Abstract
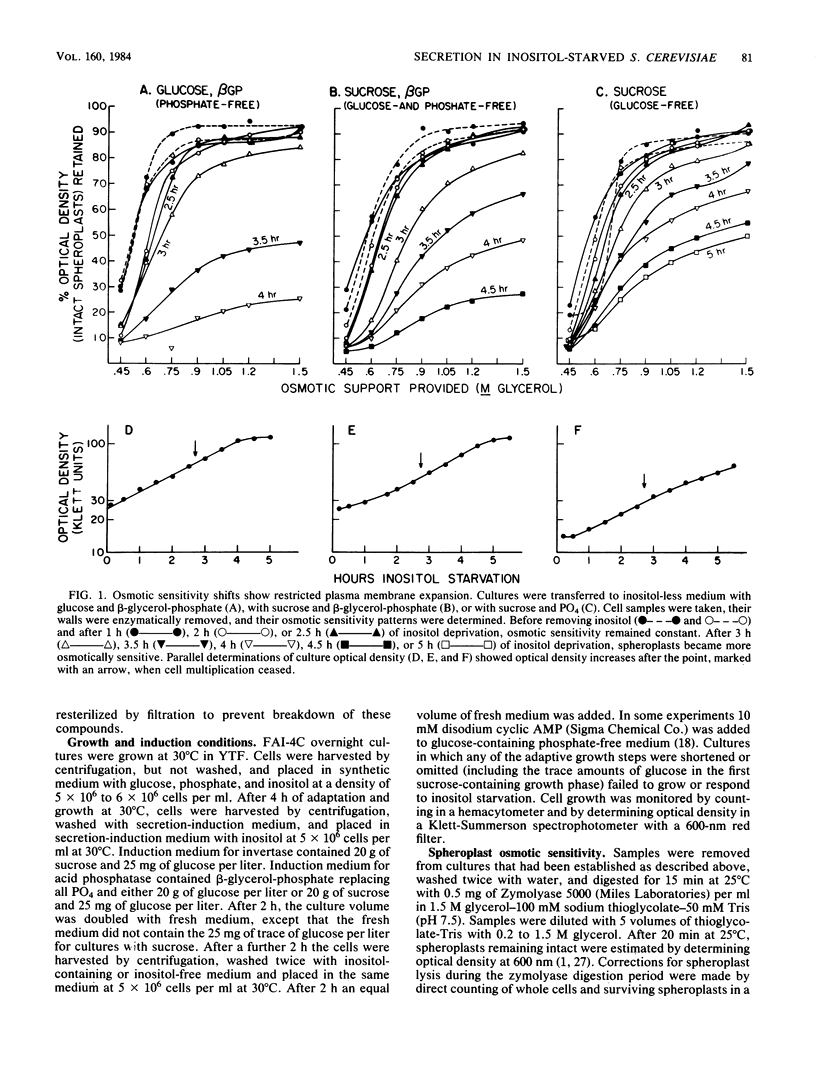
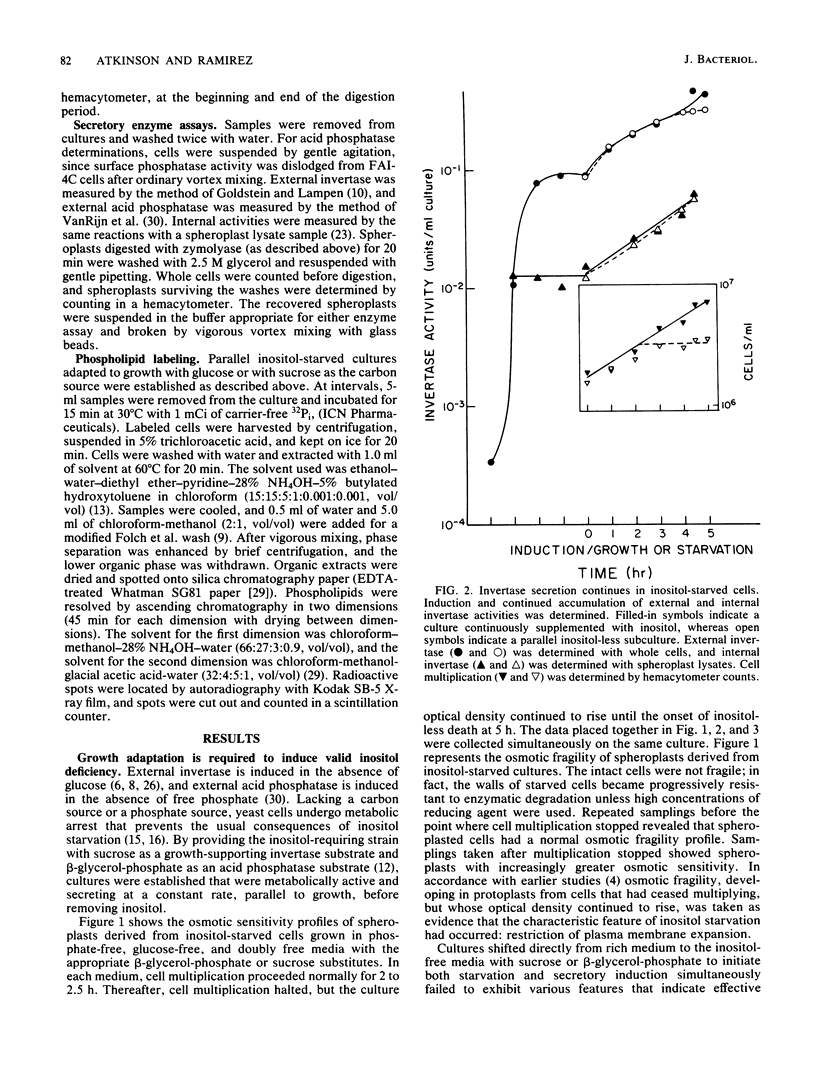
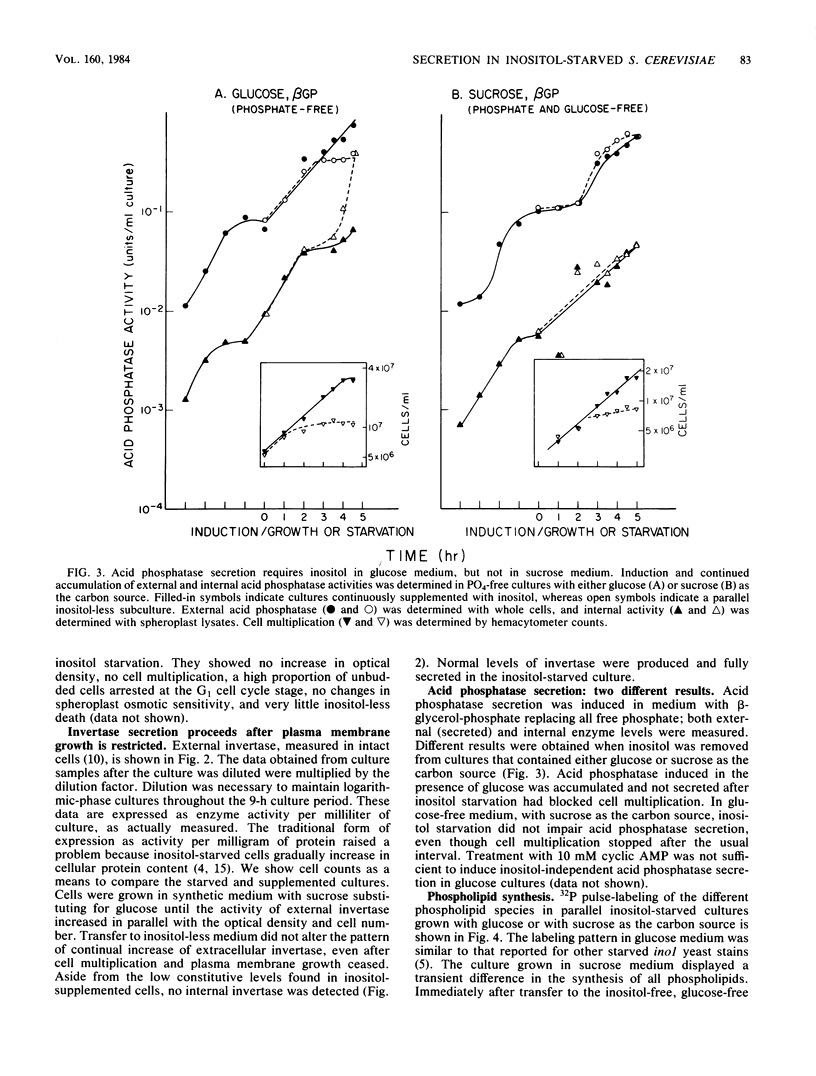
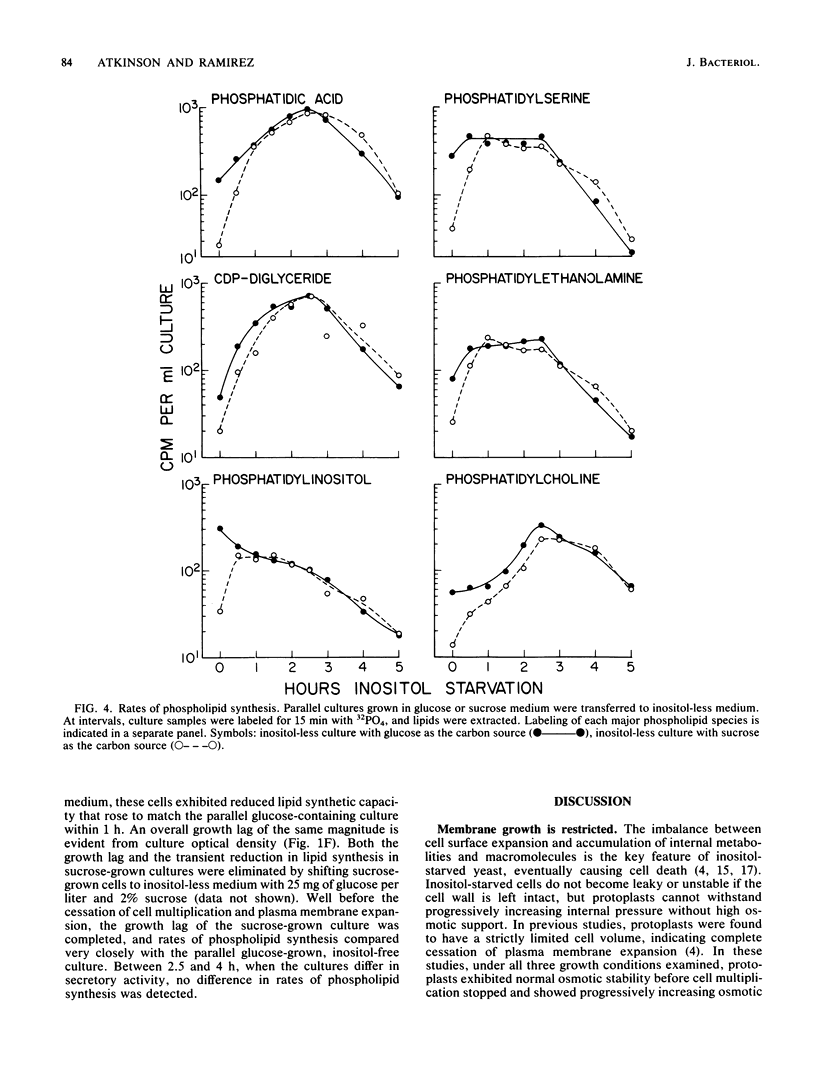
Secretion of acid phosphatase and invertase was examined in an inositol-requiring ino1 mutant of the yeast Saccharomyces cerevisiae. Inositol starvation is known to block plasma membrane expansion, presumably due to restricted membrane phospholipid synthesis. If membrane expansion and extracellular protein secretion are accomplished by the same intracellular transport process, one would expect secretion to fail coordinately with cessation of plasma membrane growth in inositol-starved cells. In glucose-grown, inositol-starved cells, plasma membrane expansion and acid phosphatase secretion stopped coordinately, and intracellular acid phosphatase accumulated. In sucrose-grown, inositol-starved cells, plasma membrane growth halted, but secretion of both acid phosphatase and invertase continued until the onset of inositol-less death. Although glucose-grown and sucrose-grown cells differ in their ability to secrete when deprived of inositol, they exhibited the same disturbances in phospholipid synthesis. Phosphatidylinositol synthesis failed, and its precursors phosphatidic acid and CDP-diglyceride accumulated equally in both cultures. Sucrose-grown yeast cells appear to accomplish normal levels of extracellular protein secretion by an inositol-independent mechanism. In glucose-grown yeasts, both plasma membrane expansion and secretion are inositol dependent.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alterthum F., Rose A. H. Osmotic lysis of sphaeroplasts from Saccharomyces cerevisiae grown anaerobically in media containing different unsaturated fatty acids. J Gen Microbiol. 1973 Aug;77(2):371–382. doi: 10.1099/00221287-77-2-371. [DOI] [PubMed] [Google Scholar]
- Angus W. W., Lester R. L. The regulated catabolism of endogenous and exogenous phosphatidylinositol by Saccharomyces cerevisiae leading to extracellular glycerophosphorylinositol and inositol. J Biol Chem. 1975 Jan 10;250(1):22–30. [PubMed] [Google Scholar]
- Angus W. W., Lester R. L. Turnover of inositol and phosphorus containing lipids in Saccharomyces cerevisiae; extracellular accumulation of glycerophosphorylinositol derived from phosphatidylinositol. Arch Biochem Biophys. 1972 Aug;151(2):483–495. doi: 10.1016/0003-9861(72)90525-5. [DOI] [PubMed] [Google Scholar]
- Atkinson K. D., Kolat A. I., Henry S. A. Osmotic imbalance in inositol-starved spheroplasts of Saccharomyces cerevisiae. J Bacteriol. 1977 Dec;132(3):806–817. doi: 10.1128/jb.132.3.806-817.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G. W., Lester R. L. Changes in phospholipids of Saccharomyces cerevisiae associated with inositol-less death. J Biol Chem. 1977 Dec 10;252(23):8684–8691. [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Culbertson M. R., Henry S. A. Inositol-requiring mutants of Saccharomyces cerevisiae. Genetics. 1975 May;80(1):23–40. doi: 10.1093/genetics/80.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODYK F., ROTHSTEIN A. FACTORS INFLUENCING THE APPEARANCE OF INVERTASE IN SACCHAROMYCES CEREVISIAE. Arch Biochem Biophys. 1964 Mar;104:478–486. doi: 10.1016/0003-9861(64)90492-8. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Goldstein A., Lampen J. O. Beta-D-fructofuranoside fructohydrolase from yeast. Methods Enzymol. 1975;42:504–511. doi: 10.1016/0076-6879(75)42159-0. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982 Jan;28(1):51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- Hansche P. E., Beres V., Lange P. Gene duplication in Saccharomyces cerevisiae. Genetics. 1978 Apr;88(4 Pt 1):673–687. [PMC free article] [PubMed] [Google Scholar]
- Hanson B. A., Lester R. L. The extraction of inositol-containing phospholipids and phosphatidylcholine from Saccharomyces cerevisiae and Neurospora crassa. J Lipid Res. 1980 Mar;21(3):309–315. [PubMed] [Google Scholar]
- Henry S. A., Atkinson K. D., Kolat A. I., Culbertson M. R. Growth and metabolism of inositol-starved Saccharomyces cerevisiae. J Bacteriol. 1977 Apr;130(1):472–484. doi: 10.1128/jb.130.1.472-484.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A. Death resulting from fatty acid starvation in yeast. J Bacteriol. 1973 Dec;116(3):1293–1303. doi: 10.1128/jb.116.3.1293-1303.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Donahue T. F., Culbertson M. R. Selection of spontaneous mutants by inositol starvation in yeast. Mol Gen Genet. 1975 Dec 30;143(1):5–11. doi: 10.1007/BF00269415. [DOI] [PubMed] [Google Scholar]
- Keith A. D., Pollard E. C., Snipes W. Inositol-less death in yeast results in a simultaneous increase in intracellular viscosity. Biophys J. 1977 Mar;17(3):205–212. doi: 10.1016/S0006-3495(77)85650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler H. R., Lin C. C. Exogenous adenosine 3': 5'-monophosphate can release yeast from catabolite repression. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1039–1047. doi: 10.1016/0006-291x(78)91500-0. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Mormeneo S., Sentandreu R. Regulation of invertase synthesis by glucose in Saccharomyces cerevisiae. J Bacteriol. 1982 Oct;152(1):14–18. doi: 10.1128/jb.152.1.14-18.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novick P., Schekman R. Export of major cell surface proteins is blocked in yeast secretory mutants. J Cell Biol. 1983 Feb;96(2):541–547. doi: 10.1083/jcb.96.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. Distinct repressible mRNAs for cytoplasmic and secreted yeast invertase are encoded by a single gene. Cell. 1981 Aug;25(2):525–536. doi: 10.1016/0092-8674(81)90071-4. [DOI] [PubMed] [Google Scholar]
- Ramirez R. M., Ishida-Schick T., Krilowicz B. L., Leish B. A., Atkinson K. D. Plasma membrane expansion terminates in Saccharomyces cerevisiae secretion-defective mutants while phospholipid synthesis continues. J Bacteriol. 1983 Jun;154(3):1276–1283. doi: 10.1128/jb.154.3.1276-1283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M. R., Lester R. L. In vitro studies of phospholipid biosynthesis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Feb 21;260(2):222–243. doi: 10.1016/0005-2760(72)90035-5. [DOI] [PubMed] [Google Scholar]
- Van Rijn H. J., Boer P., Steyn-Parvé E. P. Biosynthesis of acid phosphatase of baker's yeast. Factors influencing its production by protoplasts and characterization of the secreted enzyme. Biochim Biophys Acta. 1972 May 12;268(2):431–441. doi: 10.1016/0005-2744(72)90339-7. [DOI] [PubMed] [Google Scholar]


