Abstract
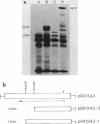
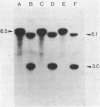
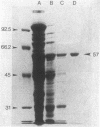
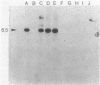
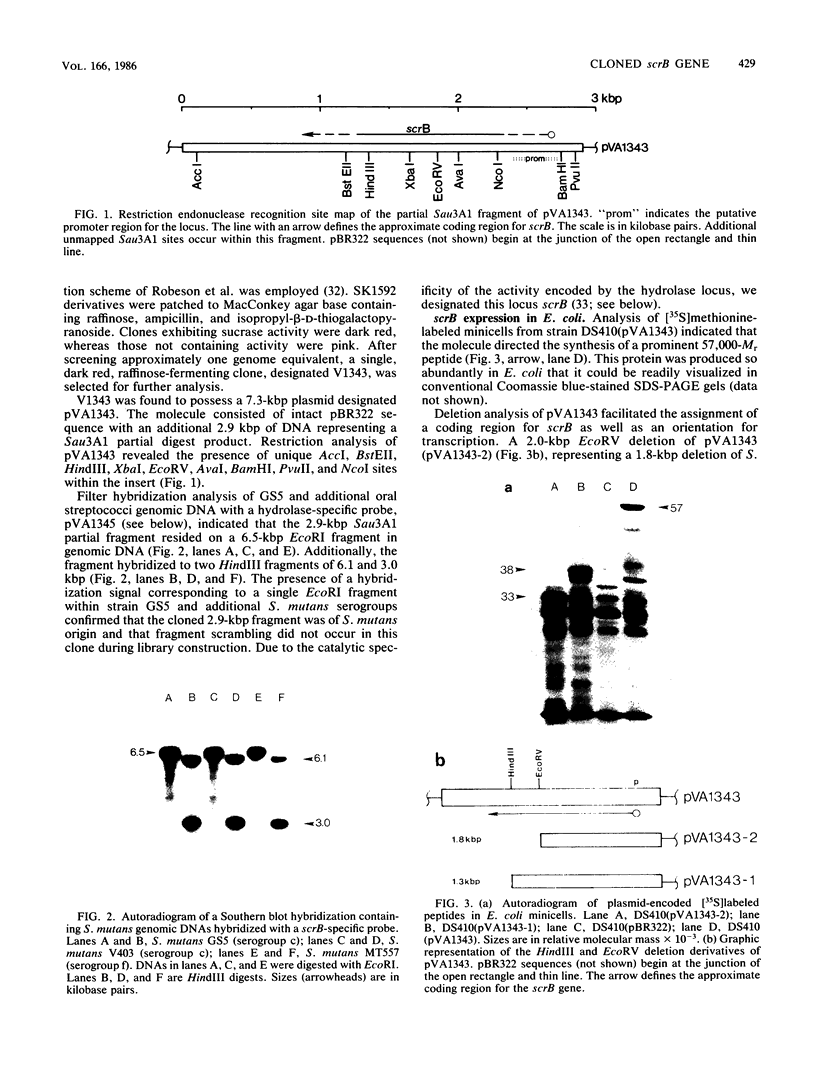
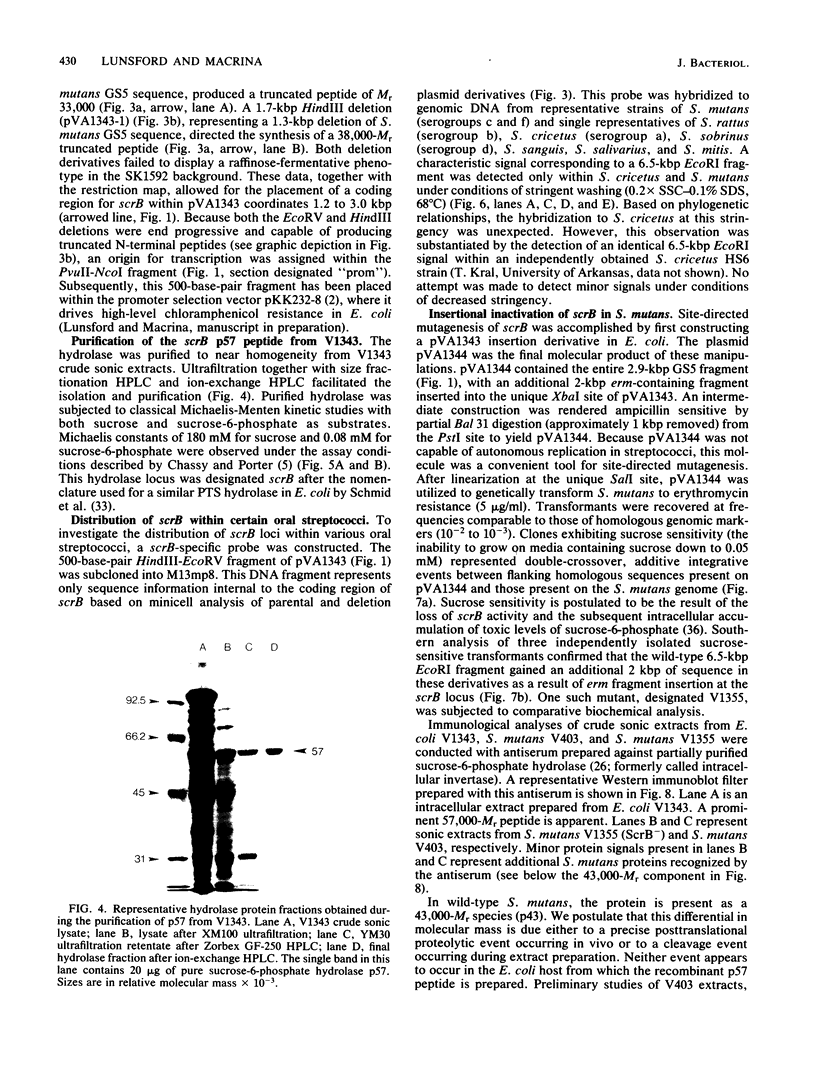
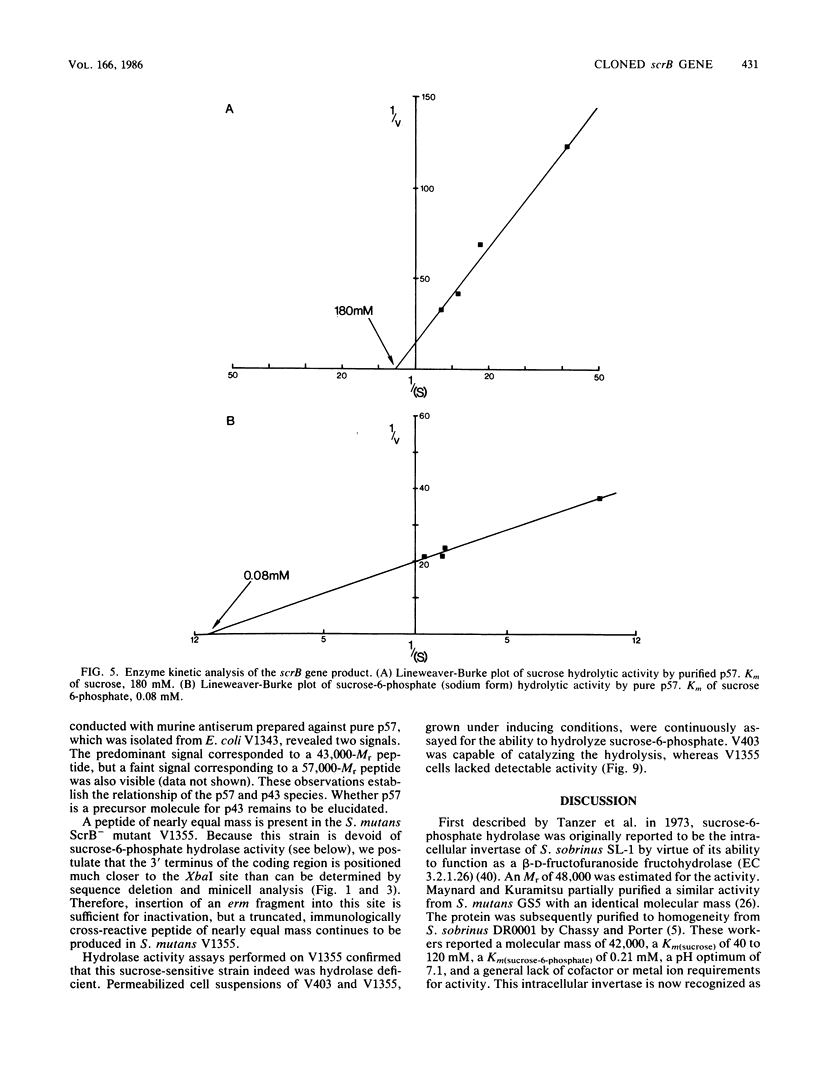
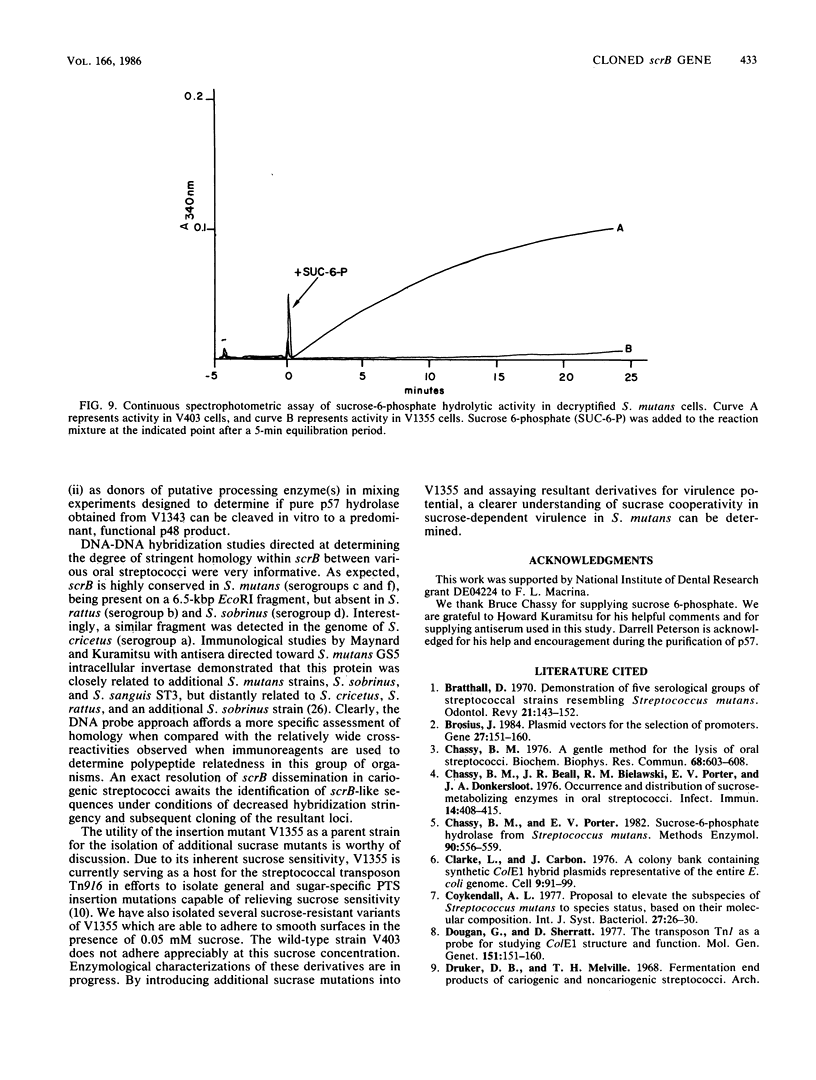
A DNA fragment encoding the sucrose-6-phosphate hydrolase component of the Streptococcus mutans phosphoenolpyruvate-dependent sucrose phosphotransferase system has been recovered from a plasmid-based genomic library of strain GS5. The locus, designated scrB, was found to reside within a 2.9-kilobase-pair restriction fragment present on the chimeric molecule pVA1343 (7.3 kilobase pairs). Minicell analysis of pVA1343-directed translation products revealed that the scrB product synthesized in Escherichia coli V1343 was a single peptide of Mr 57,000. This polypeptide was reactive with antiserum prepared against S. mutans intracellular invertase, which has been previously shown to have an Mr of 43,000 to 48,000. The basis of this difference in Mr was not established but may represent a posttranslational proteolytic event which occurred in S. mutans but not in recombinant V1343. Sucrose-6-phosphate hydrolase purified to homogeneity from V1343 exhibited Michaelis constants of 180 mM for sucrose and 0.08 mM for sucrose-6-phosphate. Deletion analysis of pVA1343 facilitated the assignment of a coding region for the hydrolase within the insert, as well as an orientation for the transcription of scrB. scrB-defective strains of S. mutans constructed by additive integration of an insertionally inactivated scrB locus exhibited the sucrose sensitivity characteristic of this mutant class. Similar loci were detected by DNA-DNA hybridization in additional strains of S. mutans and two strains of Streptococcus cricetus, but not in single strain representatives of S. rattus, S. sobrinus, S. sanguis I and II, S. salivarius, or S. mitis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Brosius J. Plasmid vectors for the selection of promoters. Gene. 1984 Feb;27(2):151–160. doi: 10.1016/0378-1119(84)90136-7. [DOI] [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Beall J. R., Bielawski R. M., Porter E. V., Donkersloot J. A. Occurrence and distribution of sucrose-metabolizing enzymes in oral streptococci. Infect Immun. 1976 Aug;14(2):408–415. doi: 10.1128/iai.14.2.408-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Porter E. V. Sucrose-6-phosphate hydrolase from Streptococcus mutans. Methods Enzymol. 1982;90(Pt E):556–559. doi: 10.1016/s0076-6879(82)90185-9. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Erratt J. A., Nasim A. Cloning and expression of a Saccharomyces diastaticus glucoamylase gene in Saccharomyces cerevisiae and Schizosaccharomyces pombe. J Bacteriol. 1986 May;166(2):484–490. doi: 10.1128/jb.166.2.484-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAFSSON B. E., QUENSEL C. E., LANKE L. S., LUNDQVIST C., GRAHNEN H., BONOW B. E., KRASSE B. The Vipeholm dental caries study; the effect of different levels of carbohydrate intake on caries activity in 436 individuals observed for five years. Acta Odontol Scand. 1954 Sep;11(3-4):232–264. doi: 10.3109/00016355308993925. [DOI] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984 Jul;159(1):214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley D. L., Jones K. R., Tobian J. A., LeBlanc D. J., Macrina F. L. Disseminated tetracycline resistance in oral streptococci: implication of a conjugative transposon. Infect Immun. 1984 Jul;45(1):13–17. doi: 10.1128/iai.45.1.13-17.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral T. A., Daneo-Moore L. Glycerol incorporation in certain oral streptococci. Infect Immun. 1980 Dec;30(3):759–765. doi: 10.1128/iai.30.3.759-765.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macrina F. L., Jones K. R., Wood P. H. Chimeric streptococcal plasmids and their use as molecular cloning vehicles in Streptococcus sanguis (Challis). J Bacteriol. 1980 Sep;143(3):1425–1435. doi: 10.1128/jb.143.3.1425-1435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Reider J. L., Virgili S. S., Kopecko D. J. Survey of the extrachromosomal gene pool of Streptococcus mutans. Infect Immun. 1977 Jul;17(1):215–226. doi: 10.1128/iai.17.1.215-226.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Scott C. L. Evidence for a disseminated plasmid in Streptococcus mutans. Infect Immun. 1978 Apr;20(1):296–302. doi: 10.1128/iai.20.1.296-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Tobian J. A., Jones K. R., Evans R. P., Clewell D. B. A cloning vector able to replicate in Escherichia coli and Streptococcus sanguis. Gene. 1982 Oct;19(3):345–353. doi: 10.1016/0378-1119(82)90025-7. [DOI] [PubMed] [Google Scholar]
- Maynard M. T., Kuramitsu H. K. Purification and antigenic properties of intracellular invertase from Streptococcus mutans. Infect Immun. 1979 Mar;23(3):873–883. doi: 10.1128/iai.23.3.873-883.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in Escherichia coli: cryogenic preservation of competent cells. J Bacteriol. 1977 Oct;132(1):349–351. doi: 10.1128/jb.132.1.349-351.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci M. J., Tew J. G., Macrina F. L. Human serum antibody response against Streptococcus mutans antigens. Infect Immun. 1986 Feb;51(2):600–606. doi: 10.1128/iai.51.2.600-606.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N. Bacteriophage infection of minicells: a general method for identification of "in vivo" bacteriophage directed polypeptide biosynthesis. Mol Gen Genet. 1977 Dec 14;158(1):73–79. doi: 10.1007/BF00455121. [DOI] [PubMed] [Google Scholar]
- Reeve J. N. Mucopeptide biosynthesis by minicells of Escherichia coli. J Bacteriol. 1977 Jul;131(1):363–365. doi: 10.1128/jb.131.1.363-365.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robeson J. P., Barletta R. G., Curtiss R., 3rd Expression of a Streptococcus mutans glucosyltransferase gene in Escherichia coli. J Bacteriol. 1983 Jan;153(1):211–221. doi: 10.1128/jb.153.1.211-221.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Schupfner M., Schmitt R. Plasmid-mediated uptake and metabolism of sucrose by Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):68–76. doi: 10.1128/jb.151.1.68-76.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Phosphoenolpyruvate-dependent sucrose phosphotransferase activity in Streptococcus mutans NCTC 10449. Infect Immun. 1979 Jun;24(3):821–828. doi: 10.1128/iai.24.3.821-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- St Martin E. J., Wittenberger C. L. Characterization of a phosphoenolpyruvate-dependent sucrose phosphotransferase system in Streptococcus mutans. Infect Immun. 1979 Jun;24(3):865–868. doi: 10.1128/iai.24.3.865-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Martin E. J., Wittenberger C. L. Regulation and function of sucrose 6-phosphate hydrolase in Streptococcus mutans. Infect Immun. 1979 Nov;26(2):487–491. doi: 10.1128/iai.26.2.487-491.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Brown A. T., McInerney M. F. Identification, preliminary characterization, and evidence for regulation of invertase in Streptococcus mutans. J Bacteriol. 1973 Oct;116(1):192–202. doi: 10.1128/jb.116.1.192-202.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M. Studies on the fate of the glucosyl moiety of sucrose metabolized by Streptococcus mutans. J Dent Res. 1972 Mar-Apr;51(2):415–423. doi: 10.1177/00220345720510023001. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]