Abstract
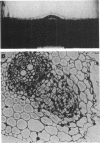
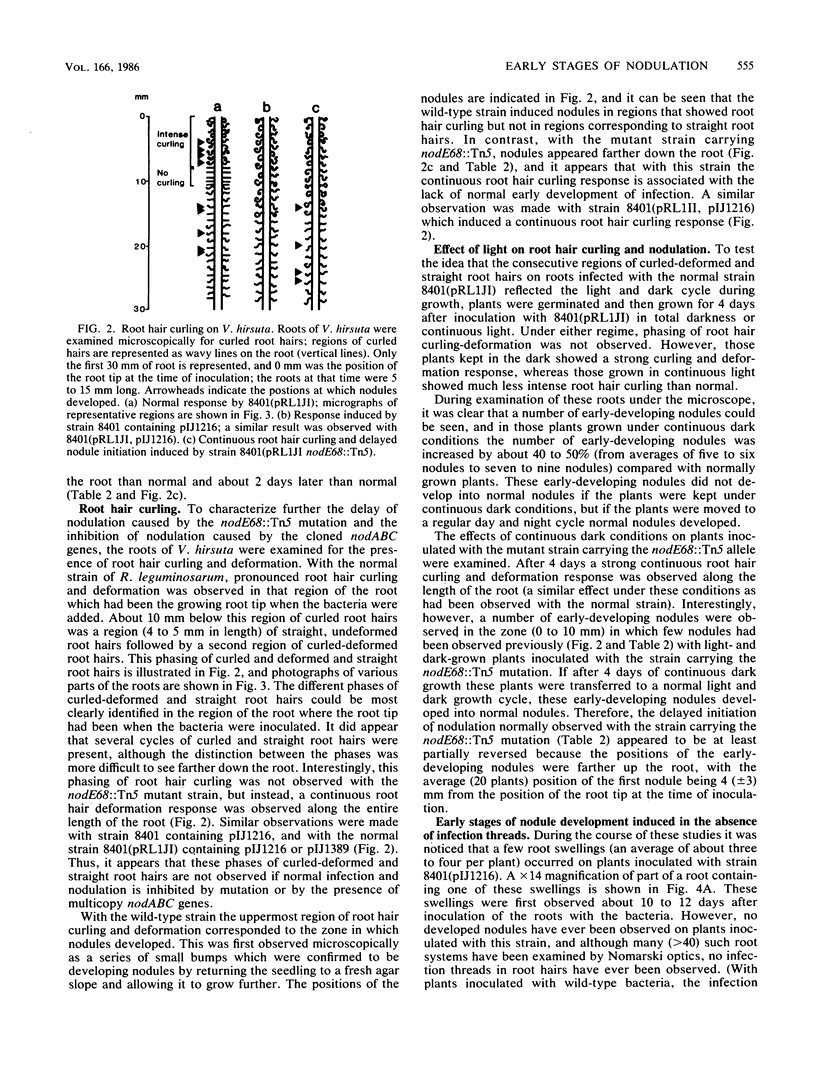
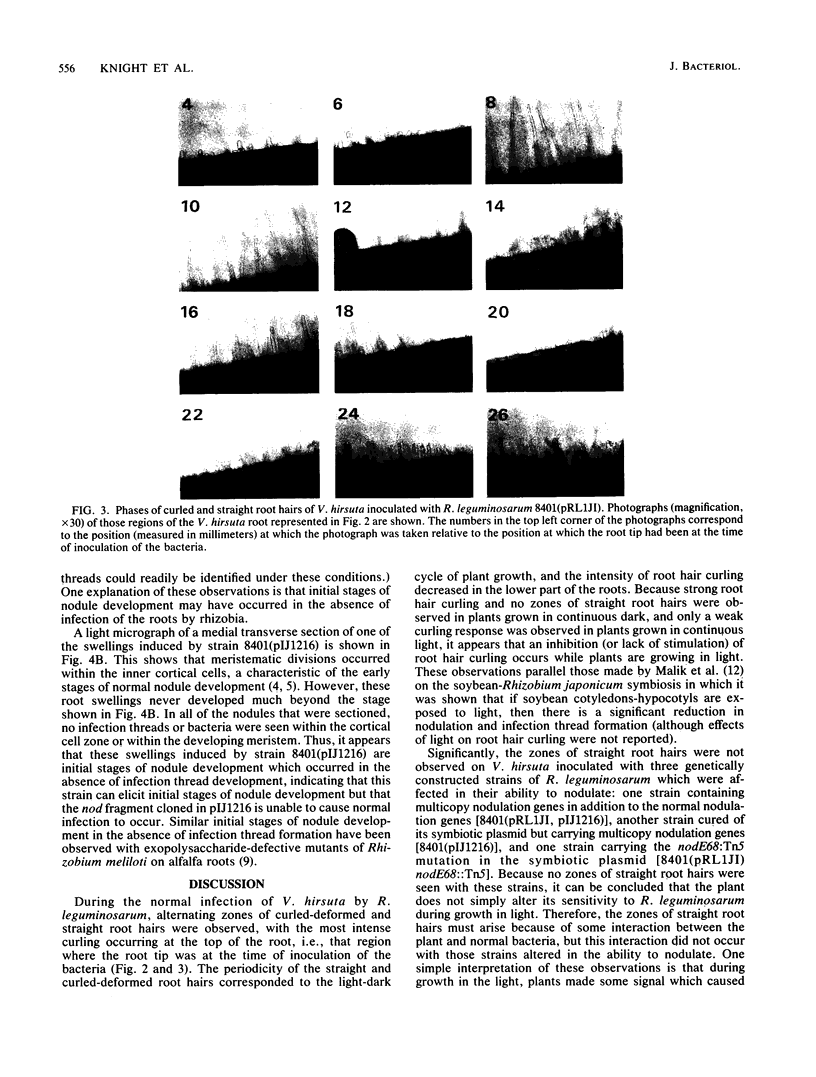
During analysis of early events in the infection and nodulation of Vicia hirsuta roots inoculated with normal and mutant strains of Rhizobium leguminosarum and strains containing cloned nodulation (nod) genes, a number of novel observations were made. (i) Alternating zones of curled and straight root hairs were seen on roots of V. hirsuta inoculated with the wild-type strain of R. leguminosarum. This phasing of root hair curling was not seen if plants were grown under continuous light or continuous dark conditions. (ii) Reduced nodulation and delayed nodule initiation was observed with a strain carrying a Tn5 mutation in the nodE gene. In addition the phased root hair curling was absent, and root hair curling was observed along the length of the root. (iii) The nodABC genes cloned on a multicopy plasmid in a wild-type strain inhibited nodulation but induced a continuous root hair curling response. Those few nodules that eventually formed were found to contain bacteria which had lost the plasmid carrying the nodABC genes. (iv) With a strain of Rhizobium cured of its indigenous symbiotic plasmid, but containing the cloned nodABCDEF genes, continuous root hair curling on V. hirsuta was observed. However, no infection threads were observed, and surprisingly, it did appear that initial stages of nodule development occurred. Observations of thin sections of these early developing nodules indicated that early nodule meristematic divisions may have occurred but that no bacteria were found within the nodules and no infection threads were observed either within the nodule bumps or within any of the root hairs. It was concluded that for normal infections to occur, precise regulation of the nod genes is required and that overexpression of the root hair curling genes inhibits the normal infection process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Ma Q. S., Knight C. D., Hombrecher G., Johnston A. W. Cloning of the symbiotic region of Rhizobium leguminosarum: the nodulation genes are between the nitrogenase genes and a nifA-like gene. EMBO J. 1983;2(6):947–952. doi: 10.1002/j.1460-2075.1983.tb01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Hirsch A. M., Leigh J. A., Johansen E., Kuldau G. A., Deegan S., Walker G. C., Signer E. R. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell. 1985 Apr;40(4):869–877. doi: 10.1016/0092-8674(85)90346-0. [DOI] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Host recognition in the Rhizobium-soybean symbiosis: detection of a protein factor in soybean root exudate which is involved in the nodulation process. Plant Physiol. 1984 Jan;74(1):84–89. doi: 10.1104/pp.74.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik N. S., Pence M. K., Calvert H. E., Bauer W. D. Rhizobium infection and nodule development in soybean are affected by exposure of the cotyledons to light. Plant Physiol. 1984 May;75(1):90–94. doi: 10.1104/pp.75.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M., Bauer W. D. A rapid regulatory response governing nodulation in soybean. Plant Physiol. 1983 Oct;73(2):286–290. doi: 10.1104/pp.73.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. G., Lyttleton P., Bullivant S., Grayston G. F. Membranes in lupin root nodules. I. The role of Golgi bodies in the biogenesis of infection threads and peribacteroid membranes. J Cell Sci. 1978 Apr;30:129–149. doi: 10.1242/jcs.30.1.129. [DOI] [PubMed] [Google Scholar]
- Rossen L., Johnston A. W., Downie J. A. DNA sequence of the Rhizobium leguminosarum nodulation genes nodAB and C required for root hair curling. Nucleic Acids Res. 1984 Dec 21;12(24):9497–9508. doi: 10.1093/nar/12.24.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen L., Shearman C. A., Johnston A. W., Downie J. A. The nodD gene of Rhizobium leguminosarum is autoregulatory and in the presence of plant exudate induces the nodA,B,C genes. EMBO J. 1985 Dec 16;4(13A):3369–3373. doi: 10.1002/j.1460-2075.1985.tb04092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J. C. Structural similarity of the membrane envelopes of rhizobial bacteroids and the host plasma membrane as revealed by freeze-fracturing. J Bacteriol. 1975 May;122(2):691–694. doi: 10.1128/jb.122.2.691-694.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]