Abstract
Dihydroxyacetone (DHA) kinase of Klebsiella pneumoniae, a gene product of the dha regulon responsible for fermentative dissimilation of glycerol and DHA, was purified 120-fold to a final specific activity of 10 mumol X min-1 X mg of protein-1 at 30 degrees C. The enzyme, a dimer of a 53,000 +/- 5,000-dalton polypeptide, is highly specific for DHA (Km, ca.4 microM). Glycerol is not a substrate at 1 mM and is not an inhibitor even at 100 mM. The enzyme is not inhibited by 5 mM fructose-1,6-diphosphate. Ca2+ gives a higher enzyme activity than Mg2+ as a cationic cofactor. Escherichia coli glycerol kinase acts on both glycerol and DHA and is allosterically inhibited by fructose-1,6-diphosphate. Antibodies raised against E. coli glycerol kinase cross-reacted with K. pneumoniae glycerol kinase but not with K. pneumoniae DHA kinase.
Full text
PDF
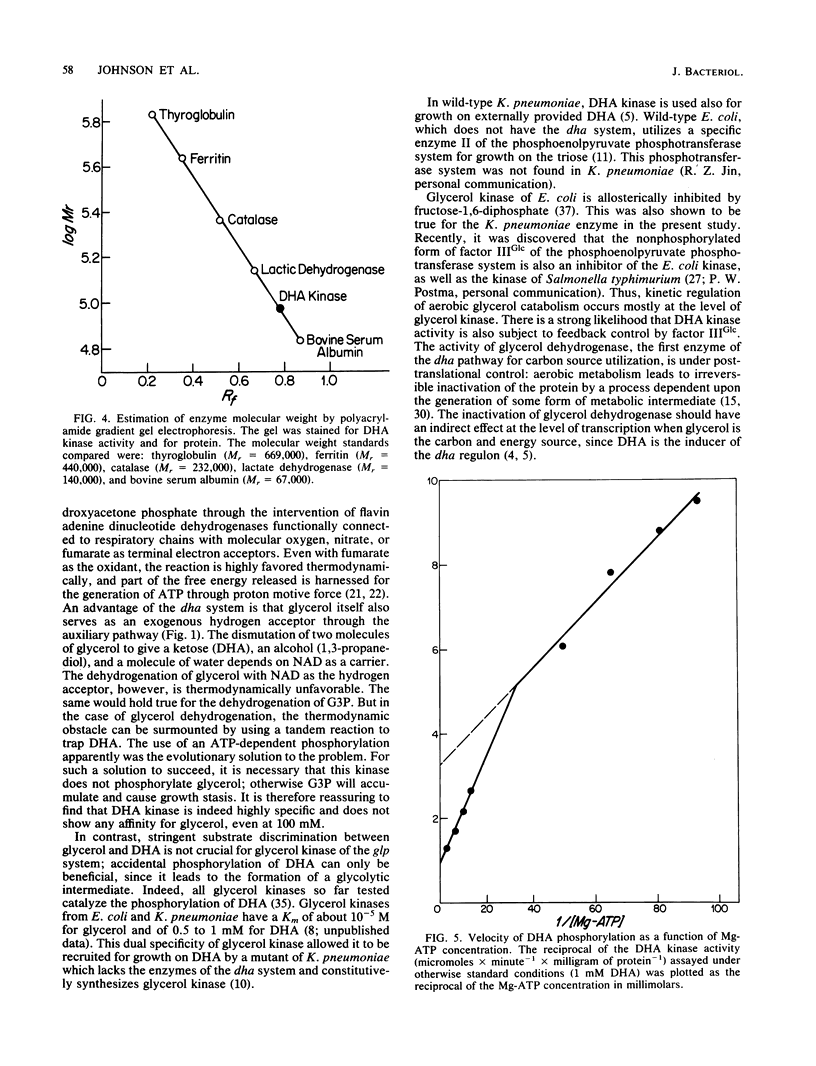

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forage R. G., Foster M. A. Glycerol fermentation in Klebsiella pneumoniae: functions of the coenzyme B12-dependent glycerol and diol dehydratases. J Bacteriol. 1982 Feb;149(2):413–419. doi: 10.1128/jb.149.2.413-419.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forage R. G., Lin E. C. DHA system mediating aerobic and anaerobic dissimilation of glycerol in Klebsiella pneumoniae NCIB 418. J Bacteriol. 1982 Aug;151(2):591–599. doi: 10.1128/jb.151.2.591-599.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedberg W. B., Kistler W. S., Lin E. C. Lethal synthesis of methylglyoxal by Escherichia coli during unregulated glycerol metabolism. J Bacteriol. 1971 Oct;108(1):137–144. doi: 10.1128/jb.108.1.137-144.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S. I., Lin E. C. Product induction of glycerol kinase in Escherichia coli. J Mol Biol. 1965 Dec;14(2):515–521. doi: 10.1016/s0022-2836(65)80200-5. [DOI] [PubMed] [Google Scholar]
- Hayashi S. I., Lin E. C. Purification and properties of glycerol kinase from Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):1030–1035. [PubMed] [Google Scholar]
- Jin R. Z., Forage R. G., Lin E. C. Glycerol kinase as a substitute for dihydroxyacetone kinase in a mutant of Klebsiella pneumoniae. J Bacteriol. 1982 Dec;152(3):1303–1307. doi: 10.1128/jb.152.3.1303-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R. Z., Lin E. C. An inducible phosphoenolpyruvate: dihydroxyacetone phosphotransferase system in Escherichia coli. J Gen Microbiol. 1984 Jan;130(1):83–88. doi: 10.1099/00221287-130-1-83. [DOI] [PubMed] [Google Scholar]
- Krymkiewicz N., Diéguez E., Rekarte U. D., Zwaig N. Properties and mode of action of a bactericidal compound (=methylglyoxal) produced by a mutant of Escherichia coli. J Bacteriol. 1971 Dec;108(3):1338–1347. doi: 10.1128/jb.108.3.1338-1347.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., LEVIN A. P., MAGASANIK B. The effect of aerobic metabolism on the inducible glycerol dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1960 Jun;235:1824–1829. [PubMed] [Google Scholar]
- LIN E. C., MAGASANIK B. The activation of glycerol dehydrogenase from Aerobacter aerogenes by monovalent cations. J Biol Chem. 1960 Jun;235:1820–1823. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B., BROOKE M. S., KARIBIAN D. Metabolic pathways of glycerol dissimilation; a comparative study of two strains of Aerobacter aerogenes. J Bacteriol. 1953 Nov;66(5):611–619. doi: 10.1128/jb.66.5.611-619.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor W. G., Phillips J., Suelter C. H. Purification and kinetic characterization of a monovalent cation-activated glycerol dehydrogenase from Aerobacter aerogenes. J Biol Chem. 1974 May 25;249(10):3132–3139. [PubMed] [Google Scholar]
- Miki K., Lin E. C. Anaerobic energy-yielding reaction associated with transhydrogenation from glycerol 3-phosphate to fumarate by an Escherichia coli system. J Bacteriol. 1975 Dec;124(3):1282–1287. doi: 10.1128/jb.124.3.1282-1287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Wilson T. H. Proton translocation associated with anaerobic transhydrogenation from glycerol 3-phosphate to fumarate in Escherichia coli. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1570–1575. doi: 10.1016/0006-291x(78)91400-6. [DOI] [PubMed] [Google Scholar]
- Neijssel O. M., Hueting S., Crabbendam K. J., Tempest D. W. Dual pathways of glycerol assimilation in Klebsiella aerogenes NCIB418: their regulation and possible functional significance. Arch Microbiol. 1975 Jun 20;104(1):83–87. doi: 10.1007/BF00447304. [DOI] [PubMed] [Google Scholar]
- Neijssel O. M., Tempest D. W. The regulation of carbohydrate metabolism in Klebsiella aerogenes NCTC 418 organisms, growing in chemostat culture. Arch Microbiol. 1975 Dec 31;106(3):251–258. doi: 10.1007/BF00446531. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Epstein W., Schuitema A. R., Nelson S. O. Interaction between IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system and glycerol kinase of Salmonella typhimurium. J Bacteriol. 1984 Apr;158(1):351–353. doi: 10.1128/jb.158.1.351-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch F. E., Jr, Lin E. C., Kowit J. D., Tang C. T., Goldberg A. L. In vivo inactivation of glycerol dehydrogenase in Klebsiella aerogenes: properties of active and inactivated proteins. J Bacteriol. 1980 Mar;141(3):1077–1085. doi: 10.1128/jb.141.3.1077-1085.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch F. E., Lengeler J., Lin E. C. Regulation of glycerol catabolism in Klebsiella aerogenes. J Bacteriol. 1974 Jul;119(1):50–56. doi: 10.1128/jb.119.1.50-56.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch F. E., Lin E. C. Independent constitutive expression of the aerobic and anaerobic pathways of glycerol catabolism in Klebsiella aerogenes. J Bacteriol. 1975 Oct;124(1):348–352. doi: 10.1128/jb.124.1.348-352.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. T., Ruch F. E., Jr, Lin C. C. Purification and properties of a nicotinamide adenine dinucleotide-linked dehydrogenase that serves an Escherichia coli mutant for glycerol catabolism. J Bacteriol. 1979 Oct;140(1):182–187. doi: 10.1128/jb.140.1.182-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner J. W., Paulus H. Catalytic and allosteric properties of glycerol kinase from Escherichia coli. J Biol Chem. 1973 Jun 10;248(11):3922–3932. [PubMed] [Google Scholar]
- Thorner J. W., Paulus H. Composition and subunit structure of glycerol kinase from Escherichia coli. J Biol Chem. 1971 Jun 25;246(12):3885–3894. [PubMed] [Google Scholar]
- Toraya T., Kuno S., Fukui S. Distribution of coenzyme B12-dependent diol dehydratase and glycerol dehydratase in selected genera of Enterobacteriaceae and Propionibacteriaceae. J Bacteriol. 1980 Mar;141(3):1439–1442. doi: 10.1128/jb.141.3.1439-1442.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaig N., Lin E. C. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science. 1966 Aug 12;153(3737):755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]