Abstract
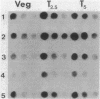
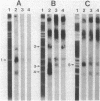
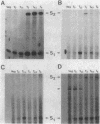
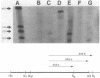
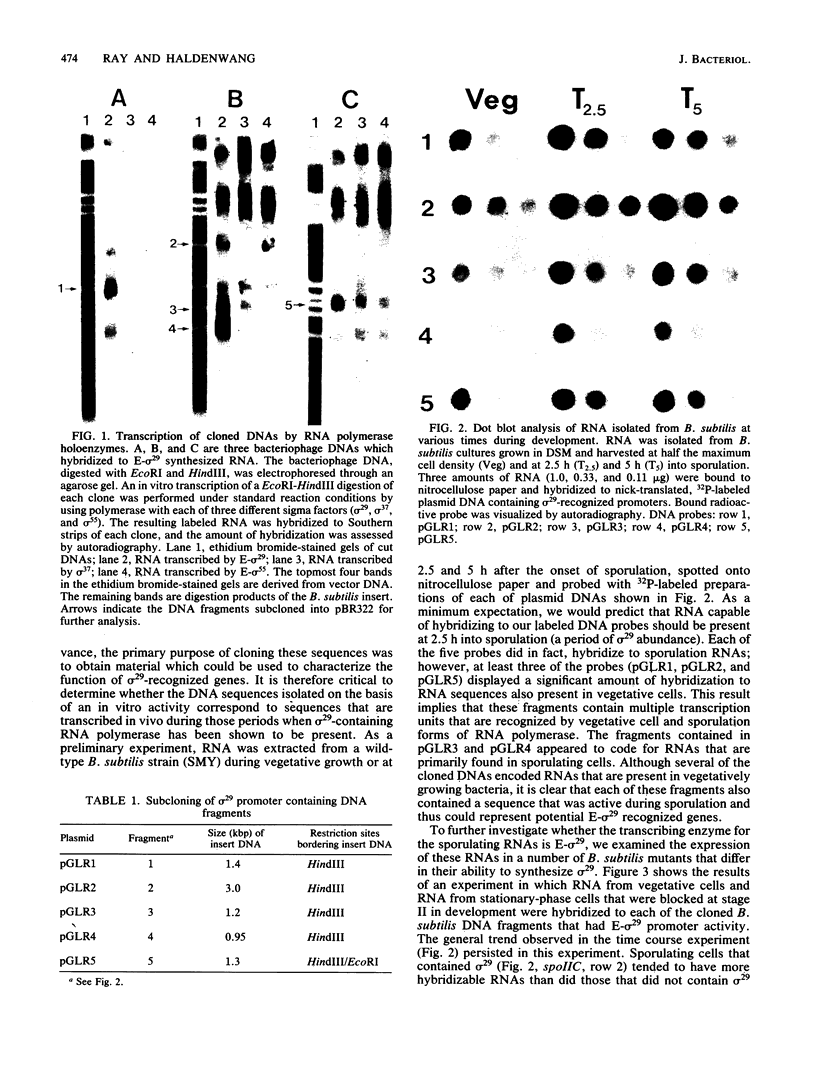
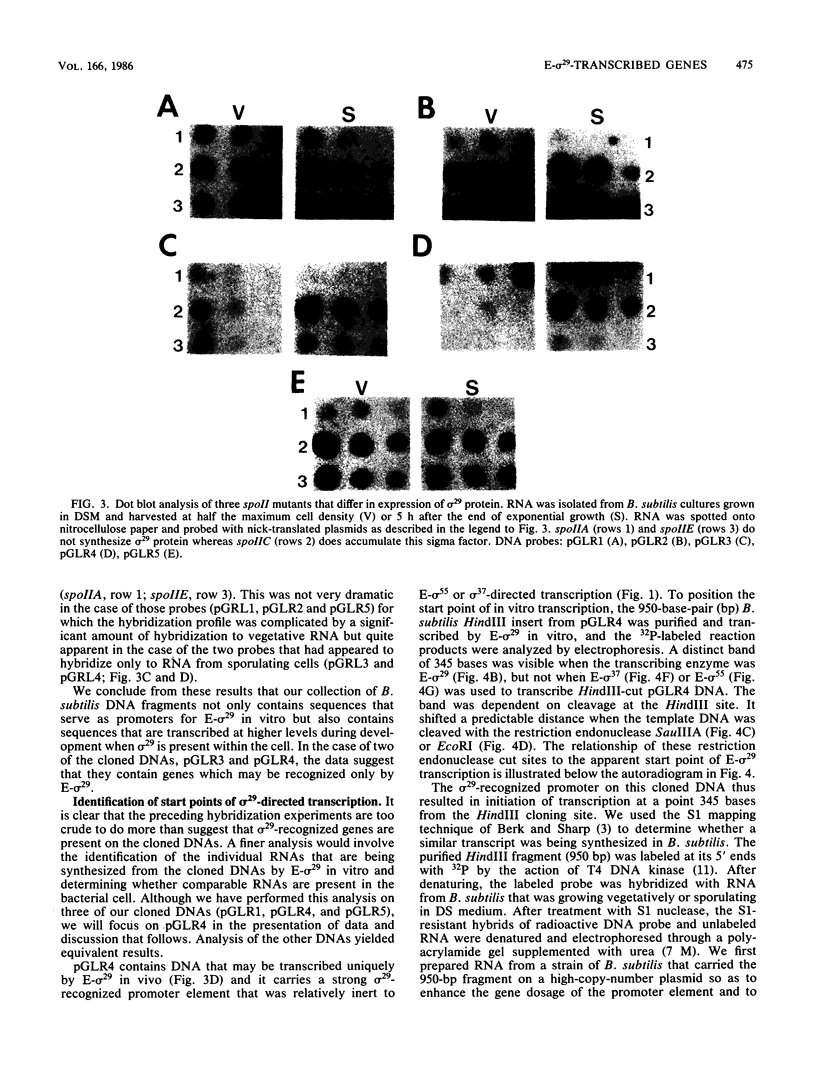
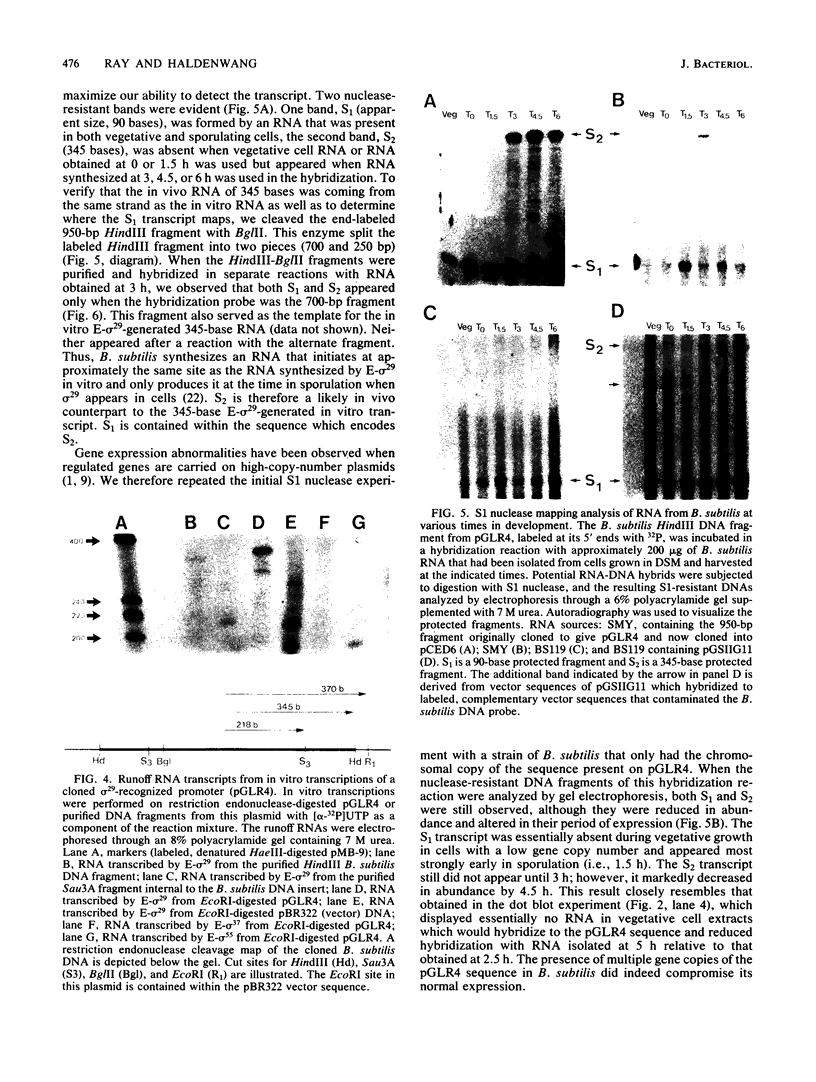
As a means of determining the function of sigma 29, a sporulation-essential sigma factor, we have isolated and begun to characterize genes that require sigma 29 for their expression. RNA transcribed in vitro from total Bacillus subtilis DNA by using sigma 29-containing RNA polymerase (E-sigma-29) was hybridized to a bank of B. subtilis DNA fragments that had been cloned into bacteriophage lambda. Approximately 0.25% of the cloned B. subtilis DNA fragments displayed detectable hybridization with our RNA probe. Five DNA fragments that had strong in vitro template activity for E-sigma-29 were selected for further study. The DNA fragments which contained in vitro sigma 29 promoter activity encoded RNAs that were synthesized by B. subtilis during sporulation. Mutant B. subtilis that failed to synthesize sigma 29 (spoIIA, spoIIE) made less RNA that could hybridize to these cloned DNAs than did a mutant (spoIIC) which did synthesize sigma 29 but was blocked at a similar stage in development. A detailed analysis of several of the cloned DNAs demonstrated that they encoded RNAs that were transcribed from approximately the same start site in vivo that E-sigma-29 initiated transcription in vitro. These particular transcripts were present only during the period of sigma-29 abundance (2 to 4 h after the onset of sporulation) in sporulating cells which carried a wild-type allele of the sigma-29 structural gene (spoIIG). We conclude that the isolation procedure used in this study identified genes that are transcribed by E-sigma 29, not only in vitro but also in vivo. Preliminary characterization of the cloned genes indicate that they encoded multiple overlapping RNAs which were each synthesized at unique times during growth or sporulation. This result implies that sigma 29 does not activate a unique population of genes with a novel function in sporulation but rather that it has a temporal role in spore gene control, transcribing those genes required to be active during its period of abundance regardless of their specific function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banner C. D., Moran C. P., Jr, Losick R. Deletion analysis of a complex promoter for a developmentally regulated gene from Bacillus subtilis. J Mol Biol. 1983 Aug 5;168(2):351–365. doi: 10.1016/s0022-2836(83)80023-0. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Donnelly C. E., Sonenshein A. L. Promoter-probe plasmid for Bacillus subtilis. J Bacteriol. 1984 Mar;157(3):965–967. doi: 10.1128/jb.157.3.965-967.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z., Glenn J. S., Singer V. L., Chamberlin M. J. Isolation of sigma-28-specific promoters from Bacillus subtilis DNA. Gene. 1984 Dec;32(1-2):11–20. doi: 10.1016/0378-1119(84)90027-1. [DOI] [PubMed] [Google Scholar]
- Haldenwang W. G., Lang N., Losick R. A sporulation-induced sigma-like regulatory protein from B. subtilis. Cell. 1981 Feb;23(2):615–624. doi: 10.1016/0092-8674(81)90157-4. [DOI] [PubMed] [Google Scholar]
- Haldenwang W. G., Losick R. A modified RNA polymerase transcribes a cloned gene under sporulation control in Bacillus subtilis. Nature. 1979 Nov 15;282(5736):256–260. doi: 10.1038/282256a0. [DOI] [PubMed] [Google Scholar]
- Hirochika H., Kobayashi Y., Kawamura F., Saito H. Cloning of sporulation gene spoOB of Bacillus subtilis and its genetic and biochemical analysis. J Bacteriol. 1981 May;146(2):494–505. doi: 10.1128/jb.146.2.494-505.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. C., Moran C. P., Jr, Losick R. Two RNA polymerase sigma factors from Bacillus subtilis discriminate between overlapping promoters for a developmentally regulated gene. Nature. 1983 Apr 28;302(5911):800–804. doi: 10.1038/302800a0. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorenstein R. G., Losick R. Purification and properties of the sigma subunit of ribonucleic acid polymerase from vegetative Bacillus subtilis. J Biol Chem. 1973 Sep 10;248(17):6163–6169. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stragier P., Bouvier J., Bonamy C., Szulmajster J. A developmental gene product of Bacillus subtilis homologous to the sigma factor of Escherichia coli. Nature. 1984 Nov 22;312(5992):376–378. doi: 10.1038/312376a0. [DOI] [PubMed] [Google Scholar]
- Talmadge K., Gilberg W. Construction of plasmid vectors with unique PstI cloning sites in a signal sequence coding region. Gene. 1980 Dec;12(3-4):235–241. doi: 10.1016/0378-1119(80)90105-5. [DOI] [PubMed] [Google Scholar]
- Tatti K. M., Moran C. P., Jr Utilization of one promoter by two forms of RNA polymerase from Bacillus subtilis. Nature. 1985 Mar 14;314(6007):190–192. doi: 10.1038/314190a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempy J. E., Morrison-Plummer J., Haldenwang W. G. Synthesis of sigma 29, an RNA polymerase specificity determinant, is a developmentally regulated event in Bacillus subtilis. J Bacteriol. 1985 Jan;161(1):340–346. doi: 10.1128/jb.161.1.340-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs J. L., Gilman M. Z., Chamberlin M. J. Heterogeneity of RNA polymerase in Bacillus subtilis: evidence for an additional sigma factor in vegetative cells. Proc Natl Acad Sci U S A. 1981 May;78(5):2762–2766. doi: 10.1073/pnas.78.5.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]