Abstract
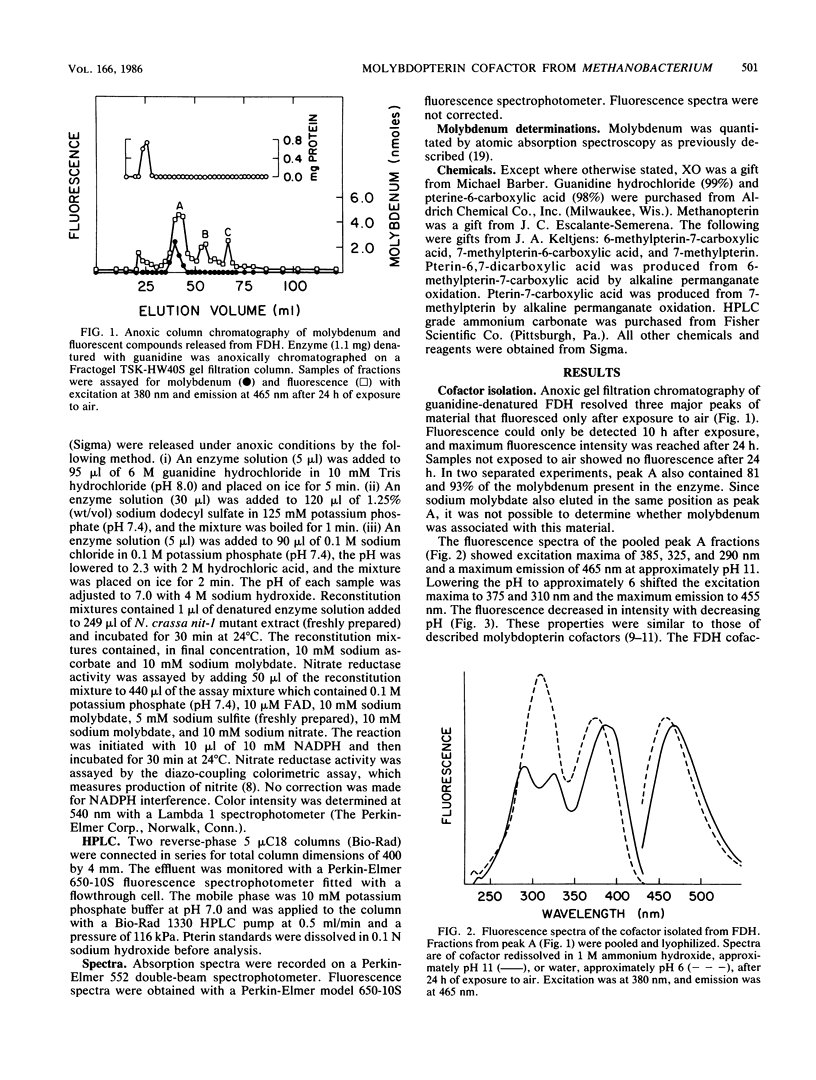
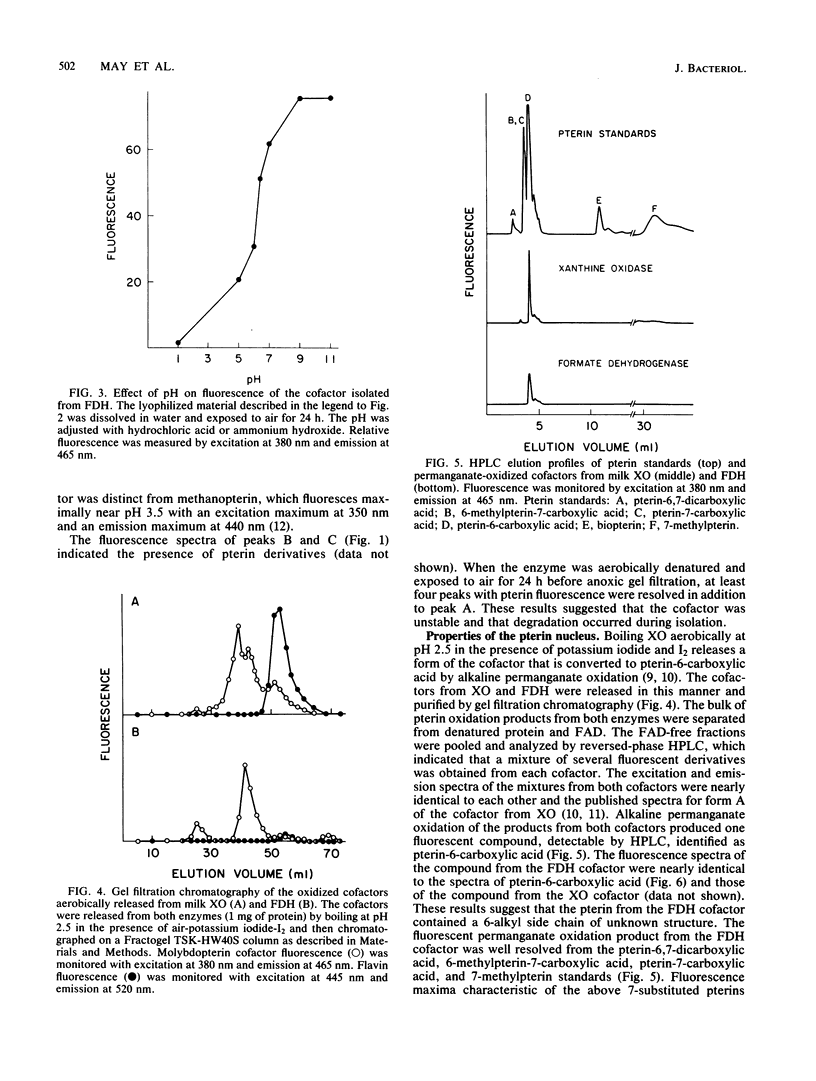
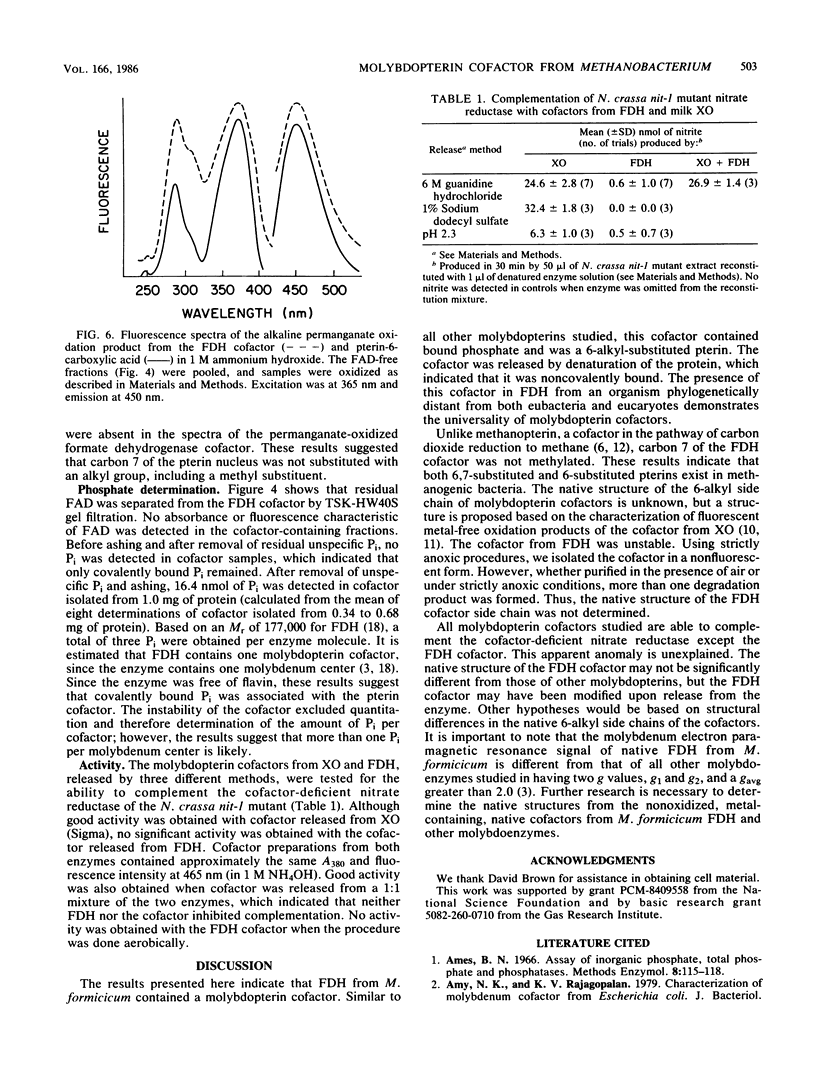
The molybdopterin cofactor from the formate dehydrogenase of Methanobacterium formicicum was studied. The cofactor was released by guanidine denaturation of homogeneous enzyme, which also released greater than 80% of the molybdenum present in the enzyme. The anoxically isolated cofactor was nonfluorescent, but after exposure to air it fluoresced with spectra similar to those of described molybdopterin cofactors. Aerobic release from acid-denatured formate dehydrogenase in the presence of I2 and potassium iodide produced a mixture of fluorescent products. Alkaline permanganate oxidation of the mixture yielded pterin-6-carboxylic acid as the only detectable fluorescent product. The results showed that the cofactor from formate dehydrogenase contained a pterin nucleus with a 6-alkyl side chain of unknown structure. Covalently bound phosphate was also present. The isolated cofactor was unable to complement the cofactor-deficient nitrate reductase of the Neurospora crassa nit-1 mutant.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber M. J., Siegel L. M., Schauer N. L., May H. D., Ferry J. G. Formate dehydrogenase from Methanobacterium formicicum. Electron paramagnetic resonance spectroscopy of the molybdenum and iron-sulfur centers. J Biol Chem. 1983 Sep 25;258(18):10839–10845. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Rinehart K. L., Jr, Wolfe R. S. Tetrahydromethanopterin, a carbon carrier in methanogenesis. J Biol Chem. 1984 Aug 10;259(15):9447–9455. [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Involvement of a B-type cytochrome in the assimilatory nitrate reductase of Neurospora crassa. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1603–1610. doi: 10.1073/pnas.58.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Hainline B. E., Rajagopalan K. V., Arison B. H. The pterin component of the molybdenum cofactor. Structural characterization of two fluorescent derivatives. J Biol Chem. 1984 May 10;259(9):5414–5422. [PubMed] [Google Scholar]
- Johnson J. L., Hainline B. E., Rajagopalan K. V. Characterization of the molybdenum cofactor of sulfite oxidase, xanthine, oxidase, and nitrate reductase. Identification of a pteridine as a structural component. J Biol Chem. 1980 Mar 10;255(5):1783–1786. [PubMed] [Google Scholar]
- Johnson J. L., Rajagopalan K. V. Structural and metabolic relationship between the molybdenum cofactor and urothione. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6856–6860. doi: 10.1073/pnas.79.22.6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltjens J. T., Huberts M. J., Laarhoven W. H., Vogels G. D. Structural elements of methanopterin, a novel pterin present in Methanobacterium thermoautotrophicum. Eur J Biochem. 1983 Feb 15;130(3):537–544. doi: 10.1111/j.1432-1033.1983.tb07183.x. [DOI] [PubMed] [Google Scholar]
- Meyer O., Rajagopalan K. V. Molybdopterin in carbon monoxide oxidase from carboxydotrophic bacteria. J Bacteriol. 1984 Feb;157(2):643–648. doi: 10.1128/jb.157.2.643-648.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIELSEN S. O., LEHNINGER A. L. Phosphorylation coupled to the oxidation of ferrocytochrome c. J Biol Chem. 1955 Aug;215(2):555–570. [PubMed] [Google Scholar]
- Pienkos P. T., Shah V. K., Brill W. J. Molybdenum cofactors from molybdoenzymes and in vitro reconstitution of nitrogenase and nitrate reductase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5468–5471. doi: 10.1073/pnas.74.12.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Composition of the coenzyme F420-dependent formate dehydrogenase from Methanobacterium formicicum. J Bacteriol. 1986 Feb;165(2):405–411. doi: 10.1128/jb.165.2.405-411.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Properties of formate dehydrogenase in Methanobacterium formicicum. J Bacteriol. 1982 Apr;150(1):1–7. doi: 10.1128/jb.150.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. H. 7-Methylpterin and 7-methyllumizine: oxidative degradation products of 7-methyl-substituted pteridines in methanogenic bacteria. J Bacteriol. 1985 May;162(2):516–520. doi: 10.1128/jb.162.2.516-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


