Abstract
The genes encoding the thermostable alpha-amylases of Bacillus stearothermophilus and B. licheniformis were cloned in Escherichia coli, and their DNA sequences were determined. The coding and deduced polypeptide sequences are 59 and 62% homologous to each other, respectively. The B. stearothermophilus protein differs most significantly from that of B. licheniformis in that it possesses a 32-residue COOH-terminal tail. Transformation of E. coli with vectors containing either gene resulted in the synthesis and secretion of active enzymes similar to those produced by the parental organisms. A plasmid was constructed in which the promoter and the NH2-terminal two-thirds of the B. stearothermophilus coding sequence was fused out of frame to the entire mature coding sequence of the B. licheniformis gene. Approximately 1 in 5,000 colonies transformed with this plasmid was found to secrete an active amylase. Hybridization analysis of plasmids isolated from these amylase-positive colonies indicated that the parental coding sequences had recombined by homologous recombination. DNA sequence analysis of selected hybrid genes revealed symmetrical, nonrandom distribution of loci at which the crossovers had resolved. Several purified hybrid alpha-amylases were characterized and found to differ with respect to thermostability and specific activity.
Full text
PDF
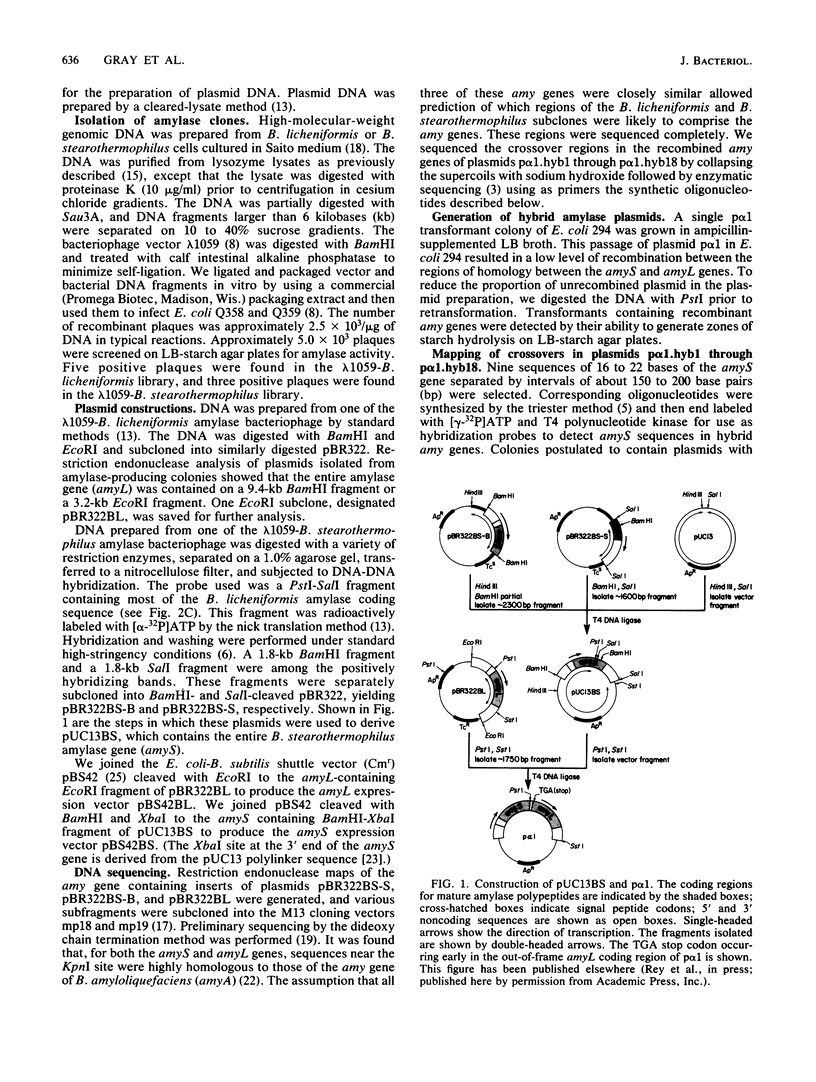

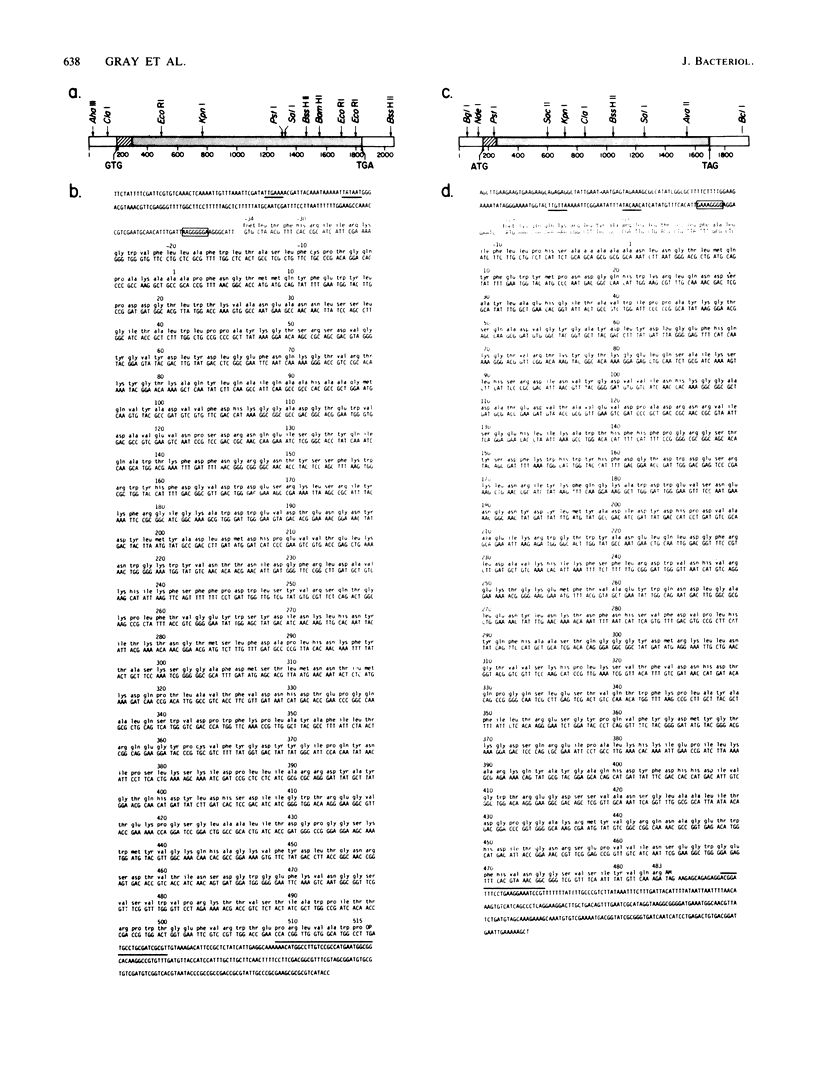

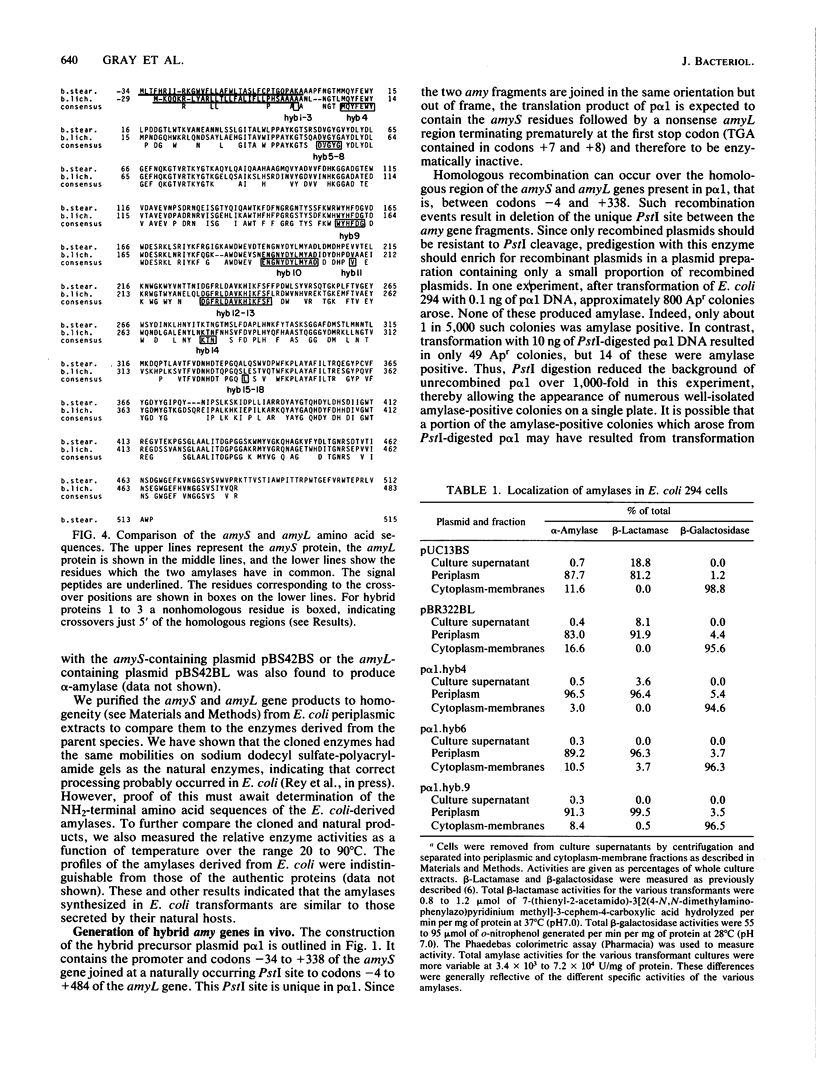
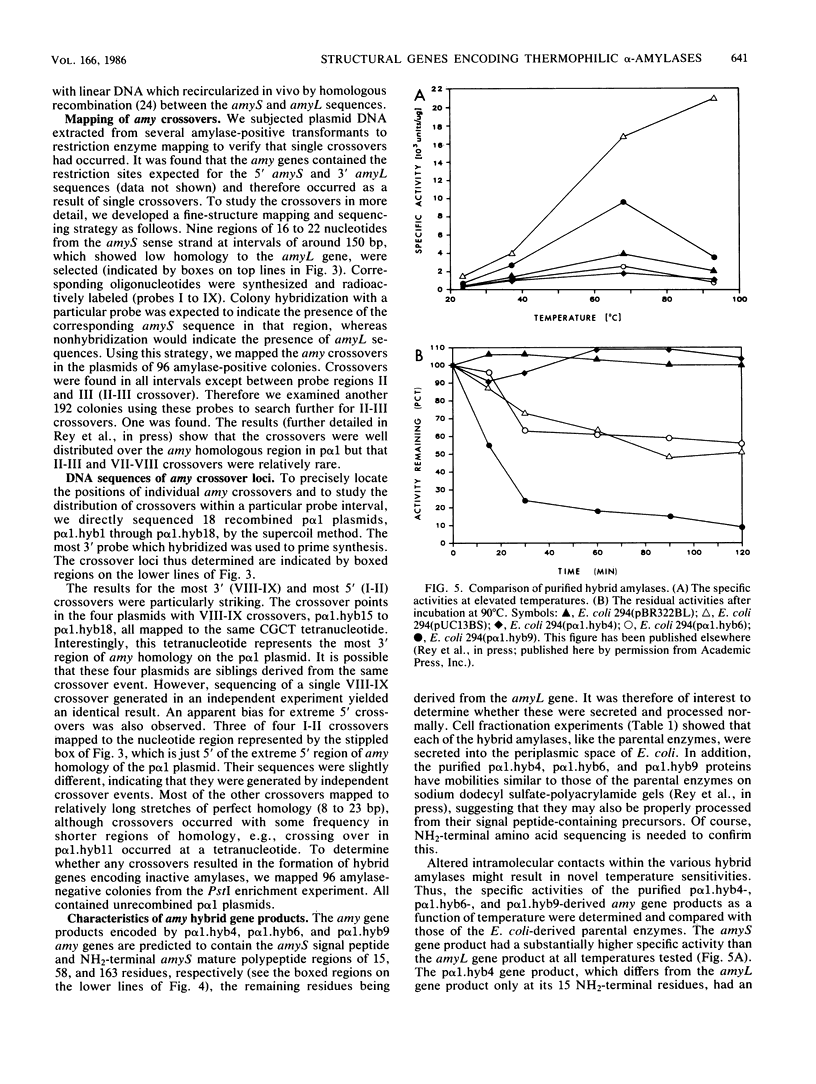


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Cornelis P., Digneffe C., Willemot K. Cloning and expression of a Bacillus coagulans amylase gene in Escherichia coli. Mol Gen Genet. 1982;186(4):507–511. doi: 10.1007/BF00337957. [DOI] [PubMed] [Google Scholar]
- Crea R., Kraszewski A., Hirose T., Itakura K. Chemical synthesis of genes for human insulin. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5765–5769. doi: 10.1073/pnas.75.12.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle M. B., Erickson R. J. Bacterial alpha-amylases. Adv Appl Microbiol. 1978;24:257–278. [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D., Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980 Jul;20(3):749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- Kuhn H., Fietzek P. P., Lampen J. O. N-terminal amino acid sequence of Bacillus licheniformis alpha-amylase: comparison with Bacillus amyloliquefaciens and Bacillus subtilis Enzymes. J Bacteriol. 1982 Jan;149(1):372–373. doi: 10.1128/jb.149.1.372-373.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meers J. L., Pedersen L. K. Nitrogen assimilation by bacillus licheniformis organisms growning in chemostat cultures. J Gen Microbiol. 1972 Apr;70(2):277–286. doi: 10.1099/00221287-70-2-277. [DOI] [PubMed] [Google Scholar]
- Mielenz J. R. Bacillus stearothermophilus contains a plasmid-borne gene for alpha-amylase. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5975–5979. doi: 10.1073/pnas.80.19.5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R., Imanaka T., Aiba S. Nucleotide sequence of the Bacillus stearothermophilus alpha-amylase gene. J Bacteriol. 1985 Jul;163(1):401–406. doi: 10.1128/jb.163.1.401-406.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Saito N. A thermophilic extracellular -amylase from Bacillus licheniformis. Arch Biochem Biophys. 1973 Apr;155(2):290–298. doi: 10.1016/0003-9861(73)90117-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M. L., Ferrari E. Replacement of the Bacillus subtilis subtilisin structural gene with an In vitro-derived deletion mutation. J Bacteriol. 1984 May;158(2):411–418. doi: 10.1128/jb.158.2.411-418.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M. A., Ortlepp S. A., Ollington J. F., McConnell D. J. Nucleotide sequence of the 5' region of the Bacillus licheniformis alpha-amylase gene: comparison with the B. amyloliquefaciens gene. J Bacteriol. 1984 Apr;158(1):369–372. doi: 10.1128/jb.158.1.369-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takkinen K., Pettersson R. F., Kalkkinen N., Palva I., Söderlund H., Käriäinen L. Amino acid sequence of alpha-amylase from Bacillus amyloliquefaciens deduced from the nucleotide sequence of the cloned gene. J Biol Chem. 1983 Jan 25;258(2):1007–1013. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weber H., Weissmann C. Formation of genes coding for hybrid proteins by recombination between related, cloned genes in E. coli. Nucleic Acids Res. 1983 Aug 25;11(16):5661–5669. doi: 10.1093/nar/11.16.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. A., Ferrari E., Henner D. J., Estell D. A., Chen E. Y. Cloning, sequencing, and secretion of Bacillus amyloliquefaciens subtilisin in Bacillus subtilis. Nucleic Acids Res. 1983 Nov 25;11(22):7911–7925. doi: 10.1093/nar/11.22.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Galizzi A., Henner D. Nucleotide sequence of the amylase gene from Bacillus subtilis. Nucleic Acids Res. 1983 Jan 25;11(2):237–249. doi: 10.1093/nar/11.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]


