Abstract
The CTLA-4 receptor is a critical inhibitory regulator of T cell proliferation and effector function. However, the mechanisms through which CTLA-4 modulates the activation of T cells remain uncertain. Initial studies, using activated human T cells, have suggested that CTLA-4 crosslinking may induce apoptosis. However, more recent experiments have demonstrated that crosslinking of the CTLA-4 receptor on the surface of resting murine T cells blocks cell cycle progression without inducing apoptosis. Here we provide evidence that CTLA-4 crosslinking on the surface of activated murine CD4+ T lymphocytes leads to death of a substantial fraction of the cells whereas in resting CD4+ T cells the same stimulation conditions induce cell cycle arrest without apoptosis. Cell death induced by CTLA-4 stimulation occurs independently of Fas and therefore may involve a novel pathway. CTLA-4-mediated apoptosis may be a means of terminating the function of previously stimulated T cells. Exploitation of this mechanism also may provide a therapeutic strategy to eliminate alloreactive or autoreactive T cells.
The elicitation of immune responses to soluble protein antigens depends on the activation of CD4+ T lymphocytes, which are induced to proliferate and to express their effector functions. As a consequence of the expansion of antigen-specific T cells, cellular and/or humoral immune reactions emerge that, in most cases, lead to the elimination of cells expressing foreign antigens. Eventually, the immune response is self-limited and wanes with time after antigen exposure (1).
Studies carried out during the past several years have established that the molecules of the B7 family, B7–1 (CD80) (2, 3) and B7–2 (CD86) (4–6), play an important role in the regulation of immune responses. These proteins control immune responses through their capacity to positively or negatively influence the activation of CD4+ T cells that are being stimulated through their T cell antigen receptor (TCR) (7, 8).
B7–1 and B7–2 each bind to two receptors on T cells, termed CD28 and CTLA-4 (CD152) (9–11). The CD28 and CTLA-4 proteins share amino acid sequences and have similar overall structure, but appear to serve different functions: principally, crosslinking of the CD28 receptor enhances (12) and crosslinking of the CTLA-4 receptor inhibits (13) T cell activation. In addition, CD28 and CTLA-4 have different characteristics of ligand binding, with CTLA-4 having a higher affinity for B7 molecules than CD28 (14).
The different functions of the CD28 and CTLA-4 receptors are illustrated most clearly by the phenotype of the respective knockout mice. CD28-deficient mice are immunodeficient when studied in a series of assays (15, 16). By contrast, CTLA-4-deficient mice are characterized by a rampant lymphoproliferative disorder that leads to death by 3–4 weeks of age (17, 18). Available evidence suggests that this breakdown of lymphoid homeostasis in CTLA-4-deficient mice results from a failure in peripheral tolerance (19).
The expression of CTLA-4 is tightly controlled. Resting T cells express very low levels of CTLA-4. However, CTLA-4 expression is up-regulated strongly upon activation of the T cell. Based on its pattern of expression, it was thought originally that CTLA-4 might function exclusively in activated T cells. More recently, a new model has been proposed that suggests that CTLA-4 may be functional on resting as well as on activated T cells (19). According to this model, presentation of antigenic peptide by an antigen-presenting cell that expresses low levels of B7 fails to stimulate resting T cells, because of inhibition by CTLA-4. CTLA-4-mediated inhibition can only be overcome if the resting T cell encounters an antigen-presenting cell that expresses high levels of B7 molecules, effectively resulting in CD28 stimulation. However, once the T cell has become fully activated it expresses high levels of CTLA-4, which may be able to override signals transduced by CD28 even if the T cell encounters an antigen-presenting cell that expresses high levels of antigen and B7 molecules (20). Therefore, CTLA-4 may serve two functions: on resting T cells it may serve to attenuate signals mediated by the TCR and CD28 and thus contribute to peripheral tolerance. By contrast, on activated T cells CTLA-4 may serve to terminate T cell activation.
It is currently unresolved through which mechanisms CTLA-4 inhibits the activation of CD4+ T cells. Initial studies, using activated human T cells, suggested that CTLA-4 crosslinking may induce apoptosis. Cell death was postulated to occur through a novel CTLA-4 counter-receptor, distinct from B7–1 and B7–2 (21). By contrast, more recent studies have demonstrated that signaling through the CTLA-4 receptor can block the proliferative response of resting murine T cells to a synergistic combination of anti-CD3 and anti-CD28 mAbs by inhibiting the production of IL-2 and thus eliciting an arrest in cell cycle progression from G0/G1 (13, 22–25).
In the current study we have examined the effects of CTLA-4 crosslinking on activated murine CD4+ T cells. Our results indicate that crosslinking of CTLA-4 by a biotinylated anti-CTLA-4 mAb and avidin can induce apoptosis of previously stimulated T cells; in contrast to its effect on activated T cells, crosslinking of CTLA-4 on the surface of freshly isolated T cells leads to cell cycle arrest in G0/G1 without apoptosis. Cell death induced by CTLA-4 crosslinking occurs in a Fas-independent fashion.
MATERIALS AND METHODS
Mice.
BALB/c mice were purchased from Charles River Breeding Laboratories. C57BL/6 and C57BL/6-lpr/lpr mice, originally obtained from The Jackson Laboratory, were kindly provided by David Gray, Imperial College School of Medicine, Hammersmith Hospital, London.
Antibodies.
The following antibodies were purchased in purified form: phycoerythrin (PE)-conjugated hamster anti-mouse-CTLA-4 mAb (UC10–4F10–11; PharMingen); PE-conjugated hamster anti-TNP mAb (clone G235–2356; PharMingen); and unconjugated hamster anti-mouse-Fas (clone Jo2; PharMingen). The anti-CD28 mAb-secreting hybridoma 37.51 (26, 27) was kindly provided by Jim Allison, University of California at Berkeley. The anti-I-Ab,d,q, anti-IEd,k hybridoma M5/114 (28) and the anti-CD8.2 hybridoma ADH4 (29) were obtained from the American Type Culture Collection (Manassas, VA). The anti-CTLA-4 mAb-secreting hybridoma UC10–4F10–11 (13) was kindly provided by Jeff Bluestone, University of Chicago. Anti-CD28 and anti-CTLA-4 mAbs were affinity-purified from mouse ascites. Purified anti-CTLA-4 mAb was biotinylated with sulfo-NHS-biotin (Pierce), according to the manufacturer’s instructions.
Lymphocyte Purification and Bulk Cell Culture.
Mice were killed by CO2 narcosis followed by cervical dislocation. Single-cell suspensions were prepared from harvested spleens. Erythrocytes were depleted by incubating the cells with buffer containing 17 mM Tris/140 mM NH4Cl, pH 7.65 on ice for 5 min. The cells were washed and resuspended in complete culture medium (RPCCM). RPCCM consisted of RPMI 1640 medium (GIBCO/BRL), supplemented with 10% FCS/4 mM l-glutamine (GIBCO/BRL)/10 mM Hepes (GIBCO/BRL)/nonessential amino acids (Sigma)/100 units/ml penicillin G (GIBCO/BRL)/100 μg/ml streptomycin sulfate (GIBCO/BRL)/250 ng/ml amphotericin B (GIBCO/BRL)/50 μM 2-mercaptoethanol (Sigma) (30). In most experiments, splenocytes were stimulated with 2.5 μg/ml Con A (Sigma) at a density of 106 cells per ml. After 3 days the cells were harvested and CD4+ cells were purified as previously described by negative depletion (30). Briefly, the splenocytes were incubated with hybridoma supernatants of M5/114 (anti-mouse Iab,d,q, IEd,k) and ADH4 (anti-mouse CD8.2) on ice for 30 min, washed and incubated with a suspension of goat anti-mouse IgG magnetic beads (PerSeptive Biosystems) according to the manufacturer’s instructions. After removal of bead-bound cells by a magnet, the procedure was repeated once with the remaining cells. This procedure typically results in >98% pure CD4+ cells as evidenced by FACS analysis (ref. 30; data not shown). CD4+ T cells were either subjected directly to cell cycle analysis or restimulated in a secondary culture. For experiments with freshly isolated CD4+ T cells, erythrocyte-depleted splenocytes were subjected immediately to negative depletion as outlined above.
Proliferation Assays.
Microcultures (200 μl) were set up in triplicate as described (30), except that RPCCM contained biotin-free RPMI 1640 medium (GIBCO/BRL) instead of regular RPMI 1640. Where indicated, culture media were supplemented with one or several of the following additives: Con A, anti-CD28 mAb 37.51, biotinylated anti-CTLA-4 mAb UC10–4F10–11, and avidin-D (Vector Laboratories). Where indicated, avidin was presaturated with biotin by incubation for 1 h at 37°C with at 10-fold molar excess of biotin (Sigma). The precise culture constituents and incubation periods are described in the respective figure legends. All cultures were pulsed with 1 μCi (37 kBq) of [3H]thymidine per well for the last 6 h of the 48-h incubation period to assay for T cell proliferation.
Restimulation of Activated CD4+ T Cells for Cell Cycle Analysis.
Spleen cells were stimulated with Con A (2.5 μg/ml) for 72 h. CD4+ T cells were purified as described above and recultured in 24-well plates at a cell density of 5 × 105 per well, in 1 ml of RPCCM medium containing biotin-free RPMI 1640 medium (GIBCO/BRL) instead of regular RPMI 1640. Where indicated, culture media were supplemented with one or several of the following additives: Con A (2.5 μg/ml), anti-CD28 mAb 37.51 (2 μg/ml), anti-Fas mAb Jo2 (5 μg/ml), biotinylated anti-CTLA-4 mAb UC10–4F10–11 (10 μg/ml), and avidin-D (Vector Laboratories) (20 μg/ml). Where indicated in the figure legends, avidin was presaturated with biotin by incubation with a 10-fold molar excess of biotin (Sigma) for 1 h at 37°C. Eighteen to 24 h after initiation of the culture, T cells were harvested for cell cycle analysis.
Flow Cytometric Analysis of Cell Surface Antigens.
To determine cell surface expression of CTLA-4, Fas, and other proteins, CD4+ T cells were analyzed by direct immunofluorescence and flow cytometry. Briefly, 106 cells were suspended in 50 μl RPMI 1640 (5% FCS/Hepes/l-glutamine) containing 10 μg/ml of the respective staining antibody. After a 40-min incubation on ice, the cells were washed twice with 500 μl of the same medium and once with PBS containing 1% BSA (Sigma). Cells were resuspended in PBS and analyzed on a Coulter Epics XL-MCL flow cytometer.
Cell Cycle Analysis.
Cell cycle analysis was carried out as previously described (31). Briefly, purified CD4+ cells, prepared as described above, were harvested and washed once in 1 ml PBS. Cells were resuspended in 0.1% Nonidet P-40/0.1% sodium citrate including 50 μg/ml propidium iodide (Sigma) and analyzed within 24 h by flow cytometry using a Coulter Epics XL-MCL instrument.
RESULTS
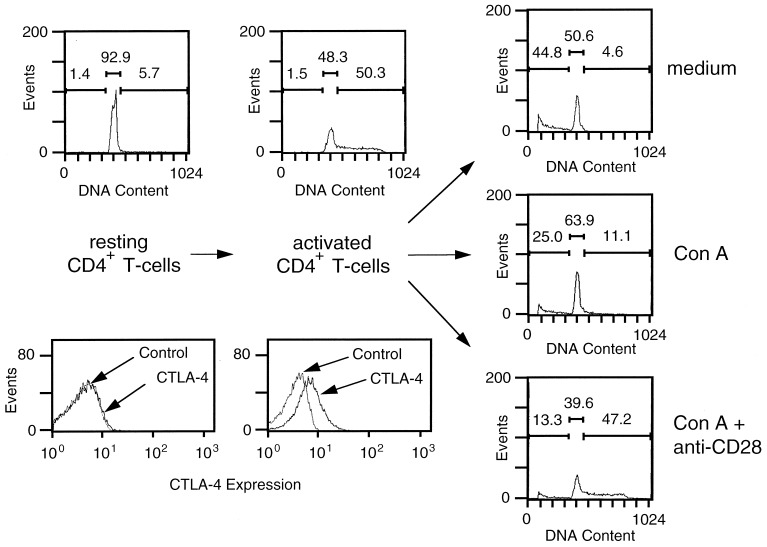
The objective of our study was to examine the functional effect of CTLA-4 crosslinking on the surface of prestimulated T lymphocytes. Our basic experimental approach is outlined in Fig. 1. Splenocytes from BALB/c or C57BL/6 mice were incubated with Con A for 3 days and CD4+ cells were subsequently purified from this population. The flow cytometry profiles in the lower graphs of Fig. 1 illustrate that, as expected, freshly isolated splenic CD4+ cells express very low amounts of CTLA-4. By contrast, after Con A stimulation in the presence of endogenous antigen-presenting cells, CTLA-4 becomes readily up-regulated (13). These preactivated CD4+ cells subsequently were restimulated under varying conditions and subjected to cell cycle analysis by staining nuclear DNA with propidium iodide and analyzing the amount of incorporated dye by flow cytometry. Typical profiles are shown in Fig. 1. As expected, nearly all resting CD4+ cells were found to be in the region corresponding to the G0/G1 phases of the cell cycle. After stimulation for 72 h in the presence of antigen-presenting cells, a significant fraction of the CD4+ cells, typically 30–50%, were in the G2/S phases of the cell cycle, indicating that they had been activated substantially. The appearance of cells in the G2/S phases depended on the simultaneous stimulation of resting CD4+ T cells by Con A and a costimulatory signal (antigen-presenting cells, anti-CD28 mAb) (data not shown; see also refs. 23 and 24).
Figure 1.
Experimental strategy used in this study. CD4+ T cells were purified from freshly prepared spleen cell suspensions, or 72 h after stimulation of spleen cells with Con A (2.5 μg/ml), as indicated in Materials and Methods. The two lower left panels are flow cytometry profiles, indicating the level of CTLA-4 surface expression vs. control staining on these two populations. In the lower left panel the two profiles essentially are superimposed. Illustrated in the two upper left panels are the cell cycle analyses for the two populations. The gated regions correspond to subdiploid quantities (left bar), G0/G1 (middle bar), and S/G2 phases (right bar), respectively. Above each bar, events are indicated as a percentage of the total number of events (5,000 = 100%). After purification, activated T cells were recultured for 24 h with either medium alone (Top Right), 2.5 μg/ml Con A (Middle Right), or Con A and 2 μg/ml anti-CD28 mAb 37.51 (Bottom Right). Illustrated are the respective cell cycle analyses for these populations.
Importantly, this requirement for a costimulatory signal was maintained when purified, activated CD4+ T cells were restimulated in a secondary culture (Fig. 1 Right). When the T cells were restimulated with Con A alone or recultured with medium alone, only a small fraction of cells remained in the G2/S region after 24 h. Moreover, a substantial fraction of the cells had an apoptotic phenotype as evidenced by a subdiploid DNA content. By contrast, restimulation of CD4+ T cells with Con A in the presence of anti-CD28 mAb kept a significant fraction of the cells in the G2/S phases, with the remaining cells mostly in the G1 phase and a small fraction (<15%) in the subdiploid region.
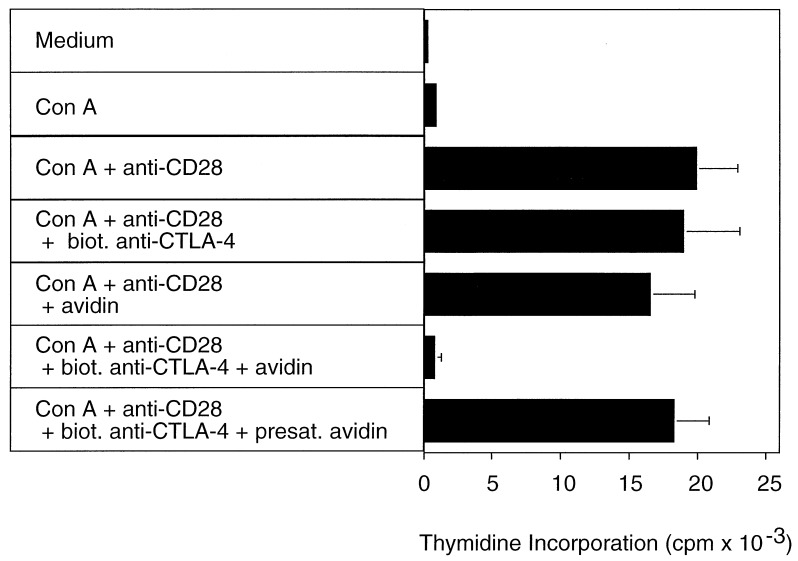
The requirement for a costimulatory signal in restimulation cultures also was observed when the incorporation of [3H]thymidine was measured as an indicator of T cell proliferation. Restimulation of purified CD4+ T cells with Con A in the absence of a costimulatory signal did not lead to cell proliferation. By contrast, significant incorporation of [3H]thymidine was observed when anti-CD28 mAb was added to the culture (Fig. 2).
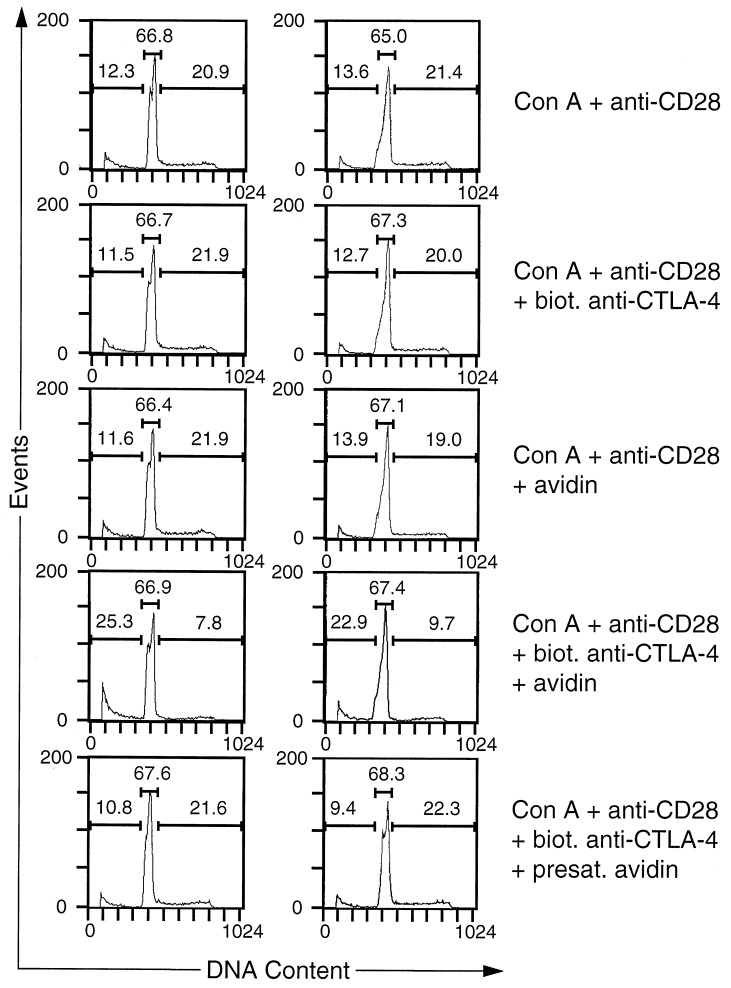
Figure 2.
CTLA-4 crosslinking inhibits proliferation of preactivated CD4+ T lymphocytes. BALB/c splenocytes were stimulated for 3 days with Con A (2.5 μg/ml) in RPCCM. After 3 days the cells were harvested and CD4+ cells were purified as described in Materials and Methods. Microcultures (200 μl) were set up with 105 purified CD4+ BALB/c T cells in biotin-free RPCCM. Where indicated, Con A (2.5 μg/ml), anti-CD28 mAb 37.51 (2 μg/ml), biotinylated anti-CTLA-4 mAb UC10–4F10–11 (10 μg/ml), and/or avidin (20 μg/ml) were added to the cultures. In one experimental group, avidin was added that had been presaturated with free biotin as detailed in Materials and Methods. To assay T cell proliferation, all cultures were pulsed with 1 μCi [3H]thymidine per well for the last 6 h of the 48-h incubation period. Indicated are the mean and SD of triplicate cultures.
CTLA-4 Triggering Induces Apoptosis in Prestimulated CD4+ T Lymphocytes.
We subsequently examined the effects of CTLA-4 stimulation on the surface of preactivated CD4+ T cells. To achieve crosslinking of the CTLA-4 receptor, we incubated preactivated CD4+ cells first with biotinylated anti-CTLA-4 mAb and subsequently with avidin. We chose this experimental approach because it has been used in other experimental systems to induce effective crosslinking of target molecules (e.g., ref. 32).
Crosslinking of CTLA-4 on the surface of preactivated CD4+ T cells abrogated the proliferation normally induced in secondary cultures by a combination of Con A and anti-CD28 mAb (Fig. 2). The inhibitory effect of CTLA-4 crosslinking was specific, because the addition of either biotinylated anti-CTLA-4 mAb or avidin alone did not inhibit T cell proliferation (Fig. 2). T cell proliferation also was not inhibited when avidin was presaturated with an excess amount (10-fold molar excess over avidin) of free biotin before addition to the culture (Fig. 2).
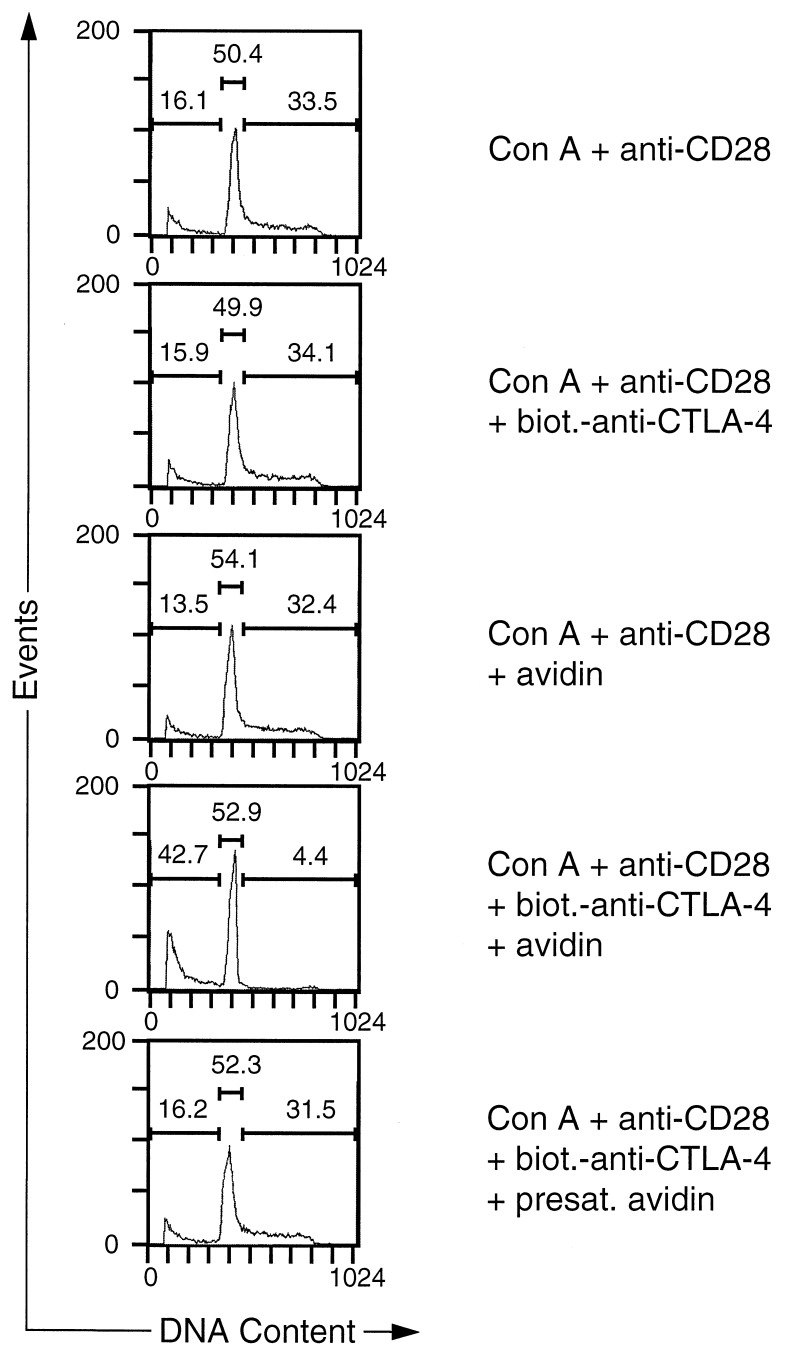
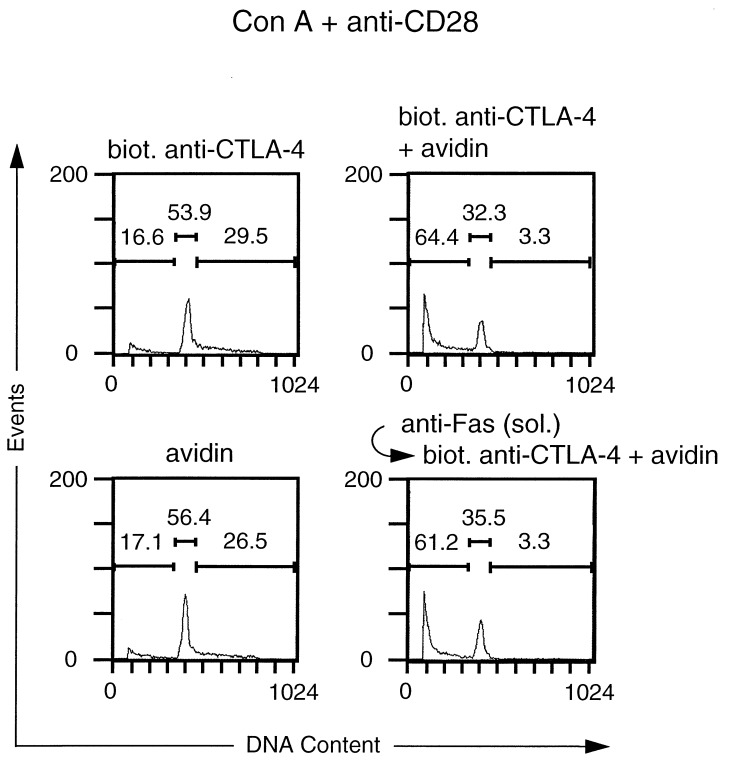
Next we examined whether CTLA-4 crosslinking had an effect on the propidium iodide staining profiles of purified activated CD4+ T cells that were restimulated with Con A and anti-CD28 mAb. Consistent with its observed effect on T cell proliferation, crosslinking of CTLA-4 lead to a dramatic reduction of cells in S/G2 (Fig. 3). Moreover, a substantial increase in the subdiploid population was observed, indicating that cells had died as a consequence of CTLA-4 engagement (Fig. 3). As in the case of the proliferative responses, the effect of CTLA-4 crosslinking on the cell cycle distribution pattern was specific, because the addition of either biotinylated anti-CTLA-4 mAb or avidin alone did not inhibit the CTLA-4-induced increase in the number of T cells with subdiploid DNA content. Increased cell death also was not observed when avidin was presaturated with an excess amount of free biotin before addition to the culture (Fig. 3) or when the T cells were incubated with an irrelevant biotinylated antibody and avidin (data not shown). The increased percentage of cells with subdiploid DNA content in cultures receiving CTLA-4 crosslinking was a result of an increase in the number of apoptotic cells because, at time of analysis (18 and 24 h), the total number of cells in control T cell cultures was only marginally different from cultures with CTLA-4 crosslinking. In 17 independent experiments, the differences in cell numbers were within a range of 25%, with only 3 experiments exceeding 15% difference. This result rules out a trivial explanation for our data, namely that all cultures contained equal numbers of apoptotic cells but such apoptotic T cells were diluted out in control groups through expansion of the remaining T cell population. It is also noteworthy that the CTLA-4-induced increase in the number of apoptotic cells cannot be the result of mere cell cycle arrest, because aphidocolin, a chemical known to induce G1 arrest, did not significantly enhance the number of apoptotic cells at concentrations that effectively inhibited cell cycle progression (data not shown). Although there was quantitative variation between different experiments, CTLA-4-induced cell death of preactivated CD4+ T cells was observed consistently: in eight independent experiments, CTLA-4 crosslinking led to a 3.5 ± 1.4 (mean ± SD) fold increase in the fraction of cells with subdiploid DNA content. These results were independently confirmed by terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL) assay (data not shown).
Figure 3.
Death of preactivated CD4+ T cells induced by CTLA-4 crosslinking. BALB/c splenocytes were stimulated for 3 days with Con A (2.5 μg/ml) in RPCCM. After 3 days the cells were harvested and CD4+ cells were purified as described in Materials and Methods. Purified CD4+ cell blasts were recultured for 24 h in biotin-free RPCCM medium in 24-well plates (1 ml) at a density of 5 × 105 per well. Where indicated, Con A (2.5 μg/ml), anti CD28 mAb 37.51 (2 μg/ml), biotinylated anti-CTLA-4 mAb UC10–4F10–11 (10 μg/ml), and/or avidin (20 μg/ml) were added to the cultures. In one experimental group, avidin was added that had been presaturated with free biotin as detailed in Materials and Methods. At the end of the incubation period the cells were harvested and washed once in PBS. Cells were resuspended in 0.1% Nonidet P-40, 0.1% sodium citrate, and 50 μg/ml propidium iodide and analyzed by flow cytometry. Events were analyzed for propidium iodide fluorescence and markers were set corresponding to subdiploid, G0/G1, and S/G2 quantities. Above each of the marked regions, events are indicated as a percentage of the total number of events (10,000 = 100%).
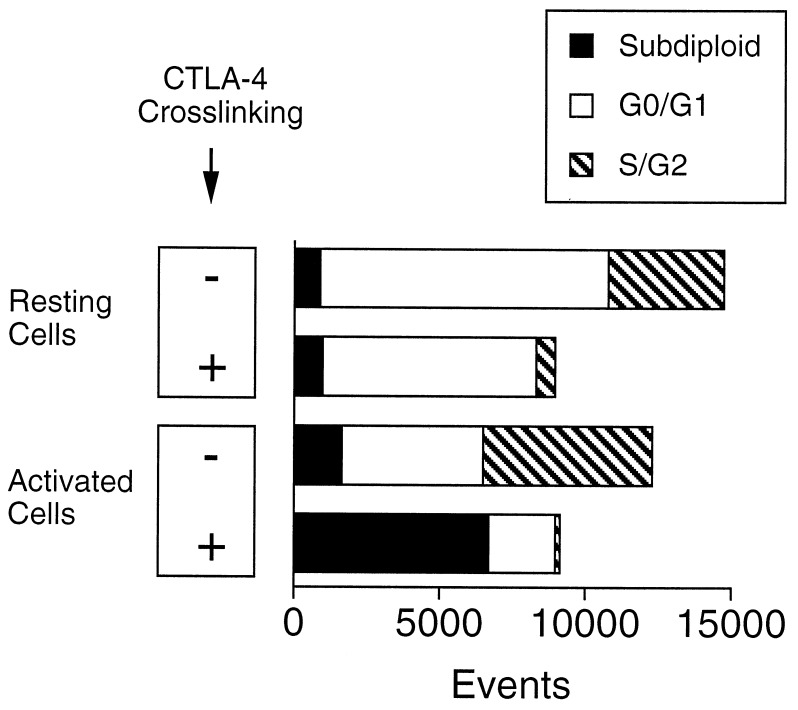
We subsequently compared directly the effects of CTLA-4 crosslinking on preactivated versus resting T cells. To carry out this comparison we had to modify the experimental approach described above, because crosslinking of CTLA-4 in freshly isolated T cells completely blocks proliferation, resulting in much diminished cell numbers at the end of the incubation period. To circumvent this problem we adopted the experimental approach developed by Krummel and Allison (23) and analyzed the cells for a fixed time period at a constant flow rate. The result of a typical experiment is shown in Fig. 4. Whereas crosslinking of CTLA-4 on the surface of preactivated T cells led to apoptosis, as shown above, crosslinking on the surface of freshly isolated CD4+ T cells inhibited cell cycle progression from G0/G1 without cell death (Fig. 4). This latter result is consistent with the observations of the Allison and Bluestone laboratories (13, 22–24).
Figure 4.
CTLA-4 crosslinking on freshly isolated CD4+ T cells inhibits cell cycle progression without apoptosis induction. Resting CD4+ cells and CD4+ blasts were prepared as described in Materials and Methods. Purified resting CD4+ cells were cultured for 72 h and preactivated CD4+ cells were recultured for 24 h in biotin-free RPCCM medium containing Con A (2.5 μg/ml), anti-CD28 mAb 37.51 (2 μg/ml). Where indicated (+), biotinylated anti-CTLA-4 mAb UC10–4F10–11 (10 μg/ml) and avidin (20 μg/ml) were added to the cultures. At the end of the incubation period the cells were harvested and washed once in PBS. Cells were resuspended in 0.1% Nonidet P-40, 0.1% sodium citrate, and 50 μg/ml propidium iodide and analyzed by flow cytometry. Events were analyzed for propidium iodide fluorescence and markers were set corresponding to subdiploid, G0/G1, and S/G2 quantities. All cells were analyzed for a fixed time period at a constant flow rate to account for differences in cell number at the end of the stimulation period (23).
T Cell Death Induced by CTLA-4 Crosslinking Is Not Blocked by Anti-Fas Antibody.
Because survival/death decisions are mediated by regulation of the Fas pathway in many systems, it was of interest to determine whether CTLA-4-induced cell death occurred in a Fas-dependent fashion. We initially examined whether CTLA-4-induced death of activated CD4+ T cells could be blocked by an anti-Fas mAb. For this purpose, we employed the anti-Fas mAb Jo2. This anti-Fas mAb can kill T cells when immobilized (33), but does not significantly affect cell viability when added in soluble form (34); moreover, in soluble form it can disrupt the Fas–Fas ligand interaction (35). As shown in Fig. 5, the CTLA-4-induced death of activated CD4+ T cells was not inhibited by the addition of saturating amounts of soluble anti-Fas mAb to the culture. This result suggested that CTLA-4-induced apoptosis was not mediated through the Fas molecule.
Figure 5.
CTLA-4-induced death of preactivated CD4+ T cells is not inhibited by anti-Fas antibody. Purified CD4+ cell blasts, prepared as outlined in the legend to Fig. 3, were recultured for 24 h in biotin-free RPCCM medium containing Con A (2.5 μg/ml) and anti CD28 mAb 37.51 (2 μg/ml), in the presence of biotinylated anti-CTLA-4 mAb UC10–4F10–11 (10 μg/ml) and/or avidin (20 μg/ml). In the experimental group depicted in the lower right graph, T cells were preincubated with anti-Fas mAb Jo2 (5 μg/ml). At the end of the incubation period the cells were harvested and washed once in PBS. Cells were resuspended in 0.1% Nonidet P-40, 0.1% sodium citrate, and 50 μg/ml propidium iodide and analyzed by flow cytometry for propidium iodide fluorescence. Soluble Jo2 mAb was functional, because it was able to inhibit apoptosis of activated CD4+ T cells induced by immobilized Jo2 mAb. In a representative control experiment, numbers of apoptotic cells were as follows: no antibody, 17.1%; soluble Jo2 mAb (5 μg/ml), 25.4%; immunobilized Jo2 mAb (1 μg/ml), 50.2%; soluble and immobilized Jo2 mAb, 21.6%.
Activated T Cells from lpr Mice Can Be Killed by CTLA-4 Crosslinking.
To further investigate this point, we examined whether CTLA-4 crosslinking could kill activated T cells that were genetically deficient in Fas expression. For this purpose, we generated activated CD4+ T cells from young C57BL/6-lpr/lpr mice as outlined above for wild-type T cells. Preactivated CD4+ cells from both mice expressed comparable amounts of CTLA-4 as assessed by flow cytometry (data not shown). As shown in Fig. 6 Left, crosslinking of CTLA-4 on the surface of activated lpr T cells led to cell death, at a frequency not lower than that observed for wild-type T cells that were analyzed in parallel (Fig. 6 Right). This result formally demonstrates that CTLA-4 crosslinking can kill activated CD4+ T cells in a Fas-independent fashion.
Figure 6.
Preactivated CD4+ T cells from Fas mutant mice die upon CTLA-4-crosslinking. Splenocytes from either C57BL/6-lpr/lpr (Left) or C57BL/6 wild-type mice (Right) were activated with Con A (2.5 μg/ml) in RPCCM. After stimulation, the cells were harvested and CD4+ cells were purified as described in Materials and Methods. Purified CD4+ cell blasts were recultured for 24 h in biotin-free RPCCM medium. Where indicated, Con A (2.5 μg/ml), anti CD28 mAb 37.51 (2 μg/ml), biotinylated anti-CTLA-4 mAb UC10–4F10–11 (10 μg/ml), and/or avidin (20 μg/ml) were added to the cultures. In one experimental group, avidin was added that had been presaturated with free biotin as detailed in Materials and Methods. At the end of the incubation period the cells were harvested and washed once in PBS. Cells were resuspended in 0.1% Nonidet P-40, 0.1% sodium citrate, and 50 μg/ml propidium iodide and analyzed as outlined in the legend to Fig. 3. Above each of the marked regions, events are indicated as a percentage of the total number of events.
DISCUSSION
Crosslinking of the CTLA-4 receptor by mAb on the surface of resting (23, 24) and activated (this report) murine T cells can inhibit T cell proliferation. What remains uncertain is how CTLA-4 mediates this function. Results from studies carried out by the Allison and Bluestone laboratories have established that CTLA-4 crosslinking on the surface of freshly isolated T cells leads to an arrest in cell cycle progression from G0/G1 without evidence of T cell death (23, 24). In this study, we provide evidence that CTLA-4 crosslinking on the surface of prestimulated murine T cells leads to the death of a substantial fraction of the T cells. By contrast, CTLA-4 crosslinking on the surface of freshly isolated T cells leads to cell cycle arrest. Therefore, the cellular responses to CTLA-4 crosslinking may be more complex than previously appreciated.
There are several possibilities to explain the differential effects of CTLA-4 crosslinking on freshly isolated versus activated T cells. (i) It is conceivable that CTLA-4 induces different biochemical signals in resting versus activated cells. Such differential receptor signaling certainly is not without precedence: It is well known that the signaling capacity of some receptors is influenced by the activation states of the cell in which it is expressed. Typically, such changes in receptor signaling are due to either modifications of the receptor itself (e.g., receptor phosphorylation) or to alterations of the cytosolic milieu in which the receptor operates. It is likely that the cytoplasmic domain of CTLA-4 is altered (e.g., phosphorylated) upon T cell stimulation, although TCR- or CD28-induced alterations have yet to be documented (20). Moreover, there are dramatic changes in the amounts and composition of cytosolic signaling modules that occur upon T cell stimulation (36) and that could account for differential signaling effects of the CTLA-4 receptor. (ii) It is possible that CTLA-4 crosslinking elicits the same biochemical signals in resting and activated T cells. According to this model, the principal effect of CTLA-4 stimulation would be to block interleukin 2 (IL-2) production; however, this block would have different consequences depending on the state of the T cell. In the case of a resting T cell, CTLA-4 stimulation would lead to arrest in cell cycle progression because progression depends on growth factor production. In the case of an activated T cell, cell death may be a consequence of depriving an activated, IL-2-dependent T cell of its growth factor. (iii) Finally, the thresholds for arresting versus apoptotic signals might be different. Specifically, it is conceivable that the induction of apoptosis might require levels of CTLA-4 that are present only on the surface of activated T cells. Currently, we cannot distinguish between these possibilities.
The results of experiments with anti-Fas antibodies as well as those using T cells from lpr mice indicate that CTLA-4-induced cell death proceeds in a Fas-independent fashion. This finding is not without precedence because receptors other than Fas have been described recently that can mediate killing of activated T cells (37–39).
Finally, studies of costimulatory pathways have enhanced our knowledge of immunologic diseases and opened up new possibilities for prophylactic and therapeutic intervention (8). In this context it is noteworthy that Colucci et al. (40) have mapped a gene responsible for apoptosis resistance of lymphocytes from nonobese diabetic mice to within 20 cM of the Ctla-4 locus; these investigators also found that CTLA-4 expression was defective in lymphocytes from these mice. In addition, the results of our study raise the possibility that crosslinking of CTLA-4 in vivo, e.g., by infusion of a biotinylated anti-CTLA-4 mAb followed by avidin, may be a possible strategy for the treatment of autoimmune diseases that are otherwise refractory to therapy. Moreover, that CTLA-4 crosslinking can induce death of activated T cells from lpr mice raises the intriguing possibility that such a regimen might be used to treat children suffering from a lymphoproliferative syndrome resulting from Fas deficiency (41, 42). These possibilities will need to be explored.
Acknowledgments
We thank Drs. Jim Allison and Jeff Bluestone for providing 37.51 and UC10–4F10–11 hybridoma cells, respectively. We thank Dr. David Gray for providing lpr mice and Drs. David Gray and Mary Ritter for reviewing this manuscript. This work was supported by the Project Grant G9628678 from the Medical Research Council (U.K.).
ABBREVIATION
- TCR
T cell antigen receptor
References
- 1.Abbas A K, Murphy K M, Sher A. Nature (London) 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Freedman A S, Freeman G, Horowitz J C, Daley J, Nadler L M. J Immunol. 1987;139:3260–3267. [PubMed] [Google Scholar]
- 3.Freeman G J, Gray G S, Gimmi C D, Lombard D B, Zhou L J, White M, Fingeroth J D, Gribben J G, Nadler L M. J Exp Med. 1991;174:625–631. doi: 10.1084/jem.174.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman G J, Gribben J G, Boussiotis V A, Ng J W, Restivo V A, Jr, Lombard D A, Gray G S, Nadler L M. Science. 1993;262:909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 5.Freeman G J, Borriello F, Hodes R J, Reiser H, Gribben J G, Ng J W, Kim J, Goldberg J M, Hathcock K, Laszlo G, et al. J Exp Med. 1993;178:2185–2192. doi: 10.1084/jem.178.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azuma M, Ito D, Yagita H, Okumura K, Phillips J H, Lanier L L, Somoza C. Nature (London) 1993;366:76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 7.Lenschow D J, Walunas T L, Bluestone J A. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 8.Reiser H, Stadecker M J. N Engl J Med. 1996;335:1369–1377. doi: 10.1056/NEJM199610313351807. [DOI] [PubMed] [Google Scholar]
- 9.Linsley P S, Clark E A, Ledbetter J A. Proc Natl Acad Sci USA. 1990;87:5031–5035. [Google Scholar]
- 10.Linsley P S, Brady W, Grosmaire L, Aruffo A, Damle N K, Ledbetter J A. J Exp Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linsley P S, Brady W, Urnes M, Grosmaire L S, Damle N K, Ledbetter J A. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara T, Fu S M, Hansen J A. J Exp Med. 1985;161:1513–1524. doi: 10.1084/jem.161.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walunas T L, Lenschow D J, Bakker C Y, Linsley P S, Freeman G J, Green J M, Thompson C B, Bluestone J A. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 14.Linsley P S, Greene J, Brady W, Bajorath J, Ledbetter J A, Peach R. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 15.Shahinian A, Pfeffer K, Lee K P, Kundig T M, Kishihara K, Wakeham A, Kawai K, Ohashi P S, Thompson C B, Mak T W. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 16.Kündig T M, Shahinian A, Kawai K, Mittrücker H W, Sebzda E, Bachmann F, Mak T W, Ohashi P S. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 17.Waterhouse P, Penninger J M, Timms E, Wakeham A, Shahinian A, Lee K P, Thompson C B, Griesser H, Mak T W. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 18.Tivol E A, Boriello F, Schweitzer A N, Lynch W P, Bluestone J A, Sharpe A H. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 19.Chambers C A, Cado D, Truong T, Allison J P. Proc Natl Acad Sci USA. 1997;94:9296–9301. doi: 10.1073/pnas.94.17.9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson C B, Allison J P. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 21.Gribben J G, Freeman G J, Boussiotis V A, Rennert P, Jellis C L, Greenfield E, Barber M, Restivo V A, Ke X, Gray G S, Nadler L M. Proc Natl Acad Sci USA. 1995;92:811–815. doi: 10.1073/pnas.92.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krummel M F, Allison J P. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krummel M F, Allison J P. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walunas T L, Bakker C Y, Bluestone J A. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blair P J, Riley J L, Levine B L, Lee K P, Craighead N, Francomano T, Perfetto S J, Gray G S, Carreno B M, June C H. J Immunol. 1998;160:12–15. [PubMed] [Google Scholar]
- 26.Gross J A, Callas E A, Allison J P. J Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
- 27.Harding F A, McArthur J G, Gross J A, Raulet D H, Allison J P. Nature (London) 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 28.Bhattacharya A, Dorf M E, Springer T A. J Immunol. 1981;127:2488–2495. [PubMed] [Google Scholar]
- 29.Gottlieb P D, Marshak-Rothstein A, Auditore-Hargreaves K, Berkoben D B, August D A, Rosche R M, Benedetto J D. Immunogenetics. 1980;10:545–555. doi: 10.1007/BF01572589. [DOI] [PubMed] [Google Scholar]
- 30.Natesan M, Razi-Wolf Z, Reiser H. J Immunol. 1996;156:2783–2791. [PubMed] [Google Scholar]
- 31.Strobel T, Swanson L, Korsmeyer S, Cannistra S A. Proc Natl Acad Sci USA. 1996;93:14094–14099. doi: 10.1073/pnas.93.24.14094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loh R K, Jabara H H, Ren C L, Fu S M, Vercelli D, Geha R S. Immunol Lett. 1995;45:99–106. doi: 10.1016/0165-2478(94)00233-h. [DOI] [PubMed] [Google Scholar]
- 33.Hiromatsu K, Aoki Y, Makino M, Matsumoto Y, Mizuochi T, Gotoh Y, Nomoto K, Ogasawara J, Nagata S, Yoshikai Y. Eur J Immunol. 1994;24:2446–2451. doi: 10.1002/eji.1830241028. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe D, Suda T, Nagata S. Int Immunol. 1995;7:1949–1956. doi: 10.1093/intimm/7.12.1949. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Mercep M, Ware C F, Ashwell J D. J Exp Med. 1995;181:1673–1682. doi: 10.1084/jem.181.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alberola-Ila J, Takaki S, Kerner J D, Perlmutter R M. Annu Rev Immunol. 1997;15:125–154. doi: 10.1146/annurev.immunol.15.1.125. [DOI] [PubMed] [Google Scholar]
- 37.Mollereau B, Deckert M, Déas O, Rieux-Laucart F, Hirsch F, Bernard A, Fischer A, Lynch D H, Charpentier B, Le Deist F, Senik A. J Immunol. 1996;156:3184–3190. [PubMed] [Google Scholar]
- 38.Screaton G R, Xu X N, Olsen A L, Cowper A E, Tan R, McMichael A J, Bell J I. Proc Natl Acad Sci USA. 1997;94:4615–4619. doi: 10.1073/pnas.94.9.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodle E S, Smith D M, Bluestone J A, Kirkman W M, III, Green D R, Skowronski E W. J Immunol. 1997;158:2156–2164. [PubMed] [Google Scholar]
- 40.Colucci F, Bergman M-L, Penha-Gonçalves C, Cilio C M, Holmberg D. Proc Natl Acad Sci USA. 1997;94:8670–8674. doi: 10.1073/pnas.94.16.8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rieux-Laucat F, Le-Deist F, Hivroz C, Roberts I A, Debatin K M, Fischer A, de Villartay J P. Science. 1996;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 42.Fisher G H, Rosenberg F J, Straus S E, Dale J K, Middleton L A, Lin A Y, Strober W, Lenardo M J, Puck J M. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]