Abstract
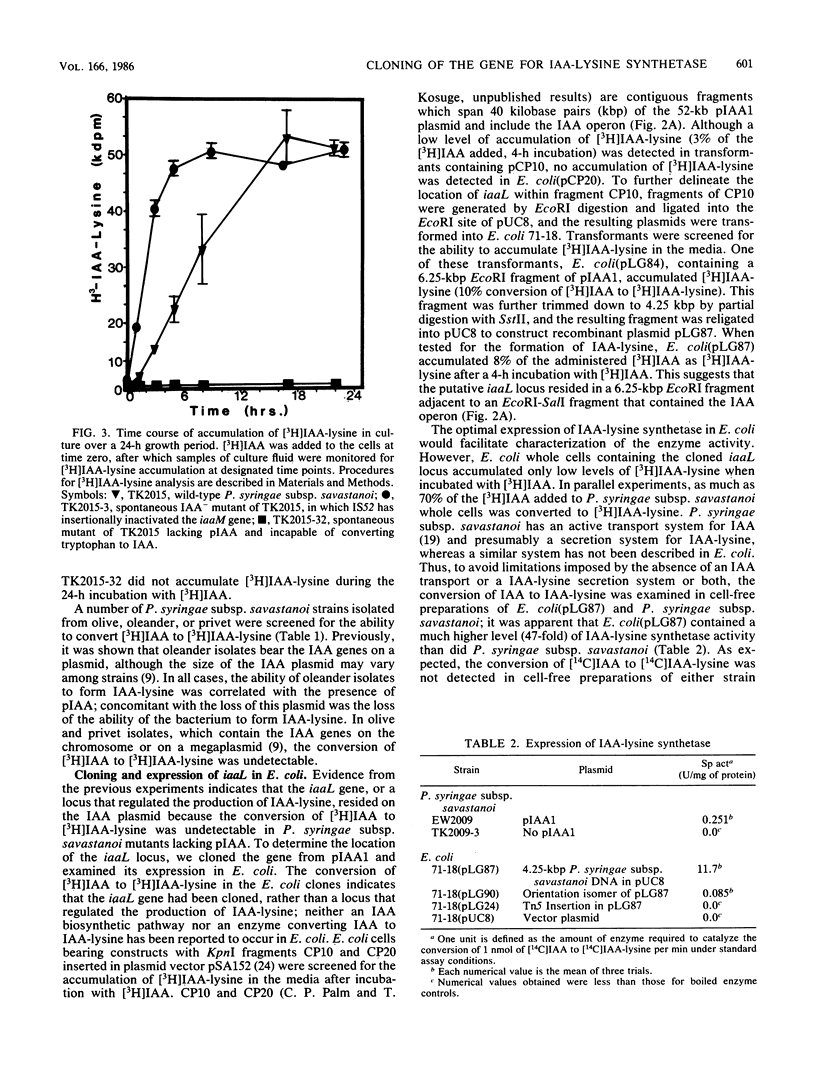
The phytopathogen Pseudomonas syringae subsp. savastanoi incites the production of galls on olive and oleander plants. Gall formation is dependent on bacterial production of the phytohormone indoleacetic acid (IAA). The genetic determinants for IAA synthesis are located on a plasmid (pIAA) and are organized in an operon in oleander strains of the bacterium. P. syringae subsp. savastanoi further converts IAA to an amino acid conjugate, 3-indole-acetyl-epsilon-L-lysine (IAA-lysine). The gene for IAA-lysine synthetase (iaaL) was found on the IAA plasmid by screening pIAA deletion mutants for the ability to convert IAA to IAA-lysine. The iaaL locus was then cloned in the vector pUC8 from a bank of P. syringae subsp. savastanoi EW2009 plasmid DNA to construct recombinant plasmid pLG87. The specific activity of IAA-lysine synthetase in Escherichia coli transformed with pLG87 was 47 times higher than that of the enzyme extract from P. syringae subsp. savastanoi. The direction of transcription of the iaaL gene was determined to be opposite to that of the IAA operon. The location of the iaaL gene on pIAA1 was mapped by Tn5 insertion mutagenesis to a 2.5-kilobase-pair fragment 2 kilobase pairs from the IAA operon.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi D. E., Morris R. O., Hinz R., Mischke B. S., Kosuge T., Garfinkel D. J., Gordon M. P., Nester E. W. Cytokinin/auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc Natl Acad Sci U S A. 1983 Jan;80(2):407–411. doi: 10.1073/pnas.80.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspart L., Got A., Delseny M., Mocquot B., Pradet A. Adaptation of ribonucleic Acid metabolism to anoxia in rice embryos. Plant Physiol. 1983 May;72(1):115–121. doi: 10.1104/pp.72.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek K., Meudt W. J., Cohen J. D. Indole-3-acetic Acid (IAA) and IAA Conjugates Applied to Bean Stem Sections: IAA Content and the Growth Response. Plant Physiol. 1983 Sep;73(1):130–134. doi: 10.1104/pp.73.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Comai L., Kosuge T. Cloning characterization of iaaM, a virulence determinant of Pseudomonas savastanoi. J Bacteriol. 1982 Jan;149(1):40–46. doi: 10.1128/jb.149.1.40-46.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Kosuge T. Involvement of plasmid deoxyribonucleic acid in indoleacetic acid synthesis in Pseudomonas savastanoi. J Bacteriol. 1980 Aug;143(2):950–957. doi: 10.1128/jb.143.2.950-957.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Kosuge T. Transposable element that causes mutations in a plant pathogenic Pseudomonas sp. J Bacteriol. 1983 Jun;154(3):1162–1167. doi: 10.1128/jb.154.3.1162-1167.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson S. W., Kosuge T. Regulation of 3-indoleacetic acid production in Pseudomonas syringae pv. savastanoi. Purification and properties of tryptophan 2-monooxygenase. J Biol Chem. 1985 May 25;260(10):6281–6287. [PubMed] [Google Scholar]
- Hutzinger O., Kosuge T. Microbial synthesis and degradation of indole-3-acetic acid. 3. The isolation and characterization of indole-3-acetyl-epsilon-L-lysine. Biochemistry. 1968 Feb;7(2):601–605. doi: 10.1021/bi00842a013. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Kosuge T., Heskett M. G., Wilson E. E. Microbial synthesis and degradation of indole-3-acetic acid. I. The conversion of L-tryptophan to indole-3-acetamide by an enzyme system from Pseudomonas savastanoi. J Biol Chem. 1966 Aug 25;241(16):3738–3744. [PubMed] [Google Scholar]
- MAGIE A. R., WILSON E. E., KOSUGE T. INDOLEACETAMIDE AS AN INTERMEDIATE IN THE SYNTHESIS OF INDOLEACETIC ACID IN PSEUDOMONAS SAVASTANOI. Science. 1963 Sep 27;141(3587):1281–1282. doi: 10.1126/science.141.3587.1281. [DOI] [PubMed] [Google Scholar]
- Marlow J. L., Kosuge T. Tryptophan and indoleacetic acid transport in the olive and oleander knot organism pseudomonas savastanoi (E.F. Smith) Stevens. J Gen Microbiol. 1972 Sep;72(2):211–219. doi: 10.1099/00221287-72-2-211. [DOI] [PubMed] [Google Scholar]
- Nester E. W., Kosuge T. Plasmids specifying plant hyperplasias. Annu Rev Microbiol. 1981;35:531–565. doi: 10.1146/annurev.mi.35.100181.002531. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yamada T., Palm C. J., Brooks B., Kosuge T. Nucleotide sequences of the Pseudomonas savastanoi indoleacetic acid genes show homology with Agrobacterium tumefaciens T-DNA. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6522–6526. doi: 10.1073/pnas.82.19.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]