Abstract
The Epstein–Barr virus oncoprotein latent infection membrane protein 1 (LMP1) is a constitutively aggregated pseudo-tumor necrosis factor receptor (TNFR) that activates transcription factor NF-κB through two sites in its C-terminal cytoplasmic domain. One site is similar to activated TNFRII in associating with TNFR-associated factors TRAF1 and TRAF2, and the second site is similar to TNFRI in associating with the TNFRI death domain interacting protein TRADD. TNFRI has been recently shown to activate NF-κB through association with TRADD, RIP, and TRAF2; activation of the NF-κB-inducing kinase (NIK); activation of the IκBα kinases (IKKα and IKKβ); and phosphorylation of IκBα. IκBα phosphorylation on Ser-32 and Ser-36 is followed by its degradation and NF-κB activation. In this report, we show that NF-κB activation by LMP1 or by each of its effector sites is mediated by a pathway that includes NIK, IKKα, and IKKβ. Dominant negative mutants of NIK, IKKα, or IKKβ substantially inhibited NF-κB activation by LMP1 or by each of its effector sites.
The Epstein–Barr virus (EBV) latent infection membrane protein 1 (LMP1) is a key effector of EBV-mediated effects on cell growth. Genetic analyses with EBV recombinants indicate that LMP1 is essential for the conversion of resting human B lymphocytes into lymphoblastoid cell lines (LCLs) (1). In non-EBV-infected Burkitt’s tumor lymphoblasts, LMP1 can induce most of the phenotypic changes associated with EBV transformation of resting human B lymphocytes including induction of adhesion molecule expression, activation of NF-κB, up-regulation of Bcl-2, and activation of stress-activated protein kinase (for review, see refs. 2–8). LMP1 can also transform immortalized rodent fibroblast cell lines to loss of contact inhibition, lower serum dependence, anchorage independence, and tumorigenicity in nude mice (9–11).
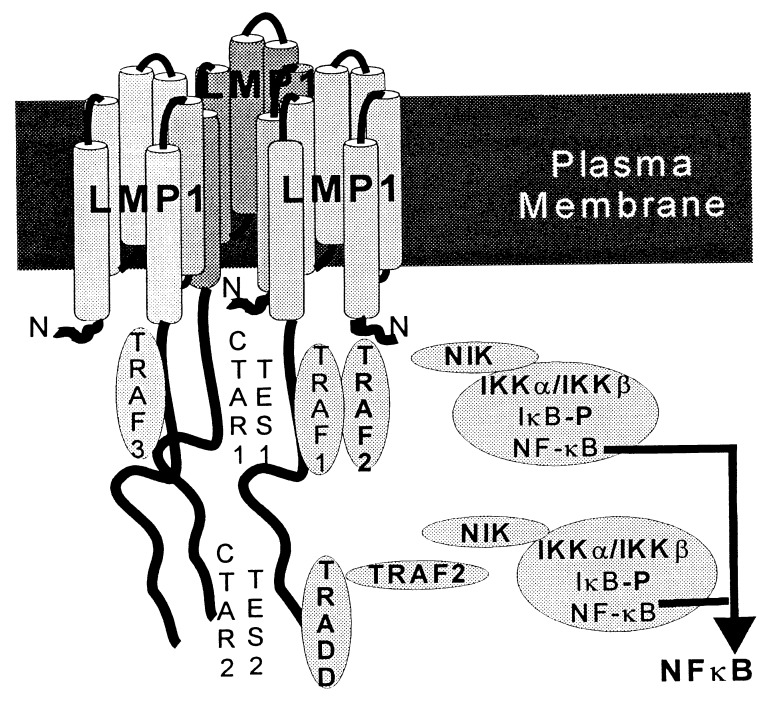
LMP1 is likely to alter cell growth by mimicking a constitutively activated tumor necrosis factor receptor (TNFR) similar to TNFRII and TNFRI, CD40, or CD30 (12–14). LMP1 has a short 24-amino acid cytoplasmic N terminus, six transmembrane domains connected by short turns, and a 200-amino acid cytoplasmic C terminus (CCT) (ref. 2 and Fig. 1). The cytoplasmic N terminus anchors the first transmembrane domain, and deletion of any specific codon does not ablate the ability of the mutated EBV recombinant to transform B lymphocytes into LCLs (15). The transmembrane domains enable LMP1 to aggregate in the plasma membrane. Aggregation in the plasma membrane is essential for activity because EBV recombinants that express a nonaggregating mutant LMP1 that diffusely distributes in the plasma membranes are unable to convert B lymphocytes into LCLs (1). Another nonaggregating mutant that diffusely distributes in all cytoplasmic membranes does not induce phenotypic changes in B lymphocytes or rodent fibroblasts or induce NF-κB (2, 4–6, 10, 11, 16). The LMP1 CCT is also essential for the transformation of resting B lymphocytes into LCLs (17). An EBV recombinant that expresses only the N terminus, the transmembrane domains, and the first 45 amino acids of the CCT can initiate B cell growth transformation and some of the infected cells can grow into long-term LCLs (17). These first 45 amino acids of the CCT include a site that can bind TNFR-associated factors (TRAFs) (12, 13, 18). These same 45 amino acids mediate a minor component of the LMP1-induced NF-κB activation and have been referred to as the C-terminal activation region 1 (CTAR1) (4, 5). A 27-codon deletion in LMP1 that includes this TRAF-binding site renders EBV recombinants incompetent for B lymphocyte growth transformation, consistent with the hypothesis that the TRAF binding site is a key transformation effector site (TES1; ref. 19).
Figure 1.
Schematic diagram of aggregated LMP1 in the cell plasma membrane. TRAF1, TRAF2, and TRAF3 constitutively associate with the LMP1-proximal NF-κB-activating region (CTAR1), which is also a key transformation effector site (TES1). TRADD constitutively associates with the LMP1 distal and major NF-κB-activating domain (CTAR2), which is a second transformation effector site (TES2). Aggregation of TRAF2 most likely activates NIK, which in turn phosphorylates and activates IKKα and IKKβ. IKKα and IKKβ then phosphorylate IκBα, leading to its ubiquitination and degradation and NF-κB activation.
A second transformation effector site (TES2) maps to the last few residues of the CCT (14). TES2 associates with the TNFR1 death domain interacting protein TRADD (14) and correlates with the major NF-κB-activating domain (4, 5, 14, 20). TES1 and TES2 are within the C-terminal NF-κB activation regions 1 and 2 (CTAR1 and CTAR2) that mediate 30% and 70%, respectively, of LMP1-induced NF-κB activation (5, 12, 16). Binding of TRAF1 and TRAF2 to TES1 mediates NF-κB activation from CTAR1, whereas TRADD binding to TES2 mediates NF-κB activation from CTAR2 (13, 14). The genetic linkage of LMP1 effector sites for cell growth transformation with effector sites for NF-κB activation and the key role of NF-κB in lymphocyte activation (21) are compatible with the working hypotheses that TRAFs and TRADD are key mediators of LMP1 effects in cell growth transformation and that NF-κB is an important component of those effects.
Pursuant to the linkage of NF-κB activation with LMP1 effects on B lymphocyte growth, we now further investigate the molecular mechanism through which LMP1 mediates NF-κB activation. TNF appears to induce NF-κB activation through a kinase cascade with two key elements: NF-κB-inducing kinase (NIK), a TRAF2-associated MAP3K homologue that is required for NF-κB activation by TNFRs and interleukin 1 (22), and IKKα/β, a NIK-associated IκBα kinase complex (23–27). To evaluate the role of this pathway in LMP1-induced NF-κB activation, we have studied the effect of dominant-negative mutants of NIK, IKKα, or IKKβ on LMP1-induced NF-κB activation.
MATERIALS AND METHODS
Plasmid Constructs.
The expression constructs of wild-type human NIK and a dominant negative “inactive” mutant of NIK with Lys-429 and Lys-430 mutated to Ala (NIK KK429–430AA) have been described (22). Full-length LMP1, LMP1 lacking the membrane distal NF-κB-activating domain (FLMPΔ232–386; referred to as F-LMPCTAR1), or LMP1 lacking the membrane-proximal NF-κB-activating domain (FLMPΔ188–351; referred to as F-LMPCTAR2) subcloned in pCDNA3 were obtained from W. Miller (28). pSG5-F-LMP1 is described elsewhere (13). F designates the presence of a FLAG epitope in the N terminus. Human IκBα and IκBα mutants were subcloned into pGEM4. The Myc-tagged wild-type IKKα and the dominant negative mutant IKKαK44A (M-IKKα and M-IKKαK44A; ref. 23) subcloned in pRK5 were obtained from M. Rothe and D. Goeddel.
Cloning of IKKβ.
The National Center for Biotechnology Information DNA database was screened for sequences similar to the IKKα sequence (23, 25). An expressed sequence tag (EST; GenBank accession no. W68756) was identified. The EST sequence aligned with the C terminus (amino acids 624–658) of IKKα. From this EST sequence, gene-specific primers were designed and used to amplify by PCR the cDNA(s) containing upstream sequences from a human B lymphoma cDNA library (Advantage cDNA polymerase mixture, CLONTECH). Two cDNA products whose sequences were similar to IKKα were fused with the downstream sequence in the EST clone (purchased from Genome Systems) to obtain pIKKβ-1 and pIKKβ-2. The sequences of pIKKβ-1 and pIKKβ-2 were later found to be identical to the corresponding regions of the human IKKβ sequence deposited in the GenBank database (accession no. AF029684) (24), except that pIKKβ-1 lacks the N-terminal 9 codons of IKKβ and pIKKβ-2 lacks the N-terminal 34 codons, the latter deletion including a portion of the predicted kinase domain. In addition, pIKKβ-2 contained the transition A → G at the second base of the codon 242 as compared with the published IKKβ sequence. The resulting IKKβ sequences from pIKKβ-1 and pIKKβ-2 were then fused in-frame downstream of the FLAG-epitope-encoding sequence in pCDNA3 to create pCDNA3-FLAG-IKKβΔ9 and pCDN3-FLAG-IKKβΔ34, respectively.
Transfections and Reporter Gene Assays.
Plasmid DNA was introduced into human embryonic kidney 293 cells by electroporation (6) or using the “superfect” kit (Qiagen, Chatsworth, CA). In the latter case, 24 h before transfection, 5 × 105 cells per well were seeded in six-well plates with 2 ml of DMEM containing 10% (D10) fetal bovine serum. Plasmid DNAs were mixed with 10 μl of superfect in 150 μl of DMEM without serum. After 10 min at room temperature, the transfection mixture was dripped to the wells. The medium was changed to D10 after 4 h. All transfections included an expression plasmid, 0.5 μg of a luciferase reporter gene containing three NF-κB sites (4), and 0.5 μg of a control β-galactosidase expression construct (pGKβgal) to normalize for transfection efficiency (6, 13). Sixteen to 24 h after transfection, cells were harvested and washed with PBS. Cell extracts were used to determine luciferase and β-gal activities, and protein expression level was determined by Western blotting.
Immune Precipitation and Western Blotting.
The 293 cells were harvested, washed once with D10 and once with PBS, transferred to a microcentrifuge tube, and incubated for 30 min on ice in 1 ml of lysis buffer [50 mM Tris⋅HCl, pH 7.4/150 mM NaCl/2 mM EDTA/0.5% Nonidet P-40/3% glycerol/1 mM phenylmethylsulfonyl fluoride/leupeptin (5 μg/ml)/aprotinin (2 μg/ml)]. Lysates were precleared with CL-6B Sepharose for 1 h and immunoprecipitated with anti-FLAG M2 beads (Kodak) for 1–2 h at 4°C. The M2 beads were used for Western blotting and for kinase assays. Proteins were separated by SDS/PAGE and were transferred to 0.45-μm (pore size) nitrocellulose sheets and detected by Western blotting with enhanced chemiluminescence. FLAG-tagged proteins were detected with the M5 mouse mAB (Eastman Kodak); non-FLAG tagged LMP1 was detected with mouse S12 anti-LMP (6).
In Vitro Kinase Assays.
The kinase reactions were in 50 μl of kinase assay buffer (50 mM Tris⋅HCl, pH 7.5/5 mM MgCl2/5 mM NaF/2 mM ATP) containing in vitro-translated 35S-labeled IκBα that was synthesized in rabbit reticulocyte lysate (TNT, Promega). Unlabeled ATP (20 μM) and [γ-32P]ATP (25 μCi) were included in the kinase reaction with IKKβΔ9. After incubation for 20 min or 1 h at 30°C, the reactions were terminated by adding 2× SDS sample buffer and boiling, followed by SDS/PAGE, Western blotting, and autoradiography.
For immunoprecipitated proteins, the M2 beads were washed three times with lysis buffer and once with kinase wash buffer (50 mM Tris⋅HCl, pH 7.5/5 mM MgCl2/5 mM NaF) before incubation in the kinase reaction buffer.
RESULTS
A Dominant-Negative NIK Mutant Inhibits LMP1-Induced NF-κB Activation.
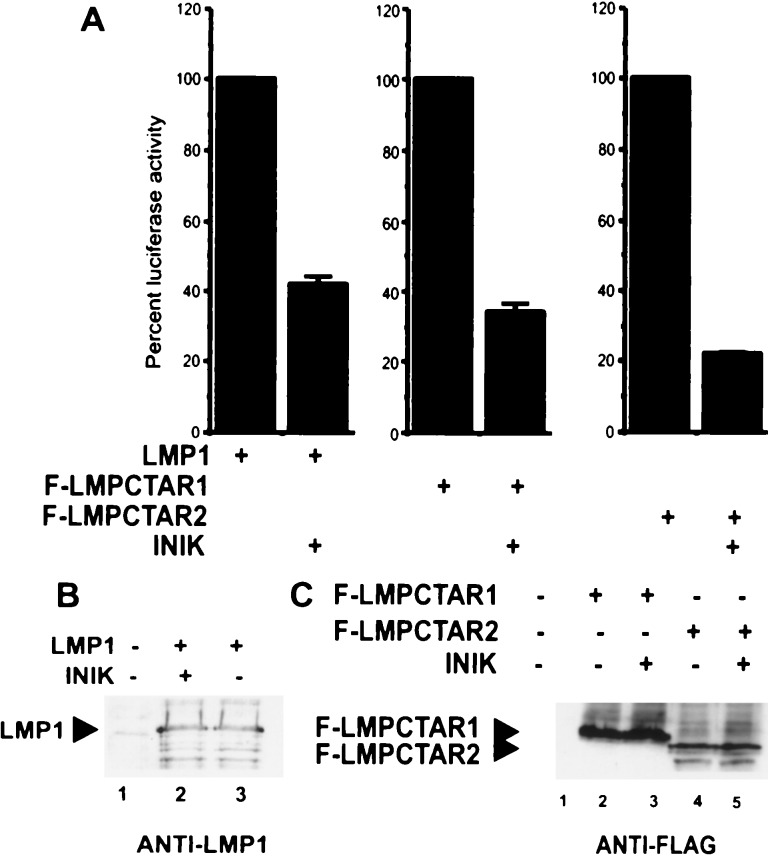
To determine whether NIK is a mediator of LMP1-induced NF-κB activation, we examined the effect of a dominant negative kinase-site mutant NIK (NIK KK429–430AA) on LMP1-induced NF-κB activation in 293 cells (Fig. 2A). Activation was measured through effects on a cotransfected luciferase reporter with three upstream NF-κB sites (4). In each experiment, LMP1 with a deletion of CTAR2 (LMPCTAR1) induced ≈25% of the total LMP1 effect, whereas LMP1 with a deletion of CTAR1 (LMPCTAR2) induced about 75% of the LMP1 effect. The dominant negative NIK inhibited 50% or more of the NF-κB-activation that was induced by full-length LMP1, by LMPCTAR1, or by LMPCTAR2 (Fig. 2A and data not shown). The inhibition of NF-κB activation by dominant negative NIK was not due to an effect of the dominant negative NIK on expression of the various LMP1 proteins (Fig. 2 B and C). These results suggest that NIK can substantially affect or mediate NF-κB activation by LMP1, LMPCTAR1, and CTAR2.
Figure 2.
Dominant negative mutant of protein kinase NIK (NIKKK429–430AA) inhibits NF-κB activation by LMP1. (A) A luciferase reporter plasmid with three upstream NF-κB binding sites was electroporated into 293 cells with a control β-galactosidase expression construct (GKβgal), and pCDNA3 (Invitrogen)-based expression plasmids for LMP1 (5 μg), F-LMPCTAR1 (10 μg), or F-LMPCTAR2 (10 μg) in the presence or absence of pCDNA3NIK KK429–430AA (referred as INIK) (10 μg). Luciferase activities were normalized for cotransfected β-galactosidase activity. Values of NF-κB activation in the presence of NIKKK429–430AA were normalized to the corresponding activities (set at 100%) obtained in the absence NIKKK429–430AA. Mean values (±SD) of relative luciferase activities from four experiments are shown. In these four experiments, NF-κB activation by full-length LMP1 was an average of 29-fold, whereas LMPCTAR1 induced 9-fold and LMPCTAR2 induced 19-fold NF-κB activation. (B) Western blot of LMP1 expression in the presence and absence of NIKKK429–430AA (INIK). LMP1 was detected with the S12 monoclonal antibody. (C) Western blot of F-LMPCTAR1 and F-LMPCTAR2 expression in the presence and absence of NIKKK429–430AA (INIK). FLAG-tagged proteins were detected with the M5 monoclonal anti-FLAG antibody.
NIK Induces IκBα Phosphorylation on Ser-32 and Ser-36 in Rabbit Reticulocyte Lysates.
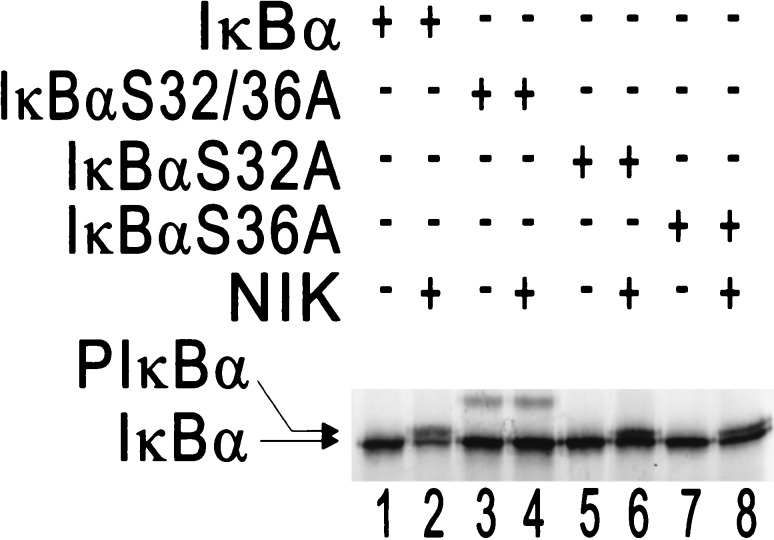
NIK activation by TNF treatment or overexpression leads to degradation of IκBα, NIK KK429–430AA blocks TNF- or interleukin 1-induced NF-κB activation (22), and phosphorylation of IκBα on Ser-32 and Ser-36 can signal its ubiquitination and degradation after TNF treatment (29). We sought to further analyze the role of NIK in NF-κB activation by evaluating whether NIK is capable of inducing phosphorylation of IκBα on Ser-32 and Ser-36, in vitro. Wild-type and Ser → Ala mutated forms of IκBα were incubated under standard kinase reaction conditions in reticulocyte lysate in the presence (Fig. 3, lanes 2, 4, 6, and 8) or absence of NIK (Fig. 3, lanes 1, 3, 5, and 7). NIK was able to induce phosphorylation of wild-type IκBα, IκBαS32A, and IκBαS36A, as judged by retarded mobility and calf intestinal phosphatase treatment (data not shown), but did not affect the mobility of IκBαS32/36A (mutated at both Ser-32 and Ser-36). These data indicate that NIK-induced phosphorylation of IκBα (as judged by gel retardation) is dependent on Ser-32 and Ser-36.
Figure 3.
NIK induces IκBα phosphorylation on Ser-32 and Ser-36. In vitro-translated 35S-labeled NIK and 35S-labeled wild-type IκBα, S32/36A, S32A, or S36A mutants were incubated in a standard kinase buffer and were analyzed by SDS/PAGE and autoradiography. The positions of IκBα, and phosphorylated IκBα (PIκBα) are indicated by arrows.
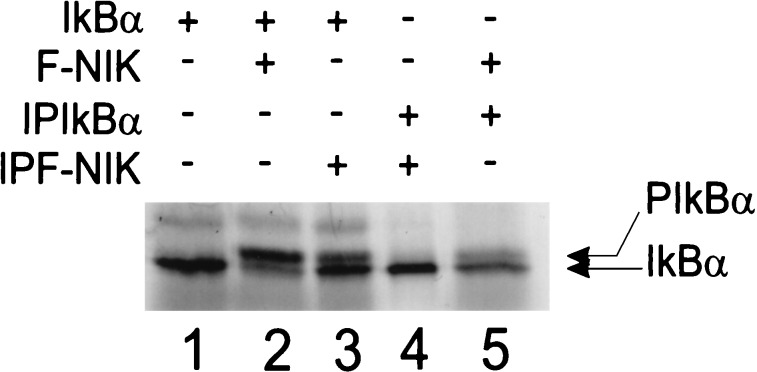
To determine whether NIK phosphorylates IκBα directly or requires other cellular factor(s) from the reticulocyte lysate, FLAG-tagged NIK (F-NIK) and IκBα were purified by immunoprecipitation and incubated in a standard kinase reaction. Incubation of in vitro-translated F-NIK with in vitro-translated IκBα in reticulocyte lysate resulted in IκBα phosphorylation (Fig. 4, compare lanes 1 and 2). In vitro-translated F-NIK could also phosphorylate immune affinity-purified in vitro-translated IκBα (Fig. 4, lane 5). However, immune affinity-purified F-NIK was unable to phosphorylate immune affinity-purified IκBα, even when the affinity-purified F-NIK was preincubated with reticulocyte lysate to restore it to an active phosphorylated state (Fig. 4, lane 4, and data not shown). Only when the immune affinity-purified, preincubated, and phosphorylated F-NIK was added to in vitro-translated IκBα in reticulocyte lysate was IκBα phosphorylated (Fig. 4, lane 3, and data not shown). These data indicate that phosphorylated NIK requires additional factor(s) from the reticulocyte lysate for IκBα phosphorylation.
Figure 4.
NIK phosphorylates IκBα in the presence but not in the absence of rabbit reticulocyte lysate. In vitro-translated 35S-labeled F-NIK and IκBα in reticulocyte lysate or, after immunoprecipitation from reticulocyte lysate (IPIκBα + IPF-NIK), were incubated in a standard kinase buffer containing 2.5 μM okadaic acid, and samples were then analyzed by SDS/PAGE and autoradiography.
Dominant Negative IKKα or IKKβ Inhibit LMP1-Induced NF-κB Activation.
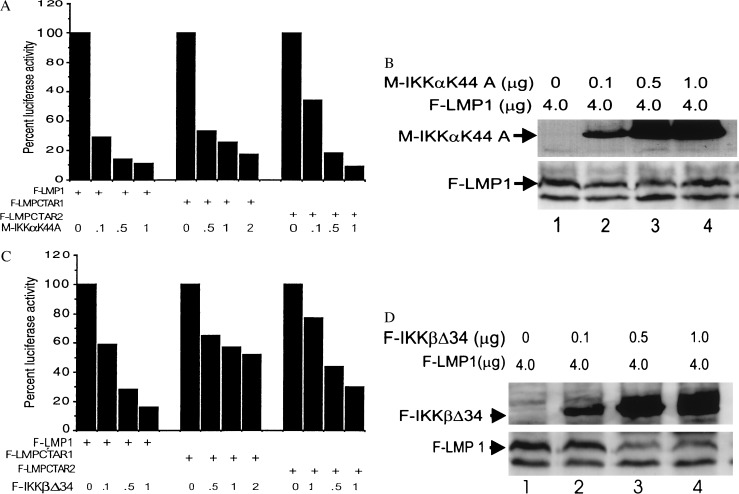
While these studies were in progress, IKKα was identified as a NIK-interacting kinase and as part of an IκB kinase complex involved in NF-κB activation (23, 25). IKKβ was identified as a homologue of IKKα and as a component of the high molecular weight complex that phosphorylates IκBα in response to treatment of cells with TNF-α (24, 26, 27). To evaluate the role of IKKα and IKKβ in LMP1-induced NF-κB activation, we examined the effect of dominant negative IKKα or IKKβ on LMP1-induced NF-κB activation in 293 cells. The dominant negative IKKα (IKKαK44A) that inhibited TNF-induced NF-κB activation (23, 25) also inhibited LMP1-, LMPCTAR1-, and LMPCTAR2-mediated NF-κB activation (Fig. 5A). The inhibition was dose-dependent and was not attributable to a direct effect of IKKαK44A on LMP1 expression because the levels of wild-type or mutant LMP1 proteins were not significantly affected by IKKαK44A expression (Fig. 5B and data not shown for LMPCTAR1 and LMPCTAR2). Thus, IKKα affects or mediates LMP1-, LMPCTAR1-, or LMPCTAR2-induced NF-κB activation.
Figure 5.
Effects of dominant negative mutants of IKKα (M-IKKαK44A) (A and B) or IKKβ (F-IKKβΔ34) (C and D) on LMP1-, LMPCTAR1-, or LMPCTAR2-induced NF-κB activation in 293 cells. F-LMP1 (4 μg) in pSG5, F-LMPCTAR1(0.5 μg), or F-LMPCTAR2 (1 μg) in pCDNA3 were cotransfected with a 3XκB luciferase reporter gene plasmid, a β-galactosidase expression construct, and increasing amounts (0.1–2 μg) of pRK5-myc-IKKαK44A, pCDN3-F-IKKβΔ34, or pCDNA3, a vector control. Because of the high expression of F-LMPCTAR1 relative to F-LMP1 and LMPCTAR2, 0.5–2 μg of myc-IKKαK44A and IKKβΔ34 were cotransfected with LMPCTAR1. Luciferase activities were normalized for β-galactosidase activities. NF-κB activation by LMP1 constructs cotransfected with IKKαK44A or IKKβΔ34 were normalized to the activities (set at 100%) obtained in the absence of IKKαK44A or IKKβΔ34. In these experiments LMP1-induced NF-κB activation averaged 76-fold, whereas LMPCTAR1-induced activation averaged 17-fold and LMPCTAR2-induced activation averaged 53-fold. Representative relative luciferase activities from at least two experiments are shown. Western blot analysis of F-LMP1, myc-IKKαK44A (B), or F-IKKβΔ34 (D) expression levels. After protein transfer, the membranes were sequentially probed with monoclonal antibodies to detect FLAG (M5) or to detect M-IKKαK44A (9E10).
To investigate the role of IKKβ in LMP1-induced NF-κB activation, two IKKβ cDNA clones were isolated from a human B cell cDNA library. One cDNA, IKKβΔ9, is missing nine N-terminal codons and activated the NF-κB-dependent reporter 15-fold (data not shown), similar to full-length IKKβ (23). A second cDNA, IKKβΔ34, is missing 34 N-terminal residues, including the ATP-binding site. IKKβΔ34 did not activate NF-κB and inhibited TNF-induced activation of NF-κB in transfected 293 cells (data not shown). IKKβΔ34 also inhibited NF-κB activation by full-length LMP1 by 80%, by LMPCTAR1 by approximately 50%, and by LMPCTAR2 by 70% (Fig. 5C). The inhibitions were dose-dependent and did not reach saturation at the highest level of transfected DNA tested (Fig. 5D and data not shown). The more modest effect of IKKβΔ34 on LMPCTAR1-induced NF-κB activation is unlikely to be due to a substantive difference in the role of IKKβ in LMPCTAR1 versus LMPCTAR2 downstream pathways and is more likely due to the higher level of LMPCTAR1 expression compared with LMPCTAR2 (Fig. 2C and data for each experiment not shown). Consistent with this hypothesis, NF-κB activation induced by smaller amounts of LMPCTAR1 expression vector was 80% inhibited by IKKβΔ34 (data not shown). These results indicate that IKKβ also affects or mediates LMP1, LMPCTAR1, and LMPCTAR2 induced NF-κB activation.
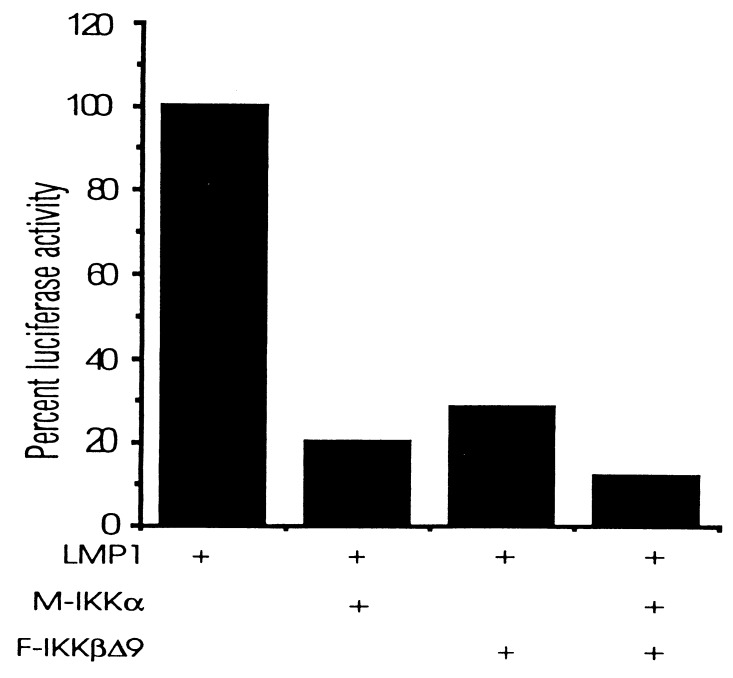
Overexpression of wild-type IKKα or IKKβ potentiates TNF-induced NF-κB activation in 293 cells, and this is consistent with their role in mediating TNF-induced NF-κB activation (23–27). Although overexpression of IKKα or IKKβ in 293 cells resulted in NF-κB activation (data not shown), high-level IKKα or IKKβ coexpression with LMP1 resulted in less NF-κB activation than was induced by LMP1 alone (Fig. 6). In contrast, lower-level expression of IKKα and/or IKKβ had neither an enhancing nor an inhibiting effect on LMP1-induced NF-κB activation (data not shown). Despite its negative effects on LMP1-induced NF-κB activation, Flag-tagged IKKβ immunoprecipitated from 293 cells was able to phosphorylate IκBα in kinase buffer, in vitro. IKKβ immunoprecipitated from LMP1 expressing cells was not more active in phosphorylating IκBα in vitro than IKKβ from LMP1-negative 293 cells (data not shown). Thus, these results are consistent with IKKα and IKKβ being important mediators of LMP1-induced NF-κB activation but raise questions about the precise stoichiometry or regulation of this pathway (see Discussion).
Figure 6.
IKKα and IKKβΔ9 inhibit LMP1-induced NF-κB activation. Luciferase reporter assays are as described in Fig. 5. Cells were transfected with 0.5 μg of expression pCDNA3-LMP1, pRK5-myc-IKKα, and pCDNA3-F-IKKβΔ9.
DISCUSSION
TNF signaling through TNFRI has been well characterized in epithelial cells, such as the 293 cells that we used (22–27, 29–37). In such cells TNF induces trimerization of TNFRI. TNFRI associates with TRADD and RIP. TRADD and RIP associate with TRAF1 and 2. TRAF2 associates and probably activates NIK. NIK activates IKKα and IKKβ. IKKα and IKKβ phosphorylate IκBα, which is then ubiquitinated and degraded, releasing active NF-κB. Although epithelial cells primarily express TNFRI, cells of bone marrow origin primarily express TNFRII. TNF also induces trimerization of TNFRII and the downstream pathways appear to only differ in the ability of trimerized TNFRII to directly engage TRAF1 and TRAF2, rather than indirectly engaging TRAF1 and TRAF2 through TRADD and RIP (22, 38, 39). For both TNFRI and TNFRII, NIK activation appears to be mediated by TRAF2 (22). Our in vitro observations that NIK indirectly induces phosphorylation of IκBα on Ser-32 and Ser-36 are consistent with and extend previous data showing that NIK induces IκBα phosphorylation and activates NF-κB in transfected 293 cells (22).
The experiments described herein indicate that the EBV-transforming protein LMP1 induces NF-κB activation through a NIK-, IKKα-, and IKKβ-kinase-dependent pathway (Fig. 1). Dominant negative forms of NIK, IKKα, or IKKβ blocked LMP1-induced NF-κB activation without affecting LMP1 expression. Because NIK and the IκBα kinases are implicated in the pathways through which TNF induces NF-κB activation, these results are consistent with previous evidence that LMP1 signals through cellular pathways that are ordinarily activated by TNF and other TNF family ligands.
LMP1 activates NF-κB from CTAR1 by associating with TRAF1 and TRAF2 and from CTAR2 by associating with TRADD (12–14, 18), in striking parallel to both TNFRII and TNFRI pathways. Because of the previous evidence linking TRAF1, TRAF2, and TRADD to LMPCTAR1- and CTAR2-mediated NF-κB activation, we anticipated that LMPCTAR1- and CTAR2-induced NF-κB activation would be inhibited by coexpression of dominant negative forms of NIK, IKKα, or IKKβ. Indeed, NIK KK429–430AA inhibited LMP1-, LMPCTAR1-, or LMPCTAR2-induced NF-κB activation by at least 50% and did not inhibit LMPCTAR1 or LMPCTAR2 expression, indicating that NIK is a mediator of LMPCTAR1- and LMPCTAR2-induced NF-κB activation in 293 cells.
Because activated NIK complexes with and activates IKKα and to a lesser extent IKKβ (23–27, 36, 37), which then phosphorylate IκBα, expression of dominant negative forms of IKKα or IKKβ also blocked LMPCTAR1- and CTAR2-mediated NF-κB activation. However, LMPCTAR1-induced NF-κB activation was somewhat more resistant to IKKβΔ34 blockage than was LMPCTAR2-induced NF-κB activation. This effect was attributed to the higher level of LMPCTAR1 expression versus LMPCTAR2. Indeed, lower-level expression of LMPCTAR1 resulted in substantial blockage by IKKβΔ34. However, LMPCTAR1 was also less inhibited by IKKβΔ34 than it was by IKKα K44A. This latter effect may be due to a differential low expression of IKKβΔ34 versus IKKα K44A or to a larger pool size of wild-type IKKβ. Consistent with these possibilities, IKKβΔ34 was also less effective than IKKα K44A in inhibiting LMP1 and LMPCTAR2 as well as LMPCTAR1. Attempts to further investigate potential differences in expression levels between IKKβΔ34 and IKKα K44A have so far been unrevealing, in part due to the different epitope tags. The similar effects of dominant negative NIK, IKKα, and IKKβ on NF-κB activation mediated by LMPCTAR1 and LMPCTAR2 are altogether most consistent with the hypothesis that the fundamental differences between the effects of CTAR1/TES1 and CTAR2/TES2 on cell growth and gene activation are due to differences in biochemical interactions upstream of NIK, IKKα, and IKKβ activation.
Given the similarity of LMP1 to TNFRI and TNFRII and the previous finding that IKKα or IKKβ overexpression stimulated TNF-mediated NF-κB activation, we were surprised that overexpression of wild-type IKKα or IKKβ inhibited LMP1-induced NF-κB activation and that LMP1 expression did not potentiate IKK-induced phosphorylation of IκBα. One explanation for these results could be the possibility that high-level expression of these kinases may alter the normal stoichiometry of the signal transduction cascade. For example, overexpression of IKKα or IKKβ may sequester key regulatory factor(s) necessary for NF-κB activation. Alternatively, IKKα and IKKβ may already be in relative molar excess versus IκBα and overexpression simply increases interaction with other targets or regulators of these kinases without positively affecting the efficiency of IκBα phosphorylation. LMP1 may differ in these effects from TNFRs because of their association with other putative signaling molecules (40, 41), which may indirectly affect NF-κB activation.
The summary drawing of LMP1 induced NF-κB activation (Fig. 1) includes several areas of uncertainty. For example, although TRAFs and TRADD are constitutively associated with LMP1 in EBV-transformed B lymphocytes or transfected 293 cells (12, 13, 18), NIK, IKKα, and IKKβ are likely to be in a separate neighboring complex. By immune microscopy, TRAFs significantly colocalize to LMP1 patches in the plasma membrane (12, 18), whereas IKKα or IKKβ are much less LMP1-associated and are evident throughout the cytoplasm (data not shown). Further, although TRAFs or TRADD readily coimmunoprecipitate with LMP1 (12, 13, 14, 18), we have been unable to coimmunoprecipitate NIK, IKKα, or IKKβ with LMP1 from cells cotransfected with expression vectors for LMP1 and epitope-tagged NIK, IKKα, or IKKβ.
In summary, these experiments indicate that NIK, IKKα, and IKKβ are effectors of NF-κB activation from CTAR1/TES1 and CTAR2/TES2 and confirm the functional similarity of these sites to the TRAF and TRADD binding domains of TNFRII and TNFRI, respectively. However, although LMPCTAR2/TES2 associates with TRADD and propagates robust NF-κB activation presumably through the recruitment of TRAF1 and TRAF2 to the TRADD N terminus, LMPCTAR2/TES2 interacts with only a part of the TRADD death domain and does not propagate a discernable proapoptotic signal through TRADD (14). Thus, LMP1 differs partially from TNFRI in TRADD-mediated signaling.
Acknowledgments
We thank other members the Kieff laboratory for their helpful advice and comments, K. Lee and M. Barkett for IκBα expression plasmids, Drs. M. Rothe and D. Goeddel for IKKα expression plasmids, and Dr. W. Miller for LMP1 expression plasmids. This work was supported by Public Health Service Grant CA47006 (to E.K.) and CA71705 (to G.M.) and by the Council for Tobacco Research (to T.D.G.). B.S.S. is a visiting fellow from the International Agency for Research on Cancer, Lyon, France.
ABBREVIATIONS
- TNFR
tumor necrosis factor receptor
- TRAF
TNFR-associated factor
- TRADD
TNFR1 death domain interacting protein
- NIK
NF-κB-inducing kinase
- IKK
1κB kinase
- EBV
Epstein–Barr virus
- LMP1
latent infection membrane protein 1
- LCL
lymphoblastoid cell line
- CCT
cytoplasmic C terminus
- CTAR
C-terminal activation region
- TES
transformation effector site
- EST
expressed sequence tag
References
- 1.Kaye K, Izumi K, Kieff E. Proc Natl Acad Sci USA. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kieff E. In: in Fields Virology. Fields B N, Knipe D M, Howley P M, editors. Philadelphia: Lippincott-Raven; 1996. pp. 2343–2396. [Google Scholar]
- 3.Laherty C, Hu H, Opipari A, Wang F, Dixit V. J Biol Chem. 1992;267:24157–24160. [PubMed] [Google Scholar]
- 4.Mitchell T, Sugden B. J Virol. 1995;69:2968–2976. doi: 10.1128/jvi.69.5.2968-2976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huen D S, Henderson S A, Croom C D, Rowe M. Oncogene. 1995;10:549–560. [PubMed] [Google Scholar]
- 6.Hatzivassiliou E, Miller W E, Raab-Traub N, Kieff E, Mosialos G. J Immunol. 1998;160:1116–1121. [PubMed] [Google Scholar]
- 7.Kieser A, Kilger E, Gries O, Ueffing M, Kolch W, Hammerschmidt M. EMBO J. 1997;16:6478–6485. doi: 10.1093/emboj/16.21.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliopoulos A G, Young L S. Oncogene. 1998;16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Liebowitz D, Kieff E. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Liebowitz D, Kieff E. J Virol. 1988;62:2337–2346. doi: 10.1128/jvi.62.7.2337-2346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baichwal V R, Sugden B. Oncogene. 1989;4:67–74. [PubMed] [Google Scholar]
- 12.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 13.Devergne O, Hatzivassilliou E, Izumi K M, Kaye K M, Kleijnen M J, Kieff E D, Mosialos G. Mol Cell Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izumi K M, Kieff E D. Proc Natl Acad Sci USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izumi K, Kaye K, Kieff E. J Virol. 1994;68:4369–4376. doi: 10.1128/jvi.68.7.4369-4376.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liebowitz D, Mannick J, Takada K, Kieff E. J Virol. 1992;66:4612–4616. doi: 10.1128/jvi.66.7.4612-4616.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaye K, Izumi K, Mosialos G, Kieff E. J Virol. 1995;69:675–683. doi: 10.1128/jvi.69.2.675-683.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaye K M, Harada J, Devergne O, Isumi K M, Yalamanchili R, Kieff E D, Mosialos G. Proc Natl Acad Sci USA. 1996;93:11085–11090. doi: 10.1073/pnas.93.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izumi K M, Kaye K M, Kieff E D. Proc Natl Acad Sci USA. 1997;94:1447–1452. doi: 10.1073/pnas.94.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floettman J, Rowe M. Oncogene. 1997;15:1851–1858. doi: 10.1038/sj.onc.1201359. [DOI] [PubMed] [Google Scholar]
- 21.Sha W, Liou H, Tuomanen E, Baltimore D. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 22.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. Nature (London) 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 23.Regnier C, Song H, Gao X, Goeddel D, Cao Z, Rothe M. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 24.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 25.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. Nature (London) 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 26.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J W, Young D B, Barbosa M, Mann M. Science. 1997;278:860–886. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 27.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 28.Miller W E, Mosialos G, Kieff E D, Rabb-Traub N. J Virol. 1997;71:586–594. doi: 10.1128/jvi.71.1.586-594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z J, Parent L, Maniatis T. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 30.Stancovski I, Baltimore D. Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 31.Tartaglia L, Goeddel D. J Biol Chem. 1992;267:4304–4307. [PubMed] [Google Scholar]
- 32.Hsu H, Xiong J, Goeddel D. Cell. 1994;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 33.Hsu H, Huang J, Shu H, Baichwall V, Goeddel D. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 34.Ting A, Pimentel-Muinos F, Seed B. EMBO J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 35.Kelliher M, Grimm S, Ishida Y, Kuo F, Stanger B, Leder P. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 36.Ling L, Cao Z, Goeddel D V. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K. Proc Natl Acad Sci USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothe M, Wong W, Henzel J, Goeddel D. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 39.Rothe M, Sarma V, Dixit V, Goeddel D. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 40.Song H, Dunbar J, Zhang Y, Guo D, Donner D. J Biol Chem. 1995;270:3574–3581. [PubMed] [Google Scholar]
- 41.Schievella A, Chen J, Graham J, Lin L. J Biol Chem. 1996;272:12069–12076. doi: 10.1074/jbc.272.18.12069. [DOI] [PubMed] [Google Scholar]