Abstract
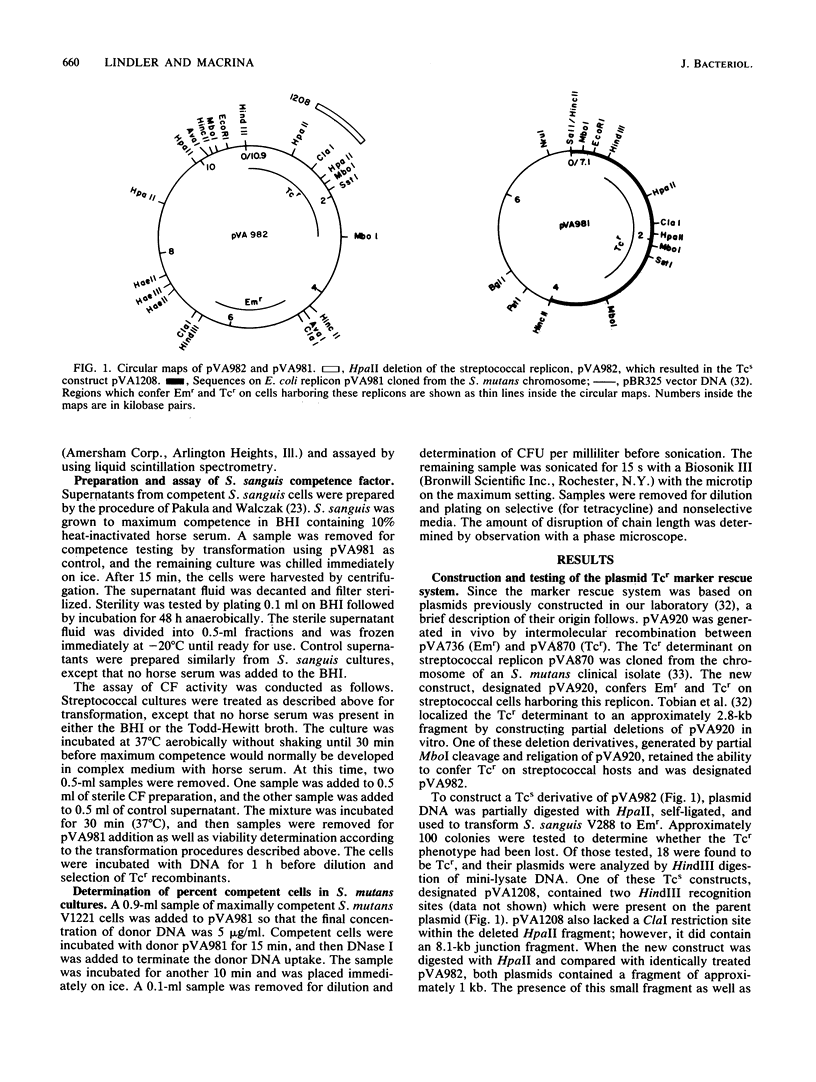
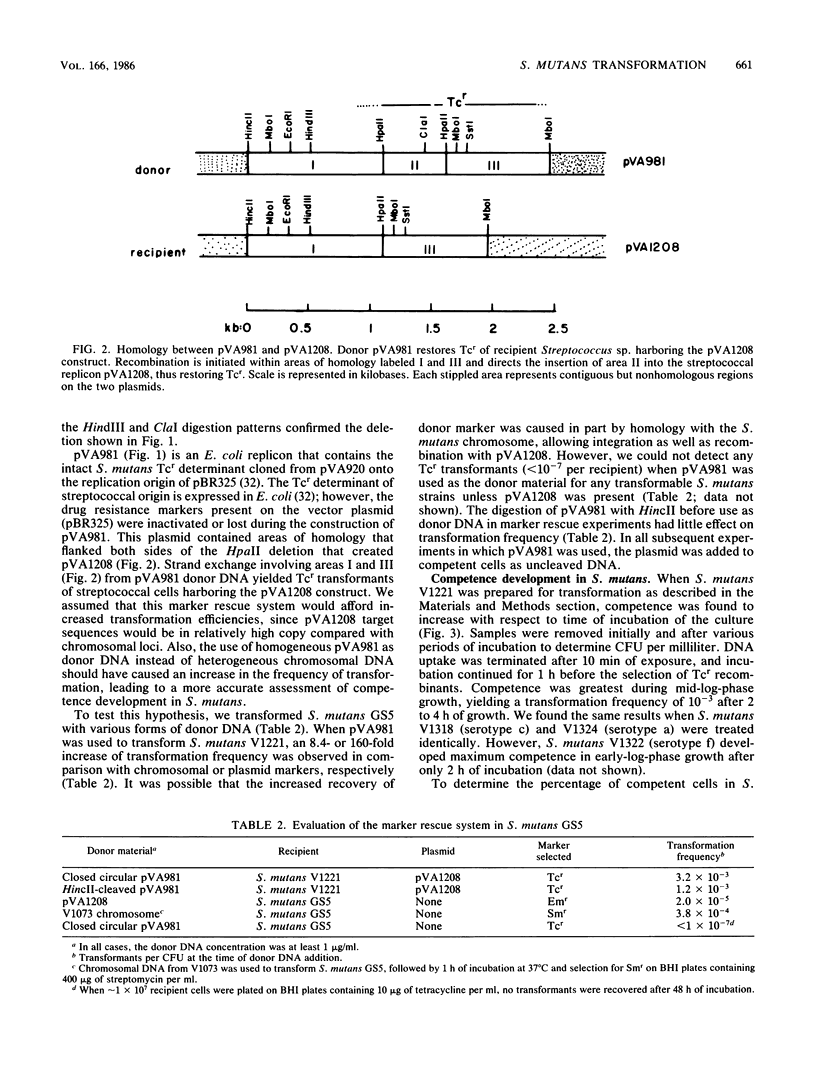
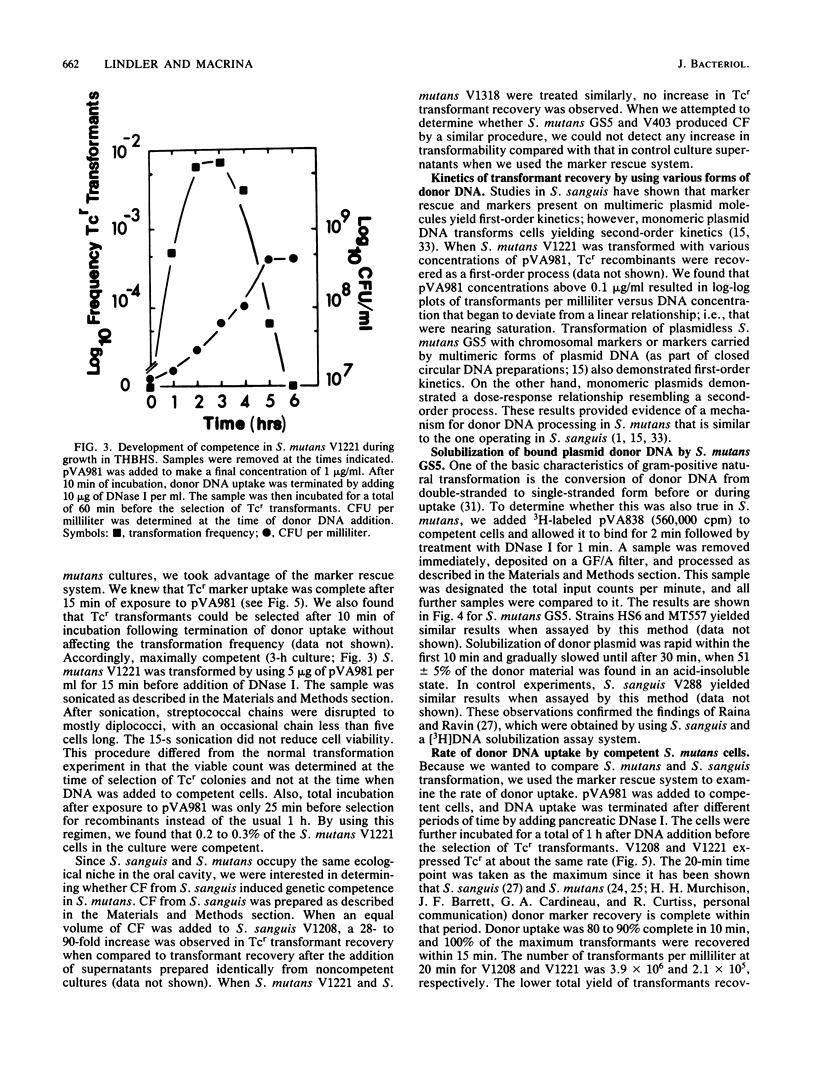
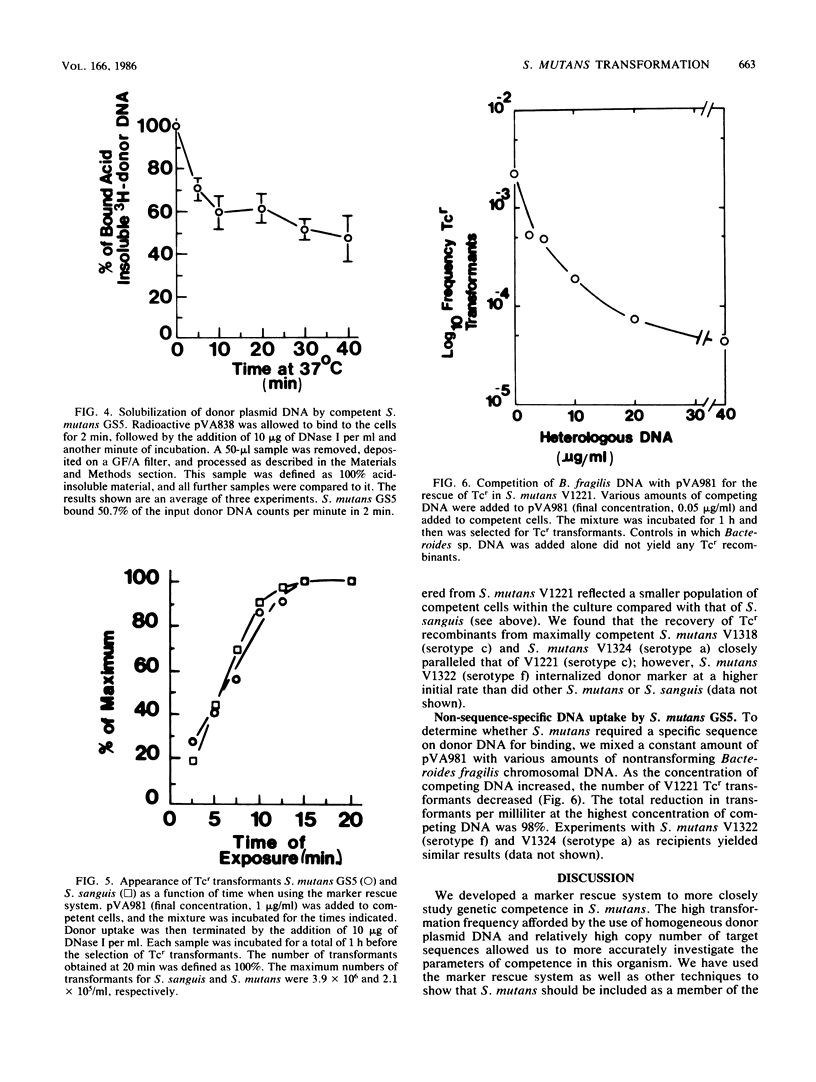
We developed a marker rescue system for study of competence development and genetic transformation in Streptococcus mutans. The system involved the recombinational rescue of a tetracycline resistance (Tcr) determinant by a homologous, inactive locus (Tcs because of a small deletion). Streptococcal cells harboring this in vitro-prepared Tcs construct (pVA1208) were restored to Tcr when plasmid (pVA981) DNA was used as donor material. pVA981 contained the intact streptococcal Tcr locus and was unable to autonomously replicate in streptococci. Marker rescue with this system followed first-order kinetics and occurred at a frequency 8- or 160-fold higher than did transformation with homologous chromosomal or plasmid DNA, respectively. By using the rescue system, we were able to confirm that competence of S. mutans appeared to be inducible. This was indicated by a sequential increase and then decrease in Tcr transformation frequencies during growth in complex medium. Also, donor DNA binding was not sequence specific, since the recovery of Tcr transformants was reduced by increasing the concentrations of heterologous DNA. We investigated the fate of donor DNA and the kinetics of plasmid establishment in the transformation of S. mutans with plasmid DNA. Monomeric plasmid molecules transformed S. mutans as a second-order process, whereas multimeric plasmid DNA and chromosomal markers were recovered as a first-order process. Approximately 50% of the initially bound donor plasmid DNA was found to remain in a trichloroacetic acid-insoluble form. Our results suggested that molecular cloning in S. mutans would be conducted most efficiently by using helper plasmid systems or shuttle vectors and that gene transfer by transformation of S. mutans occurred in a manner similar to that observed in Streptococcus sanguis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behnke D. Plasmid transformation of Streptococcus sanguis (Challis) occurs by circular and linear molecules. Mol Gen Genet. 1981;182(3):490–497. doi: 10.1007/BF00293940. [DOI] [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Gaustad P. Genetic transformation in Streptococcus sanguis. Distribution of competence and competence factors in a collection of strains. Acta Pathol Microbiol Scand B. 1979 Apr;87B(2):123–128. [PubMed] [Google Scholar]
- Gaustad P. Genetic transformation in Streptococcus sanguis. Kinetics of production in different media and specific interaction of competence factor and competence factor inactivator. Acta Pathol Microbiol Immunol Scand B. 1983 Jun;91(3):193–200. [PubMed] [Google Scholar]
- Gryczan T., Contente S., Dubnau D. Molecular cloning of heterologous chromosomal DNA by recombination between a plasmid vector and a homologous resident plasmid in Bacillus subtilis. Mol Gen Genet. 1980 Feb;177(3):459–467. doi: 10.1007/BF00271485. [DOI] [PubMed] [Google Scholar]
- Hamada S., Gill K., Slade H. D. Chemical and immunological properties of the type f polysaccharide antigen of Streptococcus mutans. Infect Immun. 1976 Jul;14(1):203–211. doi: 10.1128/iai.14.1.203-211.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral T. A., Daneo-Moore L. Glycerol incorporation in certain oral streptococci. Infect Immun. 1980 Dec;30(3):759–765. doi: 10.1128/iai.30.3.759-765.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K., Long C. M. Plasmid-mediated transformation of Streptococcus mutans. Infect Immun. 1982 Apr;36(1):435–436. doi: 10.1128/iai.36.1.435-436.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K., Trapa V. Genetic exchange between oral streptococci during mixed growth. J Gen Microbiol. 1984 Oct;130(10):2497–2500. doi: 10.1099/00221287-130-10-2497. [DOI] [PubMed] [Google Scholar]
- Lawson J. W., Gooder H. Growth and development of competence in the group H streptococci. J Bacteriol. 1970 Jun;102(3):820–825. doi: 10.1128/jb.102.3.820-825.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard C. G., Cole R. M. Purification and properties of Streptococcal competence factor isolated from chemically defined medium. J Bacteriol. 1972 Apr;110(1):273–280. doi: 10.1128/jb.110.1.273-280.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Jones K. R., Welch R. A. Transformation of Streptococcus sanguis with monomeric pVA736 plasmid deoxyribonucleic acid. J Bacteriol. 1981 May;146(2):826–830. doi: 10.1128/jb.146.2.826-830.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Jones K. R., Wood P. H. Chimeric streptococcal plasmids and their use as molecular cloning vehicles in Streptococcus sanguis (Challis). J Bacteriol. 1980 Sep;143(3):1425–1435. doi: 10.1128/jb.143.3.1425-1435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Keeler C. L., Jr, Jones K. R., Wood P. H. Molecular characterization of unique deletion mutants of the streptococcal plasmid, pAM beta 1. Plasmid. 1980 Jul;4(1):8–16. doi: 10.1016/0147-619x(80)90079-7. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Reider J. L., Virgili S. S., Kopecko D. J. Survey of the extrachromosomal gene pool of Streptococcus mutans. Infect Immun. 1977 Jul;17(1):215–226. doi: 10.1128/iai.17.1.215-226.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Scott C. L. Evidence for a disseminated plasmid in Streptococcus mutans. Infect Immun. 1978 Apr;20(1):296–302. doi: 10.1128/iai.20.1.296-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Tobian J. A., Jones K. R., Evans R. P., Clewell D. B. A cloning vector able to replicate in Escherichia coli and Streptococcus sanguis. Gene. 1982 Oct;19(3):345–353. doi: 10.1016/0378-1119(82)90025-7. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Wood P. H., Jones K. R. Simple method for demonstrating small plasmid deoxyribonucleic acid molecules in oral streptococci. Appl Environ Microbiol. 1980 May;39(5):1070–1073. doi: 10.1128/aem.39.5.1070-1073.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAKULA R., WALCZAK W. On the nature of competence of transformable streptococci. J Gen Microbiol. 1963 Apr;31:125–133. doi: 10.1099/00221287-31-1-125. [DOI] [PubMed] [Google Scholar]
- Perry D., Kuramitsu H. K. Genetic transformation of Streptococcus mutans. Infect Immun. 1981 Jun;32(3):1295–1297. doi: 10.1128/iai.32.3.1295-1297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Nilsen L. J., Kuramitsu H. K. Mapping of a cloned glucosyltransferase gene in Streptococcus mutans. Infect Immun. 1985 Oct;50(1):130–135. doi: 10.1128/iai.50.1.130-135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Wondrack L. M., Kuramitsu H. K. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983 Aug;41(2):722–727. doi: 10.1128/iai.41.2.722-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina J. L., Ravin A. W. Fate of homospecific transforming DNA bound to Streptococcus sanguis. J Bacteriol. 1978 Mar;133(3):1212–1223. doi: 10.1128/jb.133.3.1212-1223.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina J. L., Ravin A. W. Switches in macromolecular synthesis during induction of competence for transformation of Streptococcus sanguis. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6062–6066. doi: 10.1073/pnas.77.10.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C. W., Guild W. R. Pathway of plasmid transformation in Pneumococcus: open circular and linear molecules are active. J Bacteriol. 1981 May;146(2):517–526. doi: 10.1128/jb.146.2.517-526.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C. W., Guild W. R. Properties and transforming activities of two plasmids in Streptococcus pneumoniae. Mol Gen Genet. 1980;180(3):573–578. doi: 10.1007/BF00268062. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Danner D. B., Deich R. A. Genetic transformation. Annu Rev Biochem. 1981;50:41–68. doi: 10.1146/annurev.bi.50.070181.000353. [DOI] [PubMed] [Google Scholar]
- Tobian J. A., Cline M. L., Macrina F. L. Characterization and expression of a cloned tetracycline resistance determinant from the chromosome of Streptococcus mutans. J Bacteriol. 1984 Nov;160(2):556–563. doi: 10.1128/jb.160.2.556-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobian J. A., Macrina F. L. Helper plasmid cloning in Streptococcus sanguis: cloning of a tetracycline resistance determinant from the Streptococcus mutans chromosome. J Bacteriol. 1982 Oct;152(1):215–222. doi: 10.1128/jb.152.1.215-222.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Model for the mechanism controlling the expression of competent state in Pneumococcus cultures. J Bacteriol. 1966 Mar;91(3):1050–1061. doi: 10.1128/jb.91.3.1050-1061.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergren G., Emilson C. G. Transformation of streptococci to streptomycin resistance by oral streptococcal DNA. Arch Oral Biol. 1977;22(8-9):533–537. doi: 10.1016/0003-9969(77)90051-6. [DOI] [PubMed] [Google Scholar]



