Rosen J M, Matusik R J. Mol Endocrinol. 1994;8:230–239. doi: 10.1210/mend.8.2.8170479. [DOI] [PubMed] [Google Scholar]