Abstract
The utility of modified vaccinia virus Ankara (MVA) as a vector for eliciting AIDS virus-specific cytotoxic T lymphocytes (CTL) was explored in the simian immunodeficiency virus (SIV)/rhesus monkey model. After two intramuscular immunizations with recombinant MVA-SIVSM gag pol, the monkeys developed a Gag epitope-specific CTL response readily detected in peripheral blood lymphocytes by using a functional killing assay. Moreover, those immunizations also elicited a population of CD8+ T lymphocytes in the peripheral blood that bound a specific major histocompatibility complex class I/peptide tetramer. These Gag epitope-specific CD8+ T lymphocytes also were demonstrated by using both functional and tetramer-binding assays in lymph nodes of the immunized monkeys. These observations suggest that MVA may prove a useful vector for an HIV-1 vaccine. They also suggest that tetramer staining may be a useful technology for monitoring CTL generation in vaccine trials in nonhuman primates and in humans.
With accumulating evidence that virus-specific cytotoxic T lymphocytes (CTLs) are important in containing the spread of HIV-1 in infected individuals, a consensus has emerged that an HIV-1 vaccine should stimulate the generation of CTLs (1). This requirement has posed a number of challenges for HIV-1 vaccine development. A safe vaccine approach that induces high frequency, durable HIV-1-specific CTL responses has proven elusive. Moreover, because traditional methods for measuring target cell lysis are cumbersome and difficult to quantify, monitoring the efficiency of vaccine-elicited HIV-1-specific CTL generation has been problematic.
The induction of a specific CTL response generally requires the intracellular synthesis, processing, and cell-surface presentation of a short peptide in association with a major histocompatability complex (MHC) class I molecule. The ability of recombinant vaccinia viruses to efficiently prime and stimulate CTL have made them powerful research tools (2, 3). However, such replication-competent laboratory strains may be unsuited for use as vaccines, particularly in groups that are at high risk for HIV infection. Fortunately, highly attenuated strains of vaccinia virus exist. One such strain, modified vaccinia virus Ankara (MVA), acquired defects in its ability to replicate in human and most mammalian cells during >500 consecutive passages in chicken embryo fibroblasts (4–6). Remarkably, expression of recombinant proteins by MVA during an abortive infection of human cells equaled that of a fully replication competent vaccinia virus (7). Moreover, recombinant MVA-induced antibody and CTL responses were at least as good as or better than those elicited by replicating vaccinia viruses in protectively immunizing experimental animals against pathogenic viruses including influenza virus, paramyxovirus, and simian immunodeficiency virus (8–10). These observations suggest that a recombinant MVA may prove advantageous as a component of an HIV-1 vaccine.
Extensive cell manipulation and lengthy periods of in vitro cell cultivation have contributed to difficulties in evaluating CTL function by using traditional killing assays. However, the report that fluorescence dye-coupled tetrameric MHC class I-peptide complexes can specifically bind to subpopulations of epitope-specific CD8+ T cells has raised the possibility that CTL may be studied by using flow cytometry (11). In fact, recent experiments in murine systems and in humans have indicated the power and precision of this approach for analyzing virus-specific CTL (12–15). It also has proven useful in assessing Gag-specific CTL in the peripheral blood of simian immunodeficiency virus (SIV)-infected rhesus monkeys (16).
In the present study, we have evaluated the ability of a recombinant MVA-SIVSM gag pol construct to elicit SIVSM Gag-specific CTL in rhesus monkeys. We demonstrate the vaccine induction of a CTL response specific for a dominant SIVSM Gag epitope in these animals by using both functional and tetrameric MHC class I-peptide-binding assays. Moreover, we show that these CTL were present in both the peripheral blood and lymph nodes of vaccinated monkeys.
MATERIALS AND METHODS
Selection of Mamu-A*01+ Rhesus Monkeys.
Rhesus monkeys expressing the Mamu-A*01 MHC class I molecule were selected by MamuA*01-specific reverse transcription-PCR of total RNA isolated by RNeasy purification (Qiagen, Chatsworth, CA) from 5 × 106 peripheral blood mononuclear cells. Briefly, cDNA was synthesized from 5 mg of RNA in a 100-ml reaction containing 50 mM KCl, 10 mM Tris (pH 8.3), 5 mM MgCl2, 1 mM each of dATP, dCTP, dGTP, and dTTP, 2.5 mM oligo-d(T) 16, 50 units of RNase inhibitor, and 75 units of MuLV reverse transcriptase (GeneAmp RNA PCR Core Kit, Perkin–Elmer, Foster City, CA). cDNA was synthesized at 25°C for 15 min and at 42°C for 45 min followed by enzyme inactivation at 99°C for 10 min. PCR amplifications were then performed by using 20 μl of these cDNA in Hot Start reactions containing 2 mM MgCl2, 50 mM KCl, 10 mM Tris (pH 8.3), 0.5 mM of each primer, AmpliWax Gem 100, and 5 units of AmpliTaq DNA polymerase (Perkin–Elmer). The reaction was heated to 94°C for 2 min and amplified through 45 cycles: 40 sec at 90°C, 20 sec at 69°C, and 1 min at 72°C with a final extension for 7 min. Two reactions were performed with each cDNA. The first reaction used primers unique for Mamu-A*01 sequence (forward 5′-GGCGGGCTCTCACTCCATGAA-3′ and reverse 5′-ATGTGTCTTGGGGGGGTCCGT-3′) that amplified a 568-bp fragment from Mamu A*01-positive samples. The second reaction used primers conserved in all known Mamu-A, -B, and -E sequences (forward 5′-GCTACGTGGACGACACGCAGT-3′ and reverse 5′-CCGCGGAGGAGGCGCCCGTC-3′) that amplified a 197-bp fragment from all samples. Sequences of the rhesus monkey MHC class I genes (17) and sequences unique for Mamu-A*01 primers were kindly provided by David I. Watkins (Wisconsin Regional Primate Research Center and Department of Pathology, University of Wisconsin). PCR products were analyzed by electrophoresis in a 3% agarose gel (ISS Agarose HT, Integrated Separation Systems, Natick, MA), and monkeys from which both products were amplified were selected as expressing the Mamu-A*01 MHC class I molecule. Verification was achieved by direct sequencing of purified PCR products (QIAquick PCR Purification Kit, Qiagen).
Immunization of Rhesus Monkeys.
Monkeys were immunized by i.m. inoculation of 108 plaque-forming units of either MVA (n = 2) or the MVA-SIVSM gag pol (n = 4) vaccinia virus at 0 and 13 weeks, and EDTA-anticoagulated blood was collected sequentially after each immunization. Lymph node biopsies were obtained surgically at 2 weeks after the second immunization. Mononuclear cells were disrupted from lymph nodes by teasing through a Falcon cell strainer and used in subsequent cytotoxicity assays and for staining and phenotypic analysis.
Recombinant MVA Constructs.
The SIVSMH4 gag pol ORF (nucleotides 1049–5397) was cloned into the Pme I site of pMC03 (18) so that it would be regulated by the strong synthetic early/late promoter (19). The plasmid was transfected into chicken embryo fibroblasts that had been infected with MVA, and plaques that stained blue upon addition of X-Gluc (5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid, CLONTECH) were picked and plaque-purified essentially as described (18). Expression of gag-pol proteins was demonstrated by immunostaining of plaques and of blots of SDS polyacrylamide gels with sera from an SIV-infected macaque (code RH E544 1/31/94). The recombinant virus was amplified in chicken embryo fibroblasts and purified by centrifugation through a cushion of 36% sucrose (20). The titer of this stock was determined by immunostaining of infected chicken embryo fibroblasts with RH E544 serum.
Functional Cytotoxicity Assays.
Peripheral blood lymphocytes (PBLs) from Mamu-A*01+ rhesus monkeys were cultured with 10 μg/ml p11C (EGCTPYDINQML) at a density of 5 × 106 cells/ml. On day 3 of culture, the medium was supplemented with recombinant human IL-2 (20 units/ml; provided by Hoffman–La Roche), and cultures were maintained a further 4 days. PBLs were then centrifuged over Ficoll-Hypaque (Ficopaque; Pharmacia) and assessed as effector cells in a standard 51Cr-release assay by using U-bottomed microtiter plates containing 104 target cells with varying concentrations of effector cells. All wells were assayed in duplicate. Autologous B lymphoblastoid cell lines were used as target cells and were incubated with 1 μg/ml p11C, C-M (CTPYDINQM) or 1 μg/ml control peptide p11B (ALSEGCTPYDIN) during overnight 51Cr labeling. Plates were incubated in a humidified incubator at 37°C for 4 h. Specific release was calculated as [(experimental release−spontaneous release)/(maximum release−spontaneous release)] × 100. Spontaneous release was <20% of maximal release with detergent (2% Triton X-100; Sigma) in all assays.
Staining and Phenotypic Analysis of p11C-Specific CD8+ T Lymphocytes.
Soluble tetrameric Mamu-A*01/p11C, C-M complex was produced as described by Kuroda et al. (16). Phycoerythrin-labeled ExtrAvidin (Sigma) was mixed with biotinylated Mamu-A*01/p11C, C-M complex at a 1:4 molar ratio to produce the tetramers. The mAbs used for this study were directly coupled to fluorescein isothiocyanate, phycoerythrin-Texas red, or allophycocyanin.
The following mAbs were used: anti-CD8α(Leu2a)-fluorescein isothiocyanate (Becton Dickinson) and anti-CD8αβ(2ST8–5H7)-phycoerythrin-Texas red (Coulter) (which recognizes a neoantigenic determinant created by the association of the α and β chains of the CD8 molecule). The mAb FN18, which recognizes rhesus monkey CD3, a gift from D. M. Neville, Jr., NIH, Bethesda, MD, was directly coupled to allophycocyanin. The phycoerythrin-coupled tetrameric Mamu-A*01/p11C, C-M complex was used in conjunction with anti-CD8α-fluorescein isothiocyanate, anti-CD8αβ-phycoerythrin-Texas red, and anti-rhesus monkey CD3-allophycocyanin.
Lymphocytes were prepared and analyzed on a COULTER EPICS Elite ESP as described (16). Data analysis was performed by using the epics elite software version 4.02 (Coulter). Data presentation was performed by using winmdi software version 2.7 (Joseph Trotter, La Jolla, CA) and Microsoft power point software version 4.0c (Microsoft, Redmond, WA).
RESULTS
Vaccination of Rhesus Monkeys with Recombinant MVA-SIVSM gag pol Elicited a Functional Gag Epitope-Specific CTL Response.
Rhesus monkeys were vaccinated with recombinant MVA-SIVSM gag pol, and their PBLs were assessed for functional SIVSM-specific CTL activity. Because animals were selected for study that expressed the rhesus monkey MHC class I allele Mamu-A*01, it could be predicted that any SIVSM Gag-specific CTL activity generated by this vaccination would be restricted to a dominant 9-aa epitope, p11C, C-M (amino acids 181–189 of Gag) (21–23). Four monkeys received 108 plaque-forming units of MVA-SIVSM gag pol and two received 108 plaque-forming units of control MVA, both by i.m. inoculation. PBLs of these six animals were then assessed every 2 weeks for p11C, C-M-specific CTL activity after in vitro stimulation with p11C.
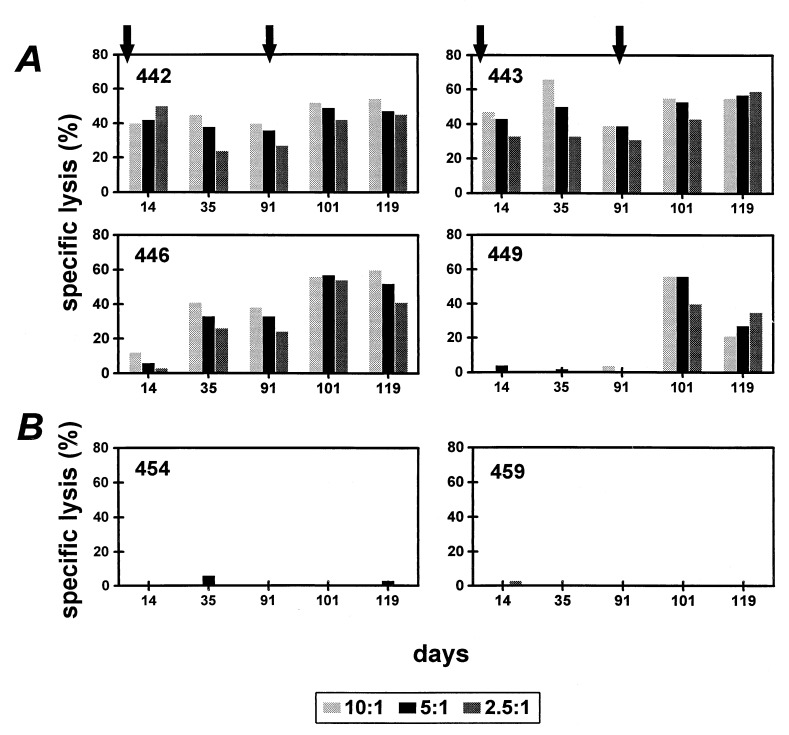
As shown in Fig. 1, three of the four monkeys vaccinated with MVA-SIVSM gag pol developed a Gag epitope-specific CTL response by 14 days after vaccination as measured after in vitro stimulation with peptide. This response was maintained until a second immunization was given on day 91. In the fourth monkey, 449, a primary CTL response was negligible. After boosting on day 91, the Gag epitope-specific CTL response increased in the three monkeys with preexisting SIVSM-specific CTL activity. Moreover, monkey 449, which until then evidenced no SIVSM-specific CTL activity, developed Gag epitope-specific CTL activity comparable with the other three monkeys. The two Mamu-A*01+ rhesus monkeys immunized with MVA alone did not develop a Gag epitope-specific CTL response, indicating that the MVA-SIVSM gag pol immunization was responsible for the induction of the Gag-specific CTL.
Figure 1.
Vaccination of rhesus monkeys with MVA-SIVSM gag pol elicited Gag epitope-specific functional CTL activity. Mamu-A*01+ rhesus monkeys were inoculated with MVA-SIVSM gag pol (A) or control MVA (B) on day 0 and again on day 91 as indicated by arrows. PBLs of these monkeys were assessed after in vitro p11C stimulation for p11C, C-M-specific lysis. Lysis was assessed at three effector:target ratios as shown.
Recombinant MVA-SIVSM Gag Pol-Vaccinated Rhesus Monkeys Developed Mamu-A*01/p11C, C-M Tetramer-Binding, CD8αβ+ Peripheral Blood T Lymphocytes.
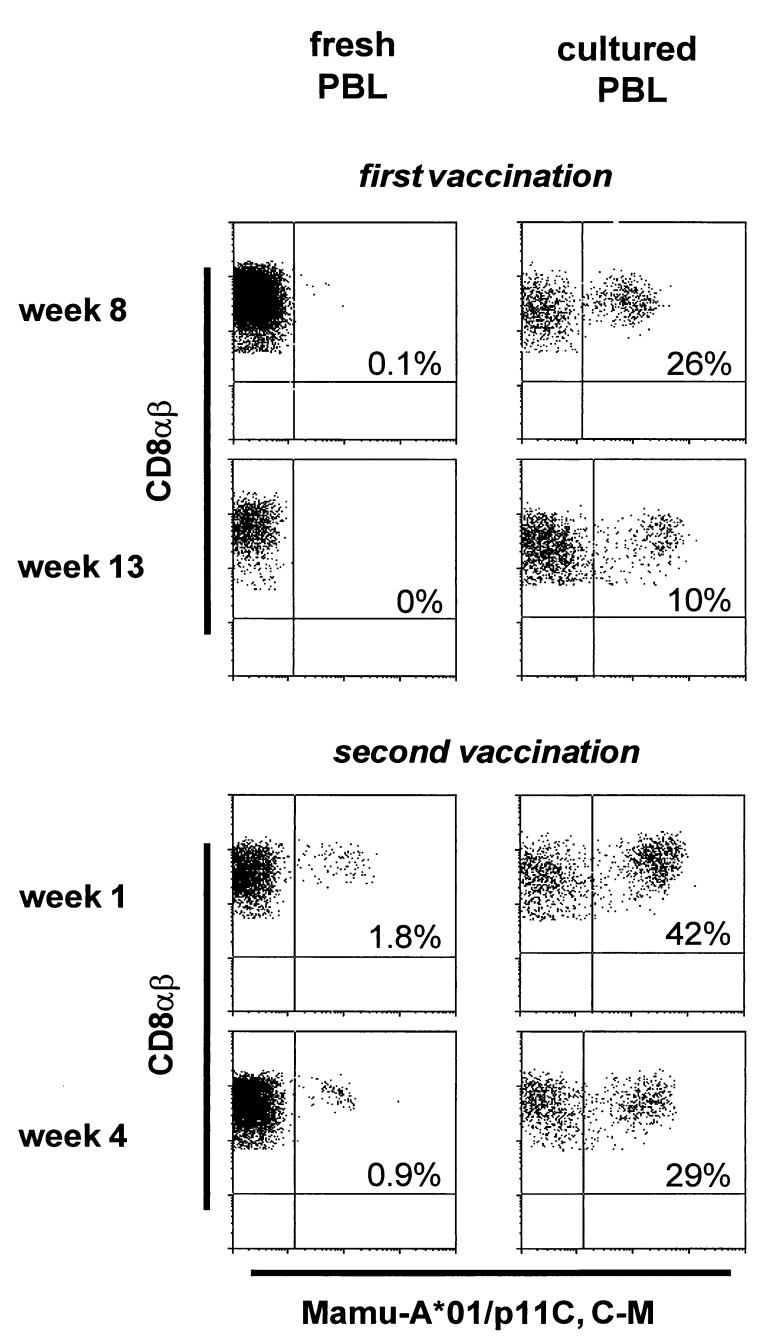
The PBLs of these vaccinated monkeys also were monitored for the development of a CD8+ T lymphocyte population that bound tetrameric Mamu-A*01/p11C, C-M. PBLs were assessed for tetramer binding both in freshly isolated whole blood and after in vitro cultivation with p11C. Data from one of the MVA-SIVSM gag pol-vaccinated Mamu-A*01+ rhesus monkeys are shown in Fig. 2. Lymphocytes in freshly isolated blood demonstrated minimal tetramer binding after the first vaccination. However, after in vitro stimulation with p11C, these same lymphocyte populations demonstrated binding to as many as 26% of CD8αβ+ T lymphocytes. By 1 week after a second vaccination, tetramer binding was demonstrable in freshly isolated whole blood to 1.8% of CD8αβ+ T lymphocytes. Moreover, after in vitro stimulation of these same PBLs with p11C, tetramer-binding T lymphocytes expanded to as many as 42% of CD8αβ+ T cells.
Figure 2.
Tetrameric Mamu-A*01/p11C, C-M complex bound to CD8αβ+ peripheral blood T lymphocytes of a vaccinated rhesus monkey (443) after immunization with MVA-SIVSM gag pol. Flow cytometry histograms illustrating tetramer binding to gated CD8αβ+ CD3+ T lymphocytes are shown for both fresh PBLs and p11C-stimulated PBLs for two time points after the first vaccination and two time points after the second vaccination. The values indicate the percentage of CD8αβ+ T lymphocytes that bound the tetramer.
Table 1 summarizes the tetramer staining and functional p11C, C-M-specific CTL data for all six Mamu-A*01+ animals 14, 35, and 91 days after the first immunization and 10 and 28 days after the second immunization. After the initial immunization, tetramer-binding CD8αβ+ T lymphocytes could be demonstrated in freshly isolated peripheral blood of only one monkey, 443, and in only 0.1%, 0.3%, and 0.1% of this monkey’s cells at days 14, 35, and 91, respectively. However, 10 days after the second immunization, a distinct population of tetramer-binding CD8αβ+ T cells was demonstrable in the whole blood of all MVA-SIVSM gag pol-immunized animals. This result was greatest in monkey 443, with 4.9% of CD8αβ+ T cells exhibiting tetramer binding. After a 6-day culture of the PBLs with p11C, T cells from all 4 MVA-SIVSM gag pol-immunized monkeys showed an expansion of tetramer-binding cells, reaching a high of 19–44% of all CD8αβ+ T lymphocytes. Thus, this vaccine-elicited dominant SIVSM Gag-epitope-specific CD8+ T lymphocyte response was readily detected by the tetramer-staining technology.
Table 1.
PBL of MVA-SIVSM gag pol-vaccinated Mamu-A*01+ rhesus monkeys demonstrate binding to tetrameric Mamu-A*01/p11C, C-M complex
| Immunization | Monkey | Assay | Day post-immunization
|
||||
|---|---|---|---|---|---|---|---|
| Primary
|
Secondary
|
||||||
| 14 | 35 | 91 | 101 | 119 | |||
| MVA-SIVSMgag pol | 442 | Fresh* | 0 | 0 | 0 | 0.7 | 0.3 |
| Peptide-stimulated† | NT§ | NT | 11.3 | 21.5 | 25.9 | ||
| % Specific lysis‡ | 50.0 | 24.0 | 27.0 | 42.0 | 45.0 | ||
| 443 | Fresh | 0.1 | 0.3 | 0.1 | 4.9 | 0.7 | |
| Peptide-stimulated | NT | NT | 13.3 | 23.9 | 27.1 | ||
| % Specific lysis | 33.0 | 33.0 | 31.0 | 43.0 | 59.0 | ||
| 446 | Fresh | 0 | 0 | 0 | 0.6 | 0.6 | |
| Peptide-stimulated | NT | NT | 13.4 | 44.4 | 35.7 | ||
| % Specific lysis | 3.0 | 26.0 | 24.0 | 54.0 | 41.0 | ||
| 449 | Fresh | 0 | 0 | 0 | 1.7 | 0.5 | |
| Peptide-stimulated | NT | NT | 5.6 | 18.5 | 15.6 | ||
| % Specific lysis | 1.0 | 0 | 1.0 | 40.0 | 35.0 | ||
| Control MVA | 454 | Fresh | 0 | 0 | 0 | 0 | 0 |
| Peptide-stimulated | NT | NT | 0 | 0 | 0 | ||
| % Specific lysis | 1.0 | 1.0 | 0 | 0 | 0 | ||
| 459 | Fresh | 0 | 0 | 0 | 0 | 0 | |
| Peptide-stimulated | NT | NT | 0 | ||||
| % Specific lysis | 3.0 | 0 | 0 | 0 | 0 | ||
Lymphocytes were assessed for binding of tetramer to CD8αβ+ T cells in whole blood.
PBLs were cultured with p11C and then tetramer binding to CD8αβ+ T cells was assessed.
PBLs were cultured with p11C and 7 days later p11C, C-M-specific lysis was determined in a standard 51Cr-release assay. Data represent % specific lysis at an E:T ratio of 2.5:1.
NT, not tested.
Recombinant MVA-SIVSM Gag Pol Immunization Elicited Gag Epitope-Specific CTL in Lymph Nodes of Rhesus Monkeys.
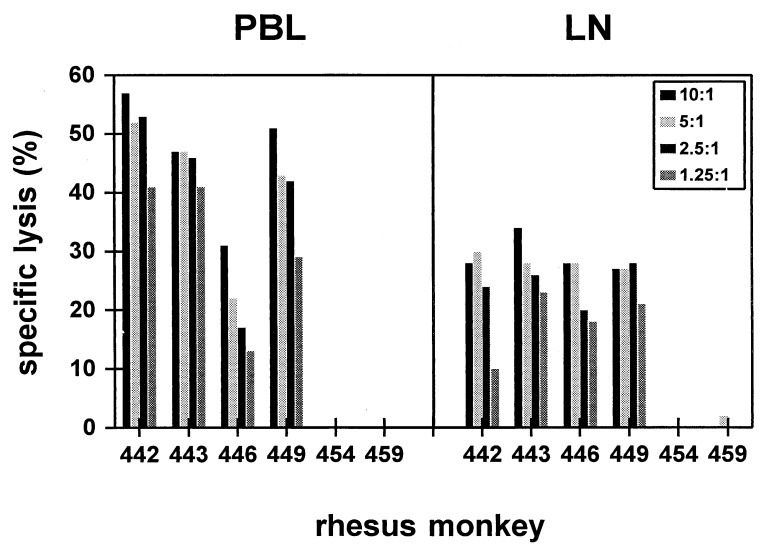
Gag epitope-specific CTL were assessed in lymph nodes of the vaccinated rhesus monkeys by using both a functional killing assay and tetramer staining. Fourteen days after the second immunization, lymph node lymphocytes and PBLs were assessed in parallel after in vitro stimulation with p11C for SIVSM Gag epitope-specific lytic activity. As shown in Fig. 3, Gag-specific lysis was demonstrated in lymph node lymphocytes of the four MVA-SIVSM gag pol-vaccinated but not the two control MVA-vaccinated monkeys. The lytic activity in the peptide-stimulated lymph node lymphocytes was consistently less than that of similarly stimulated PBLs from the same monkeys.
Figure 3.
Gag epitope-specific CTL were present in lymph nodes and peripheral blood of MVA-SIVSM gag pol-vaccinated rhesus monkeys. Lymphocytes from the peripheral blood and lymph nodes of the MVA-SIVSM gag pol-vaccinated (monkeys 442, 443, 446, and 449) and control MVA-vaccinated (monkeys 454 and 459) animals were assessed for p11C, C-M-specific lytic activity. Lysis was assessed at four effector:target ratios as shown.
These lymph node lymphocytes were similarly assessed for cells that bound tetrameric Mamu-A*01/p11C, C-M complex. As shown in Table 2, tetramer-binding cells were detected in a small percentage of freshly isolated lymphocytes from lymph nodes of the four MVA-SIVSM gag pol-vaccinated (0.1–0.5% CD8αβ+ T cells) but not the two control MVA-vaccinated monkeys. In vitro stimulation of the lymph node lymphocytes with p11C caused a substantial expansion of tetramer binding CD8αβ+ T lymphocytes to 11–16% of CD8αβ+ T cells. Thus, Gag epitope-specific CD8+ CTL were demonstrable in lymph node lymphocytes of these vaccinated monkeys by using both killing assays and tetramer staining.
Table 2.
Lymph node T lymphocytes of MVA-SIVSM gag polvaccinated Mamu-A*01+ rhesus monkeys demonstrate binding to tetrameric Mamu-A*01/p11C, C-M complex
| Immunization | Monkey | % CD8αβ tetramer binding
|
||
|---|---|---|---|---|
| Fresh* | Peptidestimulated† | % Specific lysis‡ | ||
| MVA-SIVSMgag pol | 442 | 0.1 | 10.7 | 24 |
| 443 | 0.5 | 15.8 | 26 | |
| 446 | 0.3 | 11.6 | 20 | |
| 449 | 0.3 | 13.0 | 28 | |
| MVA control | 454 | 0.0 | 0.0 | 0 |
| 459 | 0.0 | 0.0 | 0 | |
Lymph node lymphocytes were assessed for binding of tetramer to CD8αβ+ T cells.
Lymph node cells were cultured with p11C and then tetramer binding to CD8αβ+ T cells were assessed.
Lymph node cells were cultured with p11C and 7 days later p11C, C-M-specific lysis was determined in a standard 51Cr-release assay. Data represent % specific lysis at an E:T ratio of 2.5:1.
DISCUSSION
Previous studies demonstrated the ability of recombinant MVA to induce high titer antibody responses in rhesus monkeys against parainfluenza virus type 3 envelope proteins and to protectively immunize these animals against a respiratory challenge (24). In addition, recombinant MVA expressing the gag pol and env of SIV induced neutralizing antibodies and modulated viremia in rhesus monkeys after i.v. challenge with SIVSM (9). Although recombinant MVA had been shown previously to induce CTL in mice (8, 25–27), this had not been examined in primates.
The present studies indicate that a recombinant MVA-SIVSM gag pol vaccine construct can elicit a potent Gag-specific CTL response in rhesus monkeys. A number of characteristics of this response underscore the potential utility of recombinant MVA as an HIV-1 vaccine vector. First, the elicited CTL response could be boosted with the same vaccine construct. This suggests that immunity to the vector was insufficient to limit viral protein expression on repeated inoculations. Second, CTL were demonstrable in lymph nodes of the vaccinated animals. Because the major site of HIV-1 replication in infected individuals is in lymph nodes, a successful HIV-1 vaccine must elicit CTL that are active in these anatomic sites.
The ability of a replication-defective virus to induce high level humoral and cellular immunity to recombinant proteins seems counter intuitive. Based on in vitro studies with monkey cells, we anticipate limited cell-to-cell spread of MVA but high expression in those cells that are infected (6). Although not yet tested, we considered that the low number of vaccinia virus particles formed by the replication-defective MVA might lessen the host anti-viral response and thereby prolong recombinant gene expression. The complete genome sequence of MVA suggests additional explanations for its potent immunogenicity (28). Among the numerous genes deleted are many that encode proteins responsible for immune evasion; these include soluble receptors for interferon-γ, interferon-α/β, tumor necrosis factor, and CC chemokines (28, 29). Thus, the combination of high expression, more limited anti-vaccinia responses, and the absence of immunomodulatory viral proteins may compensate for the replication defects.
Monitoring HIV-1-specific CTL responses in vaccine recipients has until now proven problematic because of a dependence on nonquantitative assays. The present studies indicated that a well-tolerated vaccine modality can elicit a CTL response that is readily detected by using the tetramer-staining technology. This observation suggests that tetramer staining might complement or even supplant traditional killing assays for monitoring HIV-1 vaccine trials in nonhuman primates and in humans.
Acknowledgments
We thank Norman Cooper and Sampa Santra for help with tissue culture, Russell Byrum for assistance in collecting animal specimens, and Evelyn Gould for preparation of the manuscript. This work was supported by National Institutes of Health Grants AI35166 and AI26507.
ABBREVIATIONS
- CTL
cytotoxic T lymphocyte
- MVA
modified vaccinia virus Ankara
- SIV
simian immunodeficiency virus
- PBL
peripheral blood lymphocyte
- MHC
major histocompatibility complex
References
- 1.Letvin N L. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 2.Bennink J R, Yewdell J W, Smith G L, Moller C. Nature (London) 1984;311:578–579. doi: 10.1038/311578a0. [DOI] [PubMed] [Google Scholar]
- 3.Moss B. Proc Natl Acad Sci USA. 1996;93:11341–11348. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayr A, Hochstein-Mintzel V, Stickl H. Infection. 1975;3:6–14. [Google Scholar]
- 5.Mayr A, Stickl H, Muller H K, Danner K, Singer H. Zentralbl Bakteriol, Parasitenkd, Infektionskrankh Hyg, Abt 1: Orig, Reihe B: 1978;167:375–390. [PubMed] [Google Scholar]
- 6.Carroll M, Moss B. Virology. 1997;238:198–211. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- 7.Sutter G, Moss B. Proc Natl Acad Sci USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutter G, Wyatt L S, Foley P L, Bennink J R, Moss B. Vaccine. 1994;12:1032–1040. doi: 10.1016/0264-410x(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch V M, Fuerst T R, Sutter G, Carroll M W, Yang L C, Goldstein S, Piatak M, Jr, Elkins W R, Alvord W G, Montefiori D C, et al. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyatt L S, Shors S T, Murphy B R, Moss B. Vaccine. 1996;14:1451–1458. doi: 10.1016/s0264-410x(96)00072-2. [DOI] [PubMed] [Google Scholar]
- 11.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 12.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, et al. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 13.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 14.Gallimore A, Glithero A, Godkin A, Tissot A, Althage A, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callan M F, Tan L, Annels N, Ogg G S, Wilson J, O’Callaghan C A, McMichael A J, Rickinson A B. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuroda M J, Schmitz J E, Barouch D H, Craiu A, Allen T M, Sette A, Watkins D I, Forman M A, Letvin N L. J Exp Med. 1998;187:1373–1381. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyson J E, Shufflebotham C, Cadavid L F, Urvater J A, Knapp L A, Hughes A L, Watkins D I. J Immunol. 1996;156:4656–4665. [PubMed] [Google Scholar]
- 18.Carroll M W, Moss B. BioTechniques. 1995;19:352–356. [PubMed] [Google Scholar]
- 19.Chakrabarti S, Sisler J R, Moss B. BioTechniques. 1997;23:1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- 20.Earl P L, Moss B. In: Current Protocols in Molecular Biology. Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Vol. 2. New York: Greene & Wiley; 1991. pp. 16.17.1–16.17.16. [Google Scholar]
- 21.Yamamoto H, Miller M D, Tsubota H, Watkins D I, Mazzara G P, Stallard V, Panicali D L, Aldovini A, Young R A, Letvin N L. J Immunol. 1990;144:3385–3391. [PubMed] [Google Scholar]
- 22.Miller M D, Yamamoto H, Hughes A L, Watkins D I, Letvin N L. J Immunol. 1991;147:320–329. [PubMed] [Google Scholar]
- 23.Allen T M, Sidney J, del Guercio M F, Glickman R L, Lensmeyer G L, Wiebe D A, DeMars R, Pauza C D, Johnson R P, Sette A, Watkins D I. J Immunol. 1998;160:6062–6071. [PubMed] [Google Scholar]
- 24.Durbin, A., Wyatt, L. S., Slew, J., Moss, B. & Murphy, B. R. (1998) Vaccine, in press. [DOI] [PubMed]
- 25.Carroll M W, Overwijk W W, Chamberlain R S, Rosenberg S A, Moss B, Restifo N P. Vaccine. 1997;15:387–394. doi: 10.1016/s0264-410x(96)00195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanke T, Blanchard T J, Schneider J, Ogg G S, Tan R, Becker M, Gilbert S C, Hill A V, Smith G L, McMichael A. J Gen Virol. 1998;79:83–90. doi: 10.1099/0022-1317-79-1-83. [DOI] [PubMed] [Google Scholar]
- 27.Schneider J, Gilbert S C, Blanchard T J, Hanke T, Robson K J, Hannan C M, Becker M, Sinden R, Smith G L, Hill A V. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 28.Antoine G, Scheiflinger F, Dorner F, Falkner F G. Virology. 1998;244:365–396. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 29.Blanchard T J, Alcami A, Andrea P, Smith G L. J Gen Virol. 1998;79:1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]