Abstract
Accelerated development of the secondary immune response may be attributable in part to the rapid delivery of antigen to lymphoid follicles by circulating antibody elicited on primary immunization. Here we provide evidence indicating that the nonspecific IgM present in naive mice (natural antibody) plays a role in the acceleration of the primary response. Targeted deletion of the Ig μs polyadenylation site by use of Cre recombinase allowed the creation of mice that, although harboring a normal number of B cells expressing surface IgM, completely lacked serum IgM while retaining the other Ig isotypes. These mice retained a broadly normal B lymphocyte distribution (although containing a somewhat expanded peritoneal B1a subset) but exhibited substantial delays in mounting affinity-matured IgG responses to T cell-dependent antigens. The T cell-independent response, however, was augmented. The data indicate that the IgM present before antigen challenge (as well, possibly, as that elicited immediately after immunization) accelerates maturation of the primary response, presumably by complexing with the antigen and facilitating lymphocyte activation and/or antigen trapping.
The primary and secondary responses to antigen differ in both magnitude and kinetics. The early primary response is dominated by IgM antibody of relatively low affinity, but this response matures over days to yield higher-affinity antibody, most of which is IgG. In contrast, on secondary challenge, high-affinity IgG is produced very rapidly. The difference in kinetics of the primary and secondary responses is partly ascribable to the fact that B cells making high-affinity antibody (memory cells) will have been generated and enriched as a result of antigen priming. However, an important role also is likely played by the high-affinity serum IgG (elicited late in the primary response), which assists the trafficking and localization of antigen to sites suitable for immune-response maturation (1–4).
With regard to the primary response, a role for preimmune serum Ig (natural antibody) in assisting its development has long been discussed (5, 6). For example, the immune responsiveness of newborn piglets deprived of colostrum is deficient but can be enhanced significantly by provision of normal Ig (7, 8). A detailed understanding and confirmation of the importance of serum Ig in the development of the primary response would be assisted greatly by the generation of animals that lack natural antibody but that nevertheless are capable of mounting a humoral response. Gene targeting allows the creation of such lines.
The interaction between antigen and natural antibody is likely to be of low affinity. IgM therefore deserves particular regard. Its pentameric structure confers increased avidity on antigen interaction. Furthermore, binding of antigen to a single IgM (but not IgG) molecule can trigger complement activation (9), a factor that is likely to be of particular importance given the significance of complement recruitment in the maturation of the immune response (10–12). We therefore created mice that lack secretory IgM while retaining the membrane isoform to investigate the role of serum IgM in the development of the humoral immune response.
MATERIALS AND METHODS
Generation and Breeding of μs−/− Mice.
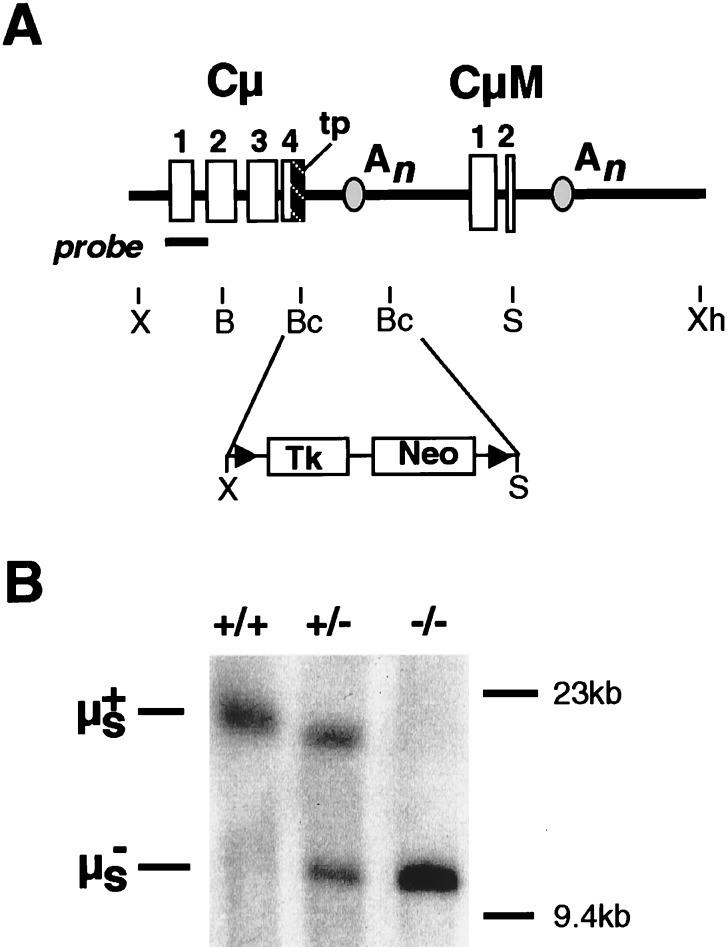
The targeting construct (Fig. 1A) was assembled from a Cμ clone that had been isolated from a 129 mouse genomic library (gift of M. Brüggemann, Babraham Institute, Cambridge, and A. Smith, Centre for Genome Research, Edinburgh, U.K.). The region between the BclI sites was replaced by a NotI linker, into which was inserted a Tk-Neo cassette (13). The BamHI/XhoI fragment of the resulting construct was transfected by electroporation into 129/Sv-derived CCB embryonic stem (ES) cells as described previously (14). Clones growing in Neo-selective medium were screened by PCR and Southern blot hybridization (XbaI digest; probe generated by PCR from the region indicated in Fig. 1B) for homologous targeting (frequency 1 in 100). A targeted clone was then transfected with a Cre-expressing plasmid (13), and, after selection in gancyclovir for Tk loss, Southern blot analysis of SpeI-digested DNA confirmed excision of the Tk-Neo cassette. Two such clones were injected into C57BL/6 blastocysts. Resultant offspring that had inherited a targeted μs allele were interbred in our barrier animal facility to generate the μs−/− and control mice analyzed here.
Figure 1.
Creation of μs−/− mice. (A) The targeting construct. Restriction endonuclease cleavage sites are abbreviated: B, BamHI; Bc, BclI; S, SpeI; X, XbaI; Xh, XhoI. The locations of the secretory μ tailpiece (tp), the μs and μm polyadenylation (An), sites and the LoxP sites (▴) are indicated. The Tk-Neo cassette was inserted between the BclI sites of Cμ. The fragment used for ES cell targeting extends from the BamHI to XhoI sites of the resulting construct. (B) Southern blot analysis of tail DNA from μs+/+, μs+/−, and μs−/− mice digested with SpeI and hybridized with the probe shown in A.
Flow Cytometric and Proliferative Analyses.
Flow cytometric analyses on 8- to 12-week-old mice, using litter-matched controls, as well as the monitoring of B cell-proliferative responses were performed as described previously (14).
Analysis of Immune Responses.
Conjugates of the hapten 4-hydroxy-3-nitrophenacetyl (NP) were obtained from Solid Phase Sciences, San Rafael, CA, and were injected i.p.: NP40-Ficoll (5 μg), NP–keyhole limpet hemocyanin (NP13-KLH; 50 μg), and alum-precipitated NP–chicken gamma globulin (NP13-CG; 20 μg). An alum-precipitated conjugate of 2-phenyloxazolone coupled to chicken serum albumin (phOx6-CSA; 5 μg) was a gift from C. Jolly (Medical Research Council, Cambridge). Natural IgM antibody (19S) was purified from pooled sera of unprimed mice by (NH4)2SO4 fractionation and gel filtration on Sephacryl S300, with the final sample being 5-fold concentrated as compared with the original serum. A parallel approach was used to purify 7S Ig from the sera of immunized mice. Antigen-specific titers were determined by ELISA on hapten–BSA-coated plates using biotinylated anticlass, subclass, or allotype reagents (PharMingen) as indicated. Titers, given in arbitrary units, were calculated by multiplying the OD value obtained in the ELISA by the relevant serum-dilution factor. Nonimmune mice gave serum background values of 1–5 × 10−2. Assays were calibrated by using the P8.86.9 monoclonal IgG1 anti-NP antibody (gift of T. Imanishi, Tufts University, Boston); 1 μg/ml of this antibody gave an ELISA titer in the range of 0.5–5 arbitrary units when measured on an NP2.5-BSA-coated plate with an anti-IgG1 developing reagent.
Serum Ig isotypes were determined by a capture ELISA (PharMingen) according to the manufacturer’s protocol. Immunohistochemistry of sections derived from the middle third of the spleen after fixing in buffered 4% formalin was performed by using peroxidase-conjugated peanut agglutinin. Surface plasmon resonance was carried out as described elsewhere (15).
RESULTS
Creation of μs−/− Mice.
We have shown previously that a Cμ gene directing the synthesis of the membrane (μm) but not secretory (μs) isoform of mouse Ig μ heavy chains can be created by deleting part of the secretory μ tailpiece together within the μs translation stop and polyadenylation sites; the 1.8-kb deletion extended from the BclI site in the μs tailpiece through to the BclI site in the Cμ4/M1 intron (16, 17). To create mice lacking secretory IgM expression, we introduced this same deletion into the mouse germ line. We used the Lox/Cre targeting approach of Gu et al. (13) because this allows the selective marker used for transfection to be removed from the targeted allele, avoiding the risk that its continued presence might otherwise confound correct interpretation of the resulting phenotype. A targeting vector therefore was constructed in which a Neo/Tk cassette (flanked by LoxP sites) was inserted into a segment of the Cμ gene that also carried the BclI deletion (Fig. 1). This construct was transfected into ES cells and Neor clones carrying integrations that had recombined into the endogenous Ig μ locus identified by Southern blotting. After transient transfection of such targeted clones with a plasmid-expressing Cre recombinase, derivatives that had excised the Neo/Tk cassette were selected on the basis of their Tk− phenotype; this was confirmed by Southern blotting. The resulting ES cells carrying a targeted μs− allele then were injected into C57BL/6 blastocysts and, after germ-line transmission, μs−/− homozygous mice were obtained.
B Cells and Serum Ig in μs−/− Mice.
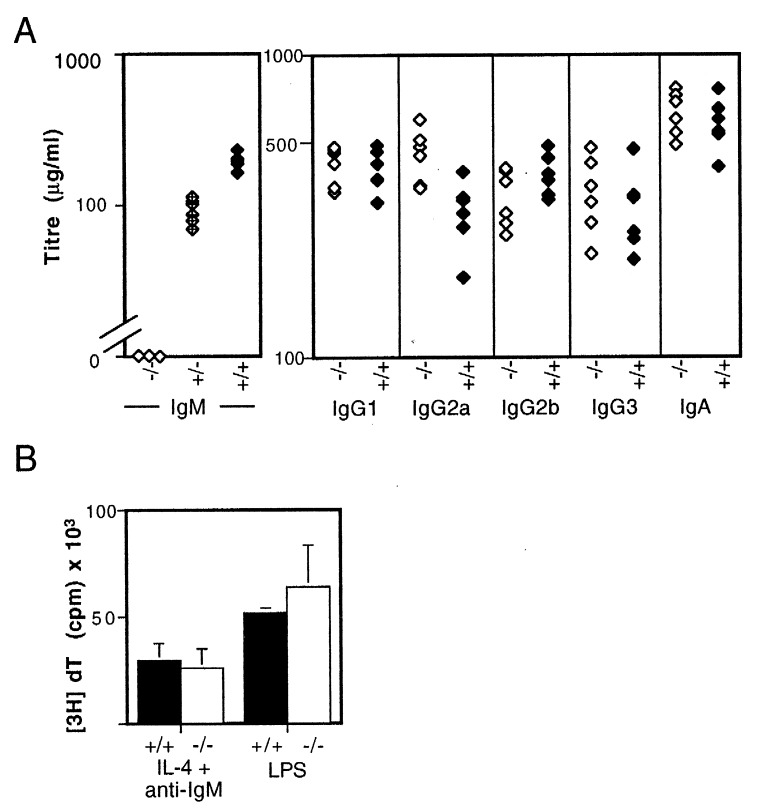
Serum IgM essentially was undetectable in the μs−/− animals and was reduced by about 50% in the heterozygotes (Fig. 2A). The serum levels of the other Ig isotypes was, however, affected little.
Figure 2.
(A) Concentrations of serum Ig in 8-week-old μs−/− (open symbols) and control mice (solid symbols) derived from breedings against C57BL/6 were determined by ELISA. The concentration of serum IgM in μs−/− mice was below the sensitivity of the ELISA (50 ng/ml); that in heterozygotes is indicated by half-filled symbols. The small difference between μs−/− and control mice seen with respect to IgG2a levels was not observed in mice in which the μs− allele was bred against BALB/c (data not shown), suggesting that the apparent difference in IgG2a levels seen here reflects differential recognition of IgG2aa and IgG2ab by the developing anti-IgG2a antiserum (also see PharMingen data sheet). (B) Proliferative responses of spleen cells from normal (solid bars) and μs−/− mice (open bars) to 9 μg/ml bacterial lipopolysaccharide (LPS) or 10 μg/ml F(ab′)2 goat anti-μ antibody + 500 units/ml interleukin 4 (IL-4) were determined by monitoring the incorporation of [3H]thymidine after 48 h in culture. Each bar depicts the mean incorporation of three individual mice; the background incorporations for individual control and μs−/− mice were below 1,600 cpm.
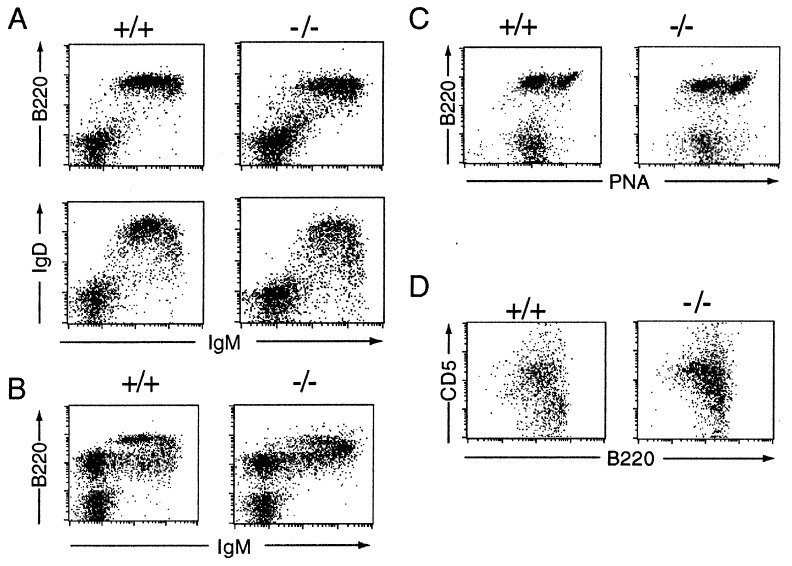
The loss of serum IgM was not accompanied by any diminution in membrane IgM expression. Indeed, flow cytometric analysis of splenic B cells revealed increased expression of surface IgM and diminished expression of IgD (Fig. 3A). A similar shift to brighter IgM staining was apparent in the IgM+ B cell populations in the bone marrow (Fig. 3B). This shift to increased IgM expression probably does not reflect a role for serum IgM in B cell maturation. Rather, we believe it is because of a cell-autonomous consequence of the μs deletion itself on the RNA-processing pattern of the primary μ-δ transcript. Evidence supporting this comes from the use of allotype-specific anti-μ antibodies to analyze IgM expression on the surface of B cells in μs+/− heterozygotes. Those B cells that express the targeted (but not the wild type) IgH allele still reveal augmented surface IgM expression although the mice do not lack serum IgM (data not shown).
Figure 3.
Flow cytometric analysis of B cell populations. The profiles are representative of results obtained with between four and six mice. (A) Spleen cells were stained with fluorescein isothiocyanate-conjugated anti-IgM and either phycoerythrin-conjugated anti-CD45R(B220) or anti-IgD. The median fluorescence of the anti-IgM staining of the CD45R(B220)+ cells in the μs−/− mice was 475 ± 80, compared with 225 ± 20 in their normal littermates. (B) Bone marrow. (C) Peyer’s patch cells were stained for B220 and peanut agglutinin (PNA). (D) Peritoneum. Analysis of CD5/CD45R(B220) staining is shown for cells gated as IgM+. In μs−/− mice, 65 ± 9% of the CD45R(B220)+ peritoneal cells were CD5+ compared with 38 ± 7% in controls.
With regard to other maturation markers, the B lineage cells in bone marrow and spleen μs−/− mice look much as those in their wild-type littermates (CD43 in bone marrow; CD21/35, CD22, CD23, and CD24 in spleen; data not shown). They also showed normal proliferative responses to bacterial lipopolysaccharide and anti-μ + IL4 (Fig. 2B); the B cells in Peyer’s patches were capable of acquiring the bright staining for peanut agglutinin characteristic of the germinal center phenotype (Fig. 3C). An unanticipated feature of the μs−/− mice, however, was that the population of peritoneal B cells was shifted toward increased CD5 expression, suggesting a relative expansion of the B1a subset (Fig. 3D).
Immune Responses in μs−/− Mice.
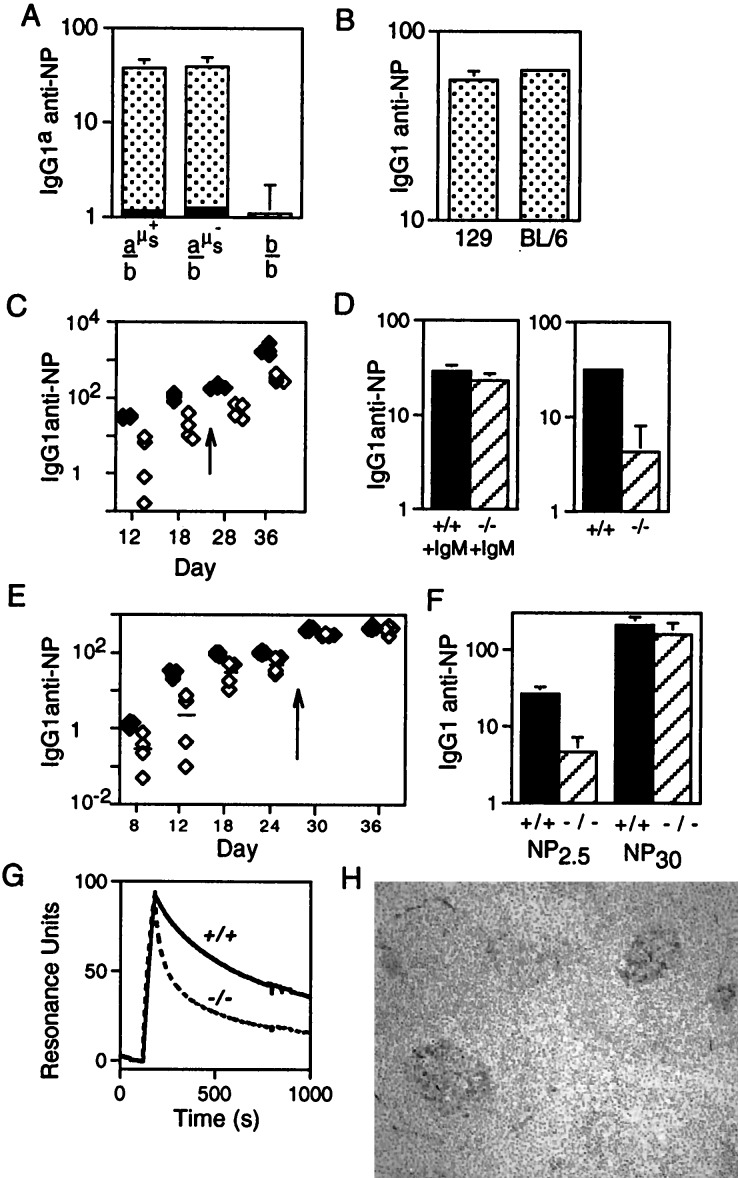
We wished to monitor whether the lack of serum IgM affected the response of the mice to a T cell-dependent antigen. To exclude the possibility that any altered responsiveness of the B cells in μs−/− mice could be attributable to a cell-autonomous B cell defect (e.g., the altered surface IgM/IgD ratio) rather than the absence of serum IgM itself, we initially compared the responses of heterozygous mice in which one IgH allele was of the b allotype and the other was either a wild-type IgHa allele or an IgHa allele carrying the targeted μs− deletion. After intraperitoneal challenge with NP13-CG, the two sets of mice gave indistinguishable titers of IgG1a anti-NP antibody (Fig. 4A). [They also gave similar titers of IgG1b antibody (not shown) and, indeed, the 129 and C57BL/6 inbred parental lines gave similar titers of total specific IgG1 (Fig. 4B). Furthermore, in keeping with the results of Toellner et al. (18), we find the majority of the IgG response to this immunization is of the IgG1 subclass.] Thus, the μs− deletion itself does not exert any cell-autonomous inhibition on the production of antigen-specific IgG1.
Figure 4.
T cell-dependent anti-NP responses. (A) Comparison of the day 12 IgG1a anti-NP response to NP-CG immunization in mice in which one IgH allele was of b allotype (derived from C57BL/6) and the other was either wild type a allotype (derived from 129; a-μs+), a allotype but carrying the targeted μs deletion (a-μs−), or b allotype (b). These mice (sets of four) were derived from breeding the ES cell-derived chimeras against C57BL/6. The solid section at the base of each histogram depicts the prebleed titer. The titers are given in arbitrary units as detailed under Materials and Methods. (B) Comparison of the day 12 total IgG1 anti-NP response to NP-CG immunization in 129 and C57BL/6 mice. (C) Comparison of the titers of IgG1 anti-NP antibody in μs−/− (open symbols) and wild-type mice (solid symbols) immunized with soluble NP-KLH and boosted (arrow) on day 24 as measured by ELISA on NP30-BSA-coated plates. Tail bleeds were obtained on the days indicated on the x axis. (D) Titers of IgG1 anti-NP antibody 12 days after NP-KLH immunization were compared in μs−/− and control mice in which the antigen challenge had been given together with pooled serum IgM from unprimed mice (an amount equivalent to the IgM present in 0.5 ml of serum). (E) Comparison of the titers of IgG1 anti-NP antibody in μs−/− (open symbols) and control littermates (solid symbols) immunized with alum-precipitated NP-CG and boosted (day 26) as monitored by ELISA on NP2.5-BSA-coated plates. (The magnitude of the day 12 IgG1 anti-NP response in NP13-CG immunized control mice is about 8-fold greater than in NP13-KLH mice when both are compared on NP30-BSA coated plates.) (F) Comparison of the titers of IgG1 anti-NP antibody in the day 12 serum of the NP13-CG immunized mice as assayed plates coated with NP2.5-BSA and NP30-BSA. (G) Analysis by surface plasmon resonance of the binding of serum antibody to NP6-BSA immobilized on the chip. The serum samples were obtained from μs−/− and μs+/+ siblings 12 days after challenge with NP-CG, and the 7S Ig fraction was purified as described in Materials and Methods. The bulk of the antibody in the μs− sample that is bound initially to the chip clearly has a dissociation rate severalfold higher than that in the control, although the heterogeneity of the serum antibody and resultant complexity of the dissociation curves preclude a simple calculation of koff. (H) Germinal centers in the spleens of μs−/− mice 12 days post-NP-CG immunization were visualized by staining for peanut agglutinin.
We then compared the responses of homozygous μs−/− and μs+/+ mice initially by monitoring the responses to soluble antigen. After intraperitoneal challenge with NP-KLH, the serum titer of IgG1 anti-NP antibody in both the primary and secondary responses were considerably (around 5-fold) reduced in μs−/− mice as compared with controls (Fig. 4C). This reduction in the specific IgG1 response could reflect a role for the serum IgM present before immunization (natural IgM) in potentiating the initiation of the response. Alternatively, the reduction could reflect a role for the antigen-specific IgM produced immediately after immunization (first-wave antibody) in enhancing the subsequent development of the response. Therefore, we investigated the effect of immunizing μs−/− mice with NP-KLH together with pooled serum IgM from nonimmunized mice (natural IgM). The result (Fig. 4D) indicates that provision of natural IgM indeed can lead to an increased specific IgG1 response in the μs−/− mice. Thus, the diminished responsiveness of μs−/− mice appears, at least in part, because of a lack of natural IgM; the data do not, however, preclude that part of the phenotype may also be attributable to a lack of first-wave IgM antibody.
We then compared the responses of μs−/− mice and their wild-type siblings to challenge with alum-precipitated NP-CG. Here again the μs−/− animals revealed a substantially decreased primary response (Fig. 4E). However, at day 12 after immunization, germinal centers were readily detectable in the spleens of μs−/− mice (Fig. 4H) and the animals were able to mount a high-titer anti-NP response on boosting. This suggested that the defect in the μs−/− mice was essentially a delay in the maturation of the response and led us to ask whether the affinities of the NP-specific antibody elicited during the early stages of the response to NP-CG were similar in μs−/− and control mice. In one approach, we compared the anti-NP IgG1 titers on plates coated with NP2.5-BSA and NP30-BSA because high-affinity antibodies bind both coats whereas low-affinity antibodies are detected preferentially on NP30-BSA (19). The results (Fig. 4F) support the proposal that the day 12 anti-NP antibody in μs−/− mice is of lower affinity than in controls. Surface plasmon resonance was used to confirm that the total 7S anti-NP antibody in these μs−/− sera has a significantly faster koff for NP than that in the sera of their control littermates (Fig. 4G).
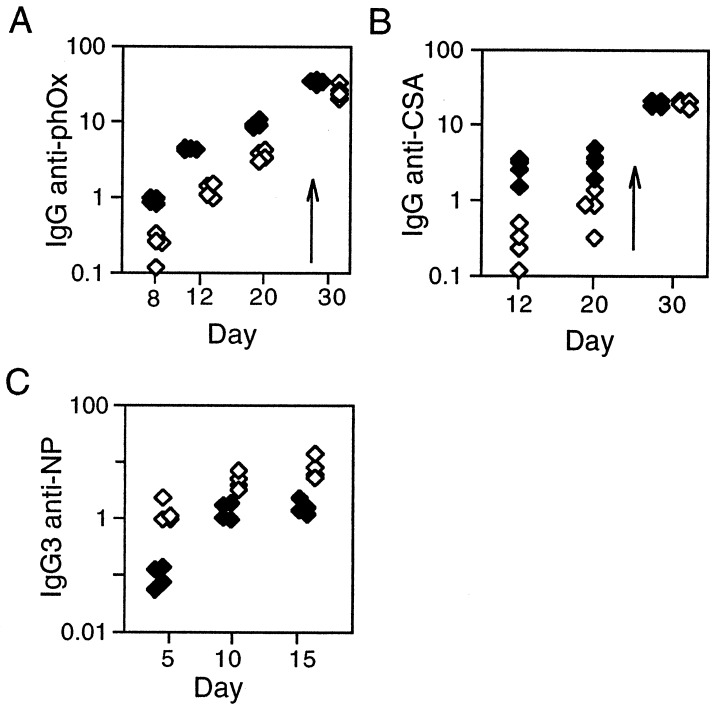
The delayed response of μs−/− mice is not unique to an anti-NP challenge. A similar delay was observed in the response to a different hapten/carrier conjugate—2-phenyloxazolone coupled to chicken serum albumin (Fig. 5 A and B). The primary response (to both hapten and carrier) again was diminished in μs−/− mice, but the animals were capable of mounting a good secondary response.
Figure 5.
Responses to phOx-CSA and NP-Ficoll. (A and B) Comparison of the serum titers of total IgG anti-phOx or anti-CSA antibody in μs−/− (open symbols) and control littermates (solid symbols) immunized with alum-precipitated phOx6-CSA and boosted (day 24). (C) Comparison of the serum titers of IgG3 anti-NP antibody in μs−/− (open symbols) and control littermates (solid symbols) immunized with NP-Ficoll.
Interestingly, however, the diminution of response to T-dependent antigens is not paralleled in the response to the T-independent antigen NP-Ficoll. Here, we found the magnitude of the response in μs−/− animals was enhanced about 3-fold (Fig. 5C). The main IgG response is of the IgG3 subclass although the IgG1, IgG2a, and IgG2b responses similarly were augmented.
DISCUSSION
The major conclusion to emanate from this work is that the mice deficient in secretory IgM exhibit a delay in the maturation of their response to T cell-dependent antigens. Compared with the controls, diminished titers of specific IgG antibody are obtained in the primary response to challenge with NP-KLH, NP-CG, and phOx-CSA. Such results imply that development of specific IgG is accelerated either by the natural IgM antibody that is present in serum before antigen encounter or by the first wave of specific IgM production that is elicited immediately after antigen challenge, or by both. That the diminution in the specific IgG response in the μs−/− mice can, at least in part, be overcome by supplementing the immunizing antigen with exogenously provided natural IgM antibody argues that at least part of the reduced responsiveness of μs−/− mice is attributable to their lack of natural IgM. Thus, the data strongly point to a role for natural IgM antibody in facilitating an acceleration of the primary immune response. An analogous role for the first wave of specific IgM antibody in acceleration of maturation of the response is not excluded. Such specific IgM may play an important role as well; indeed, some studies have revealed that the primary response can be accelerated by administration of specific IgM antibody (20–22).
It is notable that the loss of secretory IgM has a very marked effect on the maturation of the response even though the mice retain normal levels of serum IgG. This is particularly striking given that the overall IgG concentration in normal animals is severalfold higher than that of IgM.
With regard to a potentiating effect on the maturation of the response by the first wave of specific antibody, the severity of disrupting secretory IgM production can be understood readily considering that, in a normal mouse, production of low-affinity specific IgM will precede production of low-affinity IgG. Thus, if the low-affinity antibody elicited early in the immune response plays a role in accelerating the production of subsequent high-affinity antibody, then it is little wonder that disrupting secretory IgM productions delays the development of the subsequent high-affinity response.
With regard to a specific role for natural (preimmune) IgM in accelerating the maturation of the specific immune response, the rationale as to why natural IgG cannot substitute fully must be a little different. Here, the important distinction presumably lies in the increased valency of IgM together with the fact that the interaction of antigen with a single IgM (but not a single IgG) molecule can lead to activation of the complement cascade (9). Thus, natural IgM may bind and oligomerize the antigen, thereby allowing it to interact with increased avidity with the antigen receptors expressed in the primary B cell repertoire. However, probably more important, a complex of antigen with natural IgM may recruit complement and thereby cause effective B cell activation through cosignaling by the antigen receptor in conjunction with the CD19/21 complex (23, 24). In other work (15) we have noted that a threshold affinity in the range 105–106 M−1 was needed for the activation of a B cell transfectant by monovalent antigen; this threshold could be lowered by oligomerizing the antigen with soluble antibody. It will be interesting to ascertain whether the lack of secretory IgM in the μs−/− mice has increased the threshold affinity required for initiation of the primary response.
A distinct aspect of complement recruitment by IgM/antigen complexes is that such complexes could be deposited effectively at sites suitable for the development and maturation of the humoral immune response (10, 11). This could be a further function of natural IgM antibody. It therefore will be interesting to compare antigen localization and germinal center formation in the very early stages of the primary response in secretory IgM-deficient and control mice.
This work additionally has revealed an increased proportion of CD5+ B cells in the peritoneum of μs−/− mice as well as an augmented response to the T cell-independent antigen NP-Ficoll. It could be that both phenomena reflect that, in μs−/− mice, there is a lack of feedback regulation by secretory IgM (see, for example, ref. 25). Alternatively, the findings could be a consequence of the shift to brighter surface IgM expression in these animals. Some of these issues will be addressable by providing exogenous serum IgM to the μs−/− animals.
Acknowledgments
We thank the staff in the animal facility for assistance with animal husbandry and Gareth Williams and Facundo Batista for assistance and advice in measuring cell proliferation and antibody affinity. We are grateful to Marianne Brüggemann for providing a mouse Cμ clone and Klaus Rajewsky for the Neo-Tk selection cassette and Cre expression plasmid. M.R.E. was supported by a Medical Research Council Clinician Scientist award, T.O.K. was supported in part by a grant from the Oliver Bird Foundation, and S.L.D. and T.O.K. were supported in part by an International Research Scholars award from the Howard Hughes Medical Institute to M.S.N.
ABBREVIATIONS
- CG
chicken gamma globulin
- CSA
chicken serum albumin
- KLH
keyhole limpet hemocyanin
- NP
4-hydroxy-3-nitrophenacetyl
- phOx
2-phenyloxazolone
- ES
embryonic stem
References
- 1.Nossal G J V, Ada G L. Antigens, Lymphoid Cells, and the Immune Response. New York: Academic; 1971. [Google Scholar]
- 2.White R G. In: Clinical Aspects of Immunology. Gell P G H, Coombs R R A, Lachmann P J, editors. Oxford: Blackwell; 1975. pp. 411–416. [Google Scholar]
- 3.Thorbecke G J, Lerman S P. Adv Exp Biol. 1976;73A:83–100. doi: 10.1007/978-1-4684-3297-8_8. [DOI] [PubMed] [Google Scholar]
- 4.Theofilopoulos A N, Dixon F J. Adv Immunol. 1979;28:89–220. doi: 10.1016/s0065-2776(08)60800-7. [DOI] [PubMed] [Google Scholar]
- 5.Jerne N K. Proc Natl Acad Sci USA. 1955;41:849–857. doi: 10.1073/pnas.41.11.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyden S V. Adv Immunol. 1966;5:1–28. doi: 10.1016/s0065-2776(08)60271-0. [DOI] [PubMed] [Google Scholar]
- 7.Hoerlin A B. J Immunol. 1956;78:112–117. [Google Scholar]
- 8.Segre D, Kaeberle M L. J Immunol. 1962;89:782–793. [PubMed] [Google Scholar]
- 9.Borsos T, Rapp H J. Science. 1965;150:505–506. doi: 10.1126/science.150.3695.505. [DOI] [PubMed] [Google Scholar]
- 10.Pepys M B. Nat New Biol. 1972;237:157–159. doi: 10.1038/newbio237157a0. [DOI] [PubMed] [Google Scholar]
- 11.Klaus G G B, Humphrey J H, Kunkl A, Dongworth D W. Immunol Rev. 1980;53:3–69. doi: 10.1111/j.1600-065x.1980.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 12.Fearon D T, Locksley R M. Science. 1996;272:5–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 13.Gu H, Zou Y R, Rajewsky K. Cell. 1993;73:1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- 14.O’Keefe T L, Williams G T, Davies S L, Neuberger M S. Science. 1996;274:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 15.Batista F B, Neuberger M S. Immunity. 1998;8:751–759. doi: 10.1016/s1074-7613(00)80580-4. [DOI] [PubMed] [Google Scholar]
- 16.Sitia R, Neuberger M S, Milstein C. EMBO J. 1987;6:3969–3977. doi: 10.1002/j.1460-2075.1987.tb02739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teh Y-M, Neuberger M S. J Exp Med. 1997;185:1753–1758. doi: 10.1084/jem.185.10.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toellner K M, Luther S A, Sze D M Y, Choy R K W, Taylor D R, MacLennan I C M, Acha-Orbea H. J Exp Med. 1998;187:1193–1204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herzenberg L A, Black S J, Tokuhisa T, Herzenberg L A. J Exp Med. 1980;151:1071–1087. doi: 10.1084/jem.151.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry C, Jerne N K. J Exp Med. 1968;128:133–152. doi: 10.1084/jem.128.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brüggemann M, Rajewsky K. Cell Immunol. 1982;71:365–373. doi: 10.1016/0008-8749(82)90270-2. [DOI] [PubMed] [Google Scholar]
- 22.Lehner P, Hutchings P, Lydyard P M, Cooke A. Immunology. 1983;50:503–509. [PMC free article] [PubMed] [Google Scholar]
- 23.Fearon D T, Carter R H. Annu Rev Immunol. 1995;13:127–149. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 24.Ahearn J M, Fischer M B, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard R G, Rothstein T L, Carroll M C. Immunity. 1996;4:251–262. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- 25.Brodeur P H, Wortis H H. J Immunol. 1980;125:1499–1505. [PubMed] [Google Scholar]