Abstract
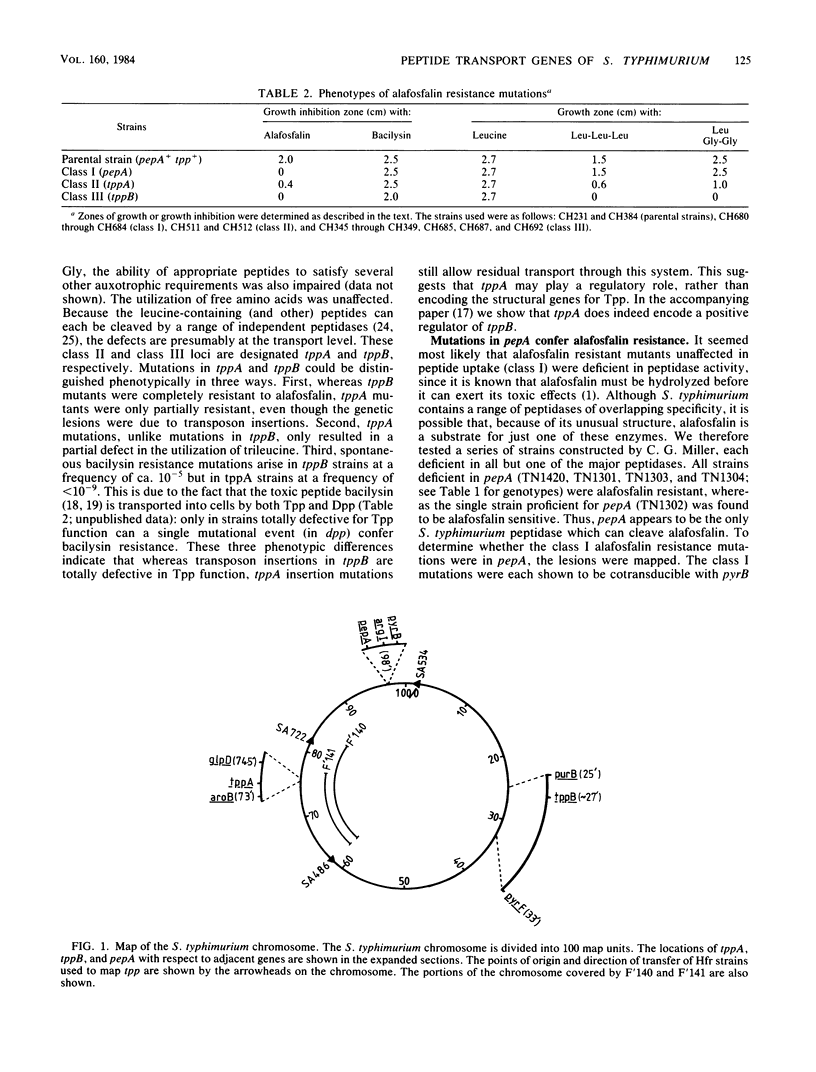
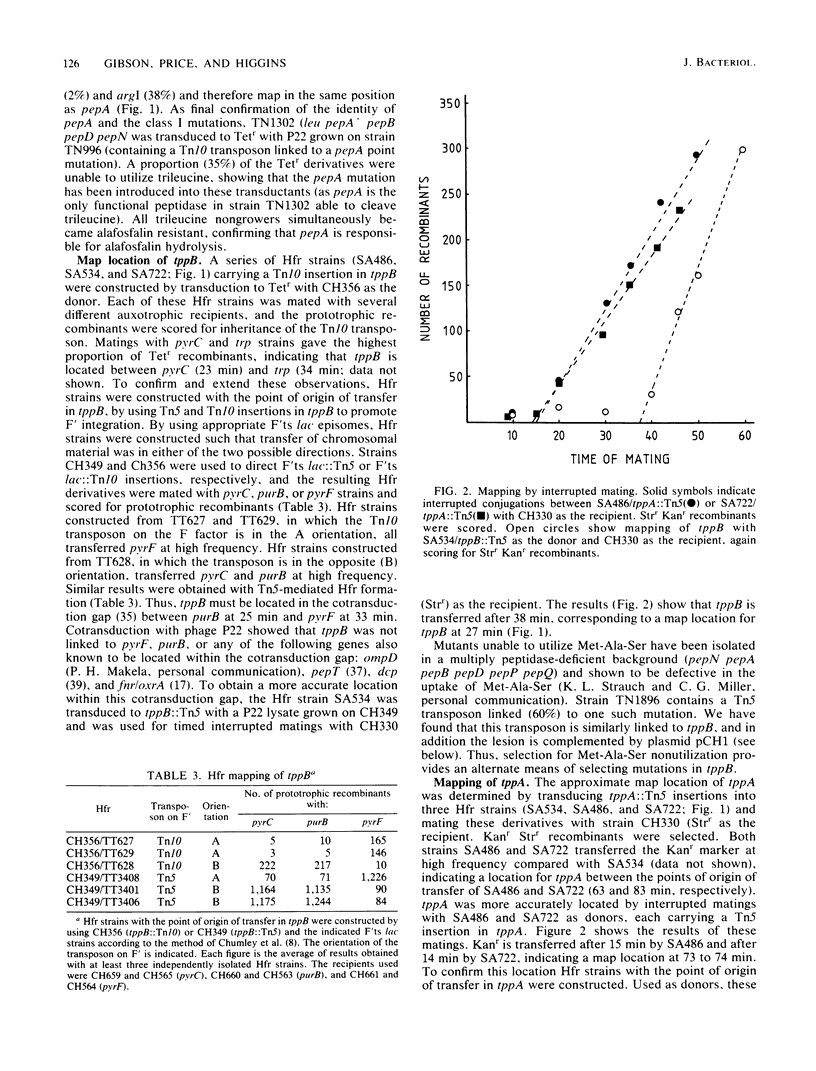
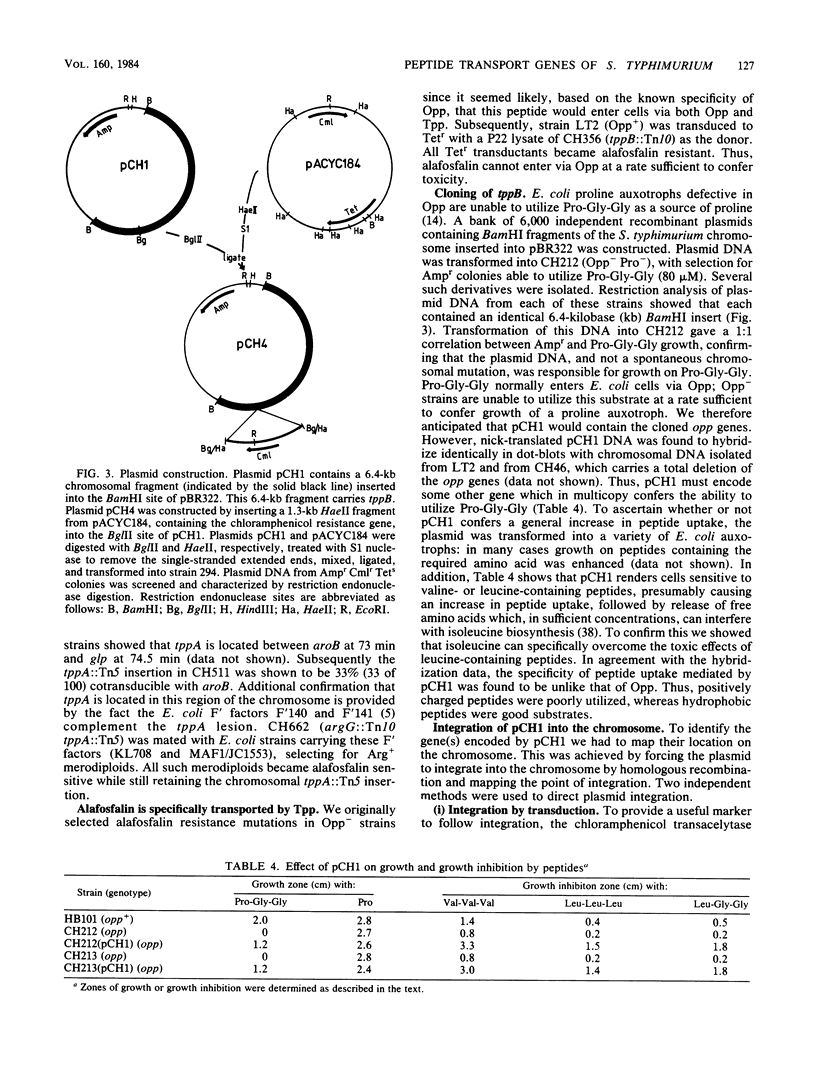
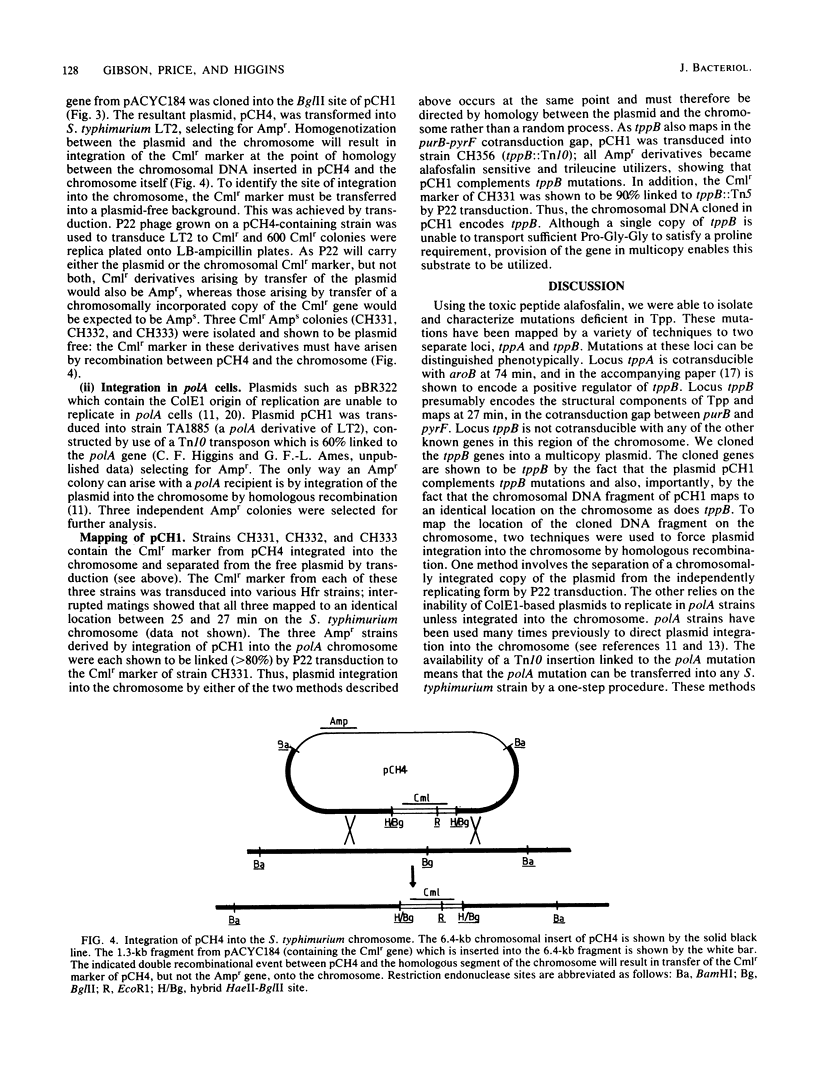
Of the three bacterial peptide transport systems only one, the oligopeptide permease, has been characterized in any detail. We have now isolated Salmonella typhimurium mutants deficient in a second transport system, the tripeptide permease (Tpp), using the toxic peptide alafosfalin. Alafosfalin resistance mutations map at three loci, the gene encoding peptidase A (pepA) and two transport-defective loci, tppA and tppB. Locus tppA has been mapped to 74 min on the S. typhimurium chromosome, cotransducible with aroB, and is a positive regulator of tppB. Locus tppB maps at 27 min in the cotransduction gap between purB and pyrF. We cloned tppB, the structural locus for the tripeptide permease. Two simple methods are described for mapping the location of cloned DNA fragments on the chromosome of S. typhimurium.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. G., Atherton F. R., Hall M. J., Hassall C. H., Holmes S. W., Lambert R. W., Nisbet L. J., Ringrose P. S. Phosphonopeptides, a new class of synthetic antibacterial agents. Nature. 1978 Mar 2;272(5648):56–58. doi: 10.1038/272056a0. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Ames G. F., Young J. D., Tsuchiya D., Lecocq J. Illicit transport: the oligopeptide permease. Proc Natl Acad Sci U S A. 1973 Feb;70(2):456–458. doi: 10.1073/pnas.70.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshir F., Higgins C. F., Ames G. F. Physical map of the Salmonella typhimurium histidine transport operon: correlation with the genetic map. J Bacteriol. 1981 Aug;147(2):401–409. doi: 10.1128/jb.147.2.401-409.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton F. R., Hall M. J., Hassall C. H., Lambert R. W., Lloyd W. J., Lord A. V., Ringrose P. S., Westmacott D. Phosphonopeptides as substrates for peptide transport systems and peptidases of Escherichia coli. Antimicrob Agents Chemother. 1983 Oct;24(4):522–528. doi: 10.1128/aac.24.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Z., Gilbarg C. Specialized peptide transport system in Escherichia coli. J Bacteriol. 1975 Jun;122(3):1200–1207. doi: 10.1128/jb.122.3.1200-1207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney J., Higgins C. F., Booth I. R. Proline uptake through the major transport system of Salmonella typhimurium is coupled to sodium ions. J Bacteriol. 1984 Oct;160(1):22–27. doi: 10.1128/jb.160.1.22-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickel T. E., Gilvarg C. Transport of impermeant substances in E. coli by way of oligopeptide permease. Nat New Biol. 1973 Feb 7;241(110):161–163. doi: 10.1038/newbio241161a0. [DOI] [PubMed] [Google Scholar]
- Greener A., Hill C. W. Identification of a novel genetic element in Escherichia coli K-12. J Bacteriol. 1980 Oct;144(1):312–321. doi: 10.1128/jb.144.1.312-321.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick D., Calvo J. M., Klopotowski T., Ames B. N. Compounds which serve as the sole source of carbon or nitrogen for Salmonella typhimurium LT-2. J Bacteriol. 1969 Oct;100(1):215–219. doi: 10.1128/jb.100.1.215-219.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterson N. I., Koshland D. E., Jr Replacement and amplification of bacterial genes with sequences altered in vitro. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4894–4898. doi: 10.1073/pnas.80.16.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Hardie M. M., Jamieson D., Powell L. M. Genetic map of the opp (Oligopeptide permease) locus of Salmonella typhimurium. J Bacteriol. 1983 Feb;153(2):830–836. doi: 10.1128/jb.153.2.830-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth B. G., Higgins C. F. Genetic organization of the oligopeptide permease (opp) locus of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1983 Mar;153(3):1548–1551. doi: 10.1128/jb.153.3.1548-1551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Anaerobic and leucine-dependent expression of a peptide transport gene in Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):131–136. doi: 10.1128/jb.160.1.131-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenig M., Abraham E. P. Antimicrobial activities and antagonists of bacilysin and anticapsin. J Gen Microbiol. 1976 May;94(1):37–45. doi: 10.1099/00221287-94-1-37. [DOI] [PubMed] [Google Scholar]
- Kenig M., Vandamme E., Abraham E. P. The mode of action of bacilysin and anticapsin and biochemical properties of bacilysin-resistant mutants. J Gen Microbiol. 1976 May;94(1):46–54. doi: 10.1099/00221287-94-1-46. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. DNA polymerase as a requirement for the maintenance of the bacterial plasmid colicinogenic factor E1. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1538–1544. doi: 10.1016/0006-291x(70)90562-0. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Chan R. K., Tye B. K., Botstein D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J Mol Biol. 1975 Oct 5;97(4):561–575. doi: 10.1016/s0022-2836(75)80059-3. [DOI] [PubMed] [Google Scholar]
- Miller C. G. Gentic mapping of Salmonella typhimurium peptidase mutations. J Bacteriol. 1975 Apr;122(1):171–176. doi: 10.1128/jb.122.1.171-176.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Mackinnon K. Peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):355–363. doi: 10.1128/jb.120.1.355-363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naider F., Becker J. M. Multiplicity of oligopeptide transport systems in Escherichia coli. J Bacteriol. 1975 Jun;122(3):1208–1215. doi: 10.1128/jb.122.3.1208-1215.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. Size restriction on peptide utilization in Escherichia coli. J Biol Chem. 1968 Dec 10;243(23):6291–6299. [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. The role of the terminal carboxyl group on peptide transport in Escherichia coli. J Biol Chem. 1968 Jan 25;243(2):335–340. [PubMed] [Google Scholar]
- Payne J. W. Oligopeptide transport in Escherichia coli. Specificity with respect to side chain and distinction from dipeptide transport. J Biol Chem. 1968 Jun 25;243(12):3395–3403. [PubMed] [Google Scholar]
- Payne J. W. Peptides and micro-organisms. Adv Microb Physiol. 1976;13:55–113. doi: 10.1016/s0065-2911(08)60038-7. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. A method for detection of phage mutants with altered transducing ability. Mol Gen Genet. 1971;110(4):378–381. doi: 10.1007/BF00438281. [DOI] [PubMed] [Google Scholar]
- Strauch K. L., Miller C. G. Isolation and characterization Salmonella typhimurium mutants lacking a tripeptidase (peptidase T). J Bacteriol. 1983 May;154(2):763–771. doi: 10.1128/jb.154.2.763-771.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavori H., Kimmel Y., Barak Z. Toxicity of leucine-containing peptides in Escherichia coli caused by circumvention of leucine transport regulation. J Bacteriol. 1981 May;146(2):676–683. doi: 10.1128/jb.146.2.676-683.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., Miller C. G. Dipeptidyl carboxypeptidase-deficient mutants of Salmonella typhimurium. J Bacteriol. 1983 Mar;153(3):1252–1258. doi: 10.1128/jb.153.3.1252-1258.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



