Abstract
Methylammonium was taken up rapidly by illuminated cells of Anacystis nidulans R-2, leading to internal concentrations of 1.3 +/- 0.1 mM within 1 min, and a gradient of up to 200 between the cells and medium. Accumulation of 14CH3NH3+ required at least 5 mM NaCl, but the uptake rate was independent of medium pH between 6.5 and 9. The kinetics of uptake could be resolved into an initial fast phase lasting less than 1 min (approximate Km, 7.2 microM; Vmax, 12.5 nmol min-1 mg of protein-1 at 15 degrees C). A second, slower phase associated with product formation was eliminated by preincubation with methionine sulfoximine, a specific inhibitor of glutamine synthetase; the rapid phase was unaffected by this treatment. Ammonium ions competed with 14CH3NH3+ for entry, and addition of 5 microM NH4+ or 100 microM CH3NH3+ released 14CH3NH3+ accumulated during the rapid phase of entry. Small additions of NH4+ made at the same time as additions of 14CH3NH3+ delayed the start of radioactivity uptake by a time which corresponded accurately with the period needed for the complete removal of the added NH4+. The effects of inhibitors on accumulation and carbocyanine dye fluorescence suggest that ATP-dependent membrane potential was needed to drive 14CH3NH3+ transport. Spheroplasts were as active as whole cells in accumulating NH4+ and 14CH3NH3+, indicating that soluble periplasmic components are not involved in the translocation. Some significant differences between the translocation of 14CH3NH3 and that of NH4+ were observed: growth with NH4+ in place of NO3- repressed 14CH3NH3+ accumulation ability without affecting the NH4+ uptake rate Na+ was not required for NH4+ uptake, and concentration of KCl inhibitory with 14C3NH3+ did not reduce NH4+ uptake.
Full text
PDF

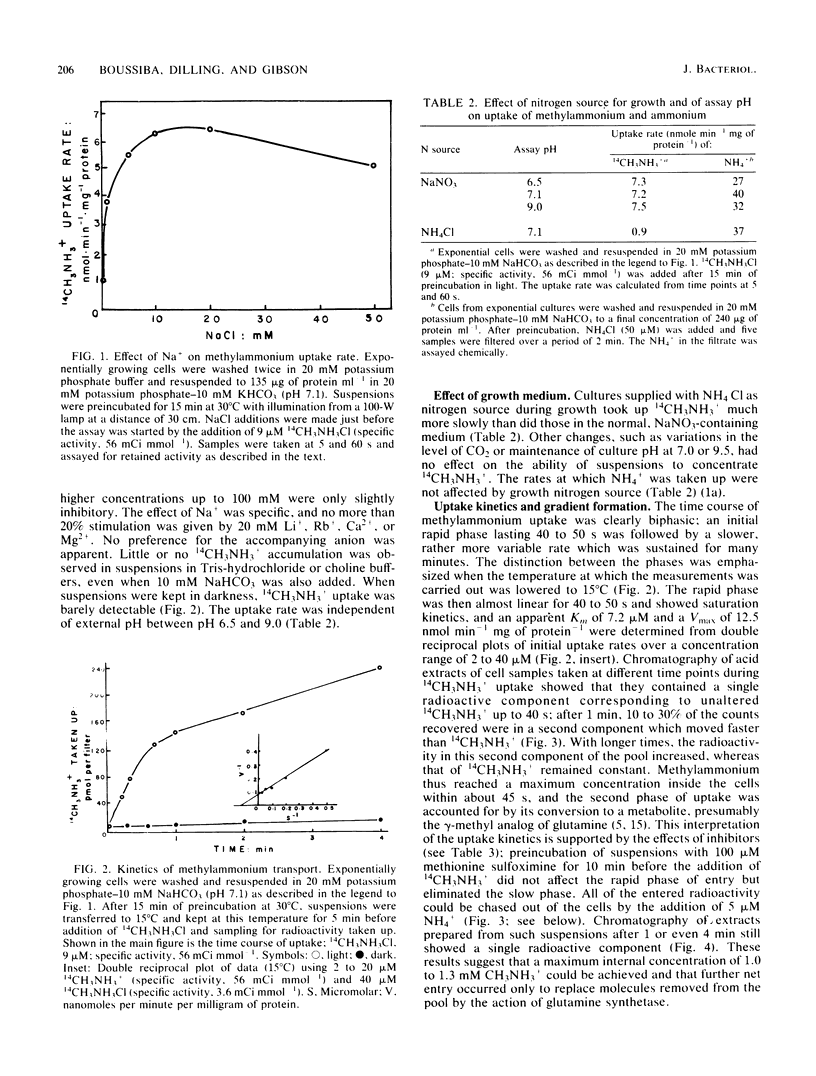
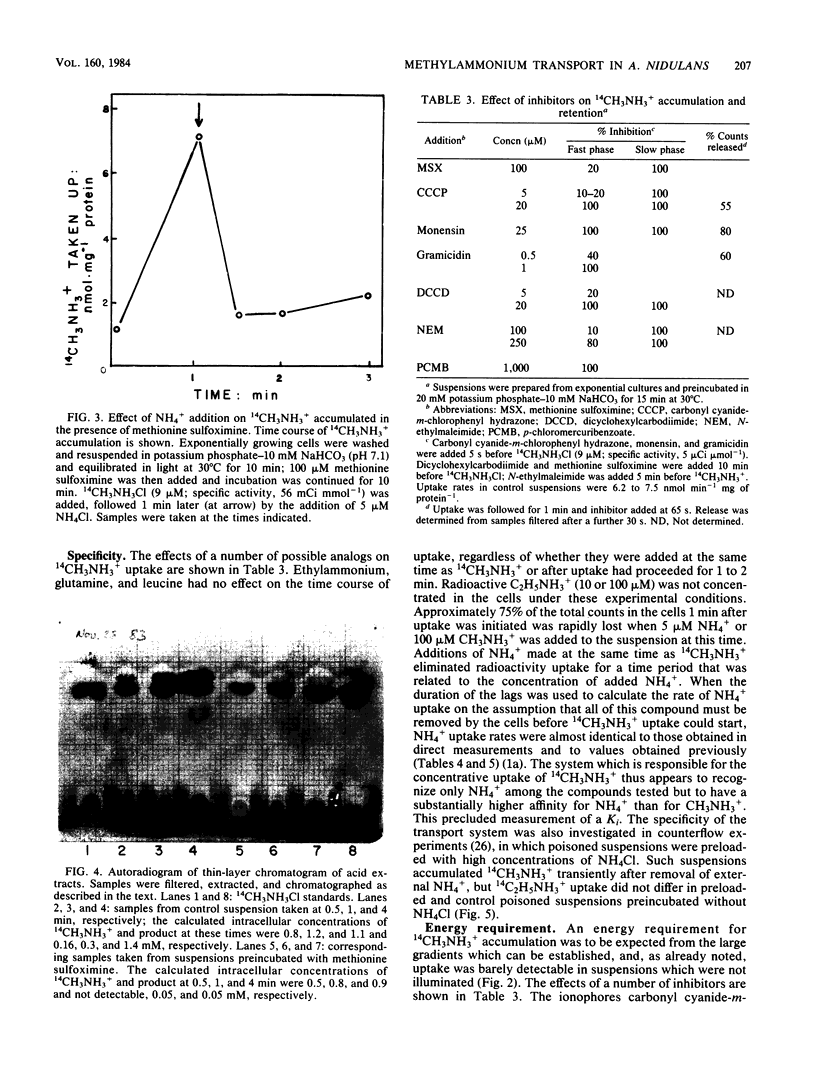
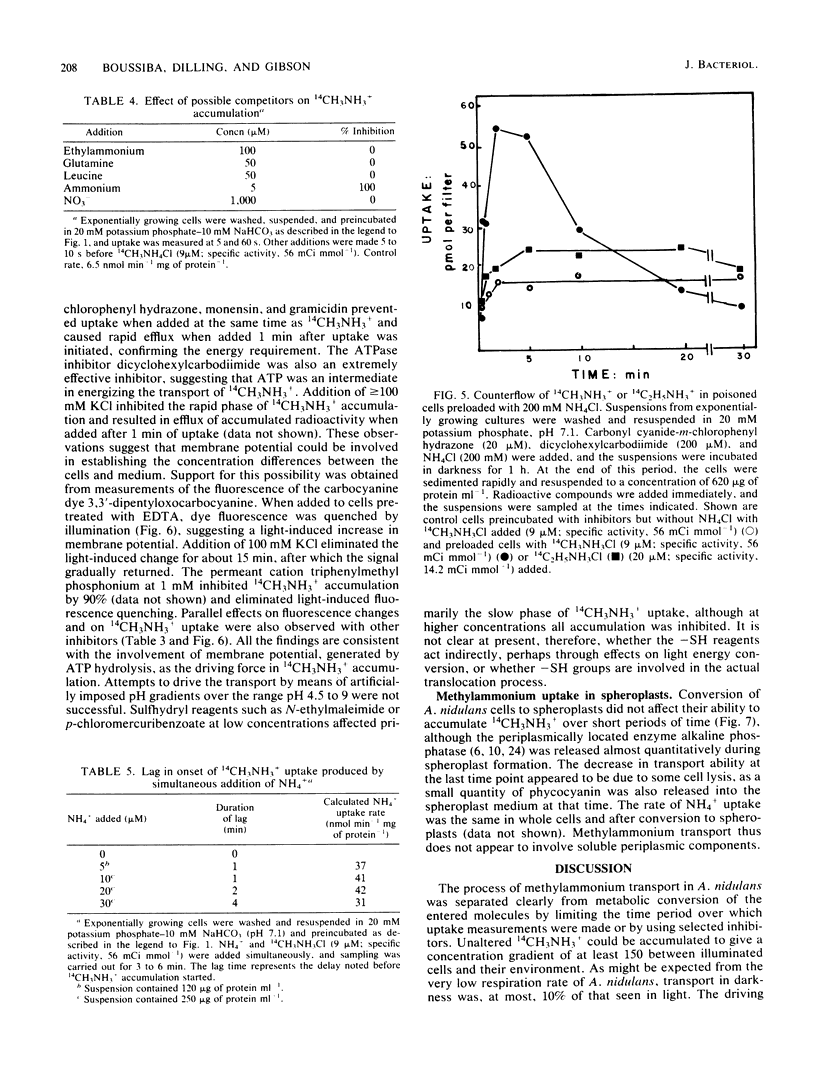
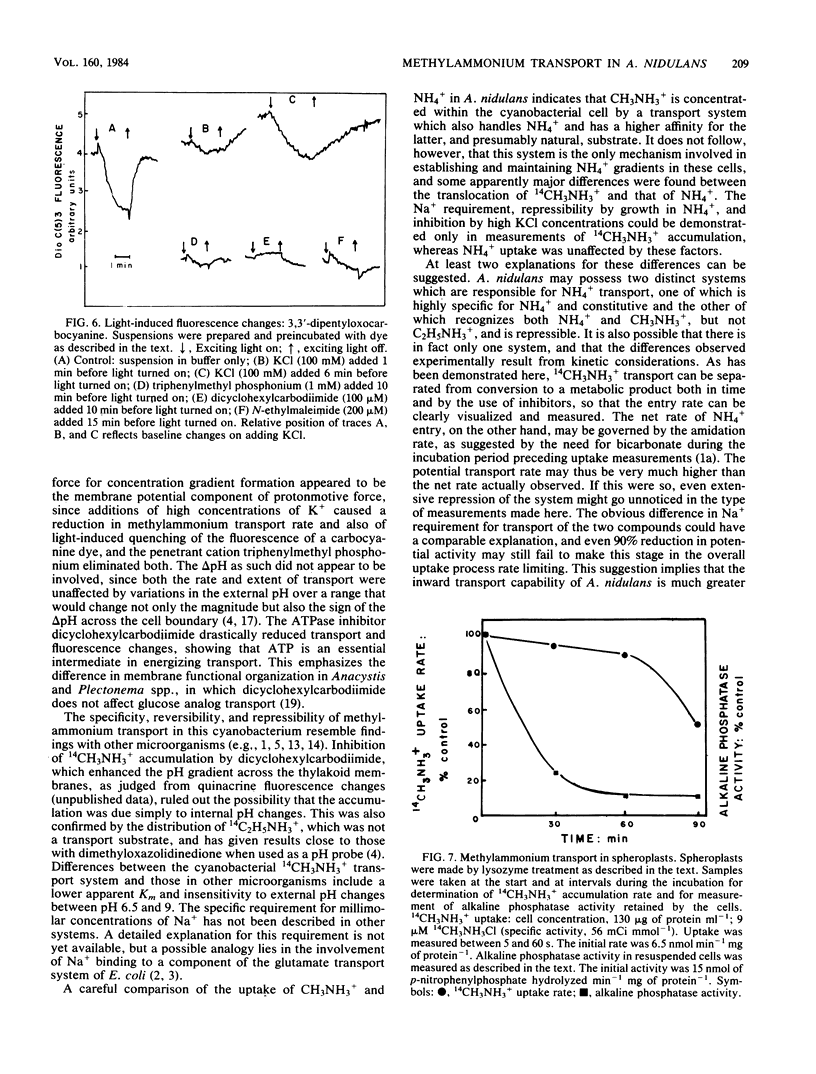

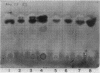
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Jr, Zimniak P. Transport of ammonium and methylammonium ions by Azotobacter vinelandii. J Bacteriol. 1981 May;146(2):512–516. doi: 10.1128/jb.146.2.512-516.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Yamato I., Anraku Y. Mechanism of glutamate transport in Escherichia coli B. 1. Proton-dependent and sodium ion dependent binding of glutamate to a glutamate carrier in the cytoplasmic membrane. Biochemistry. 1983 Apr 12;22(8):1954–1959. doi: 10.1021/bi00277a033. [DOI] [PubMed] [Google Scholar]
- Fujimura T., Yamato I., Anraku Y. Mechanism of glutamate transport in Escherichia coli B. 2. Kinetics of glutamate transport driven by artificially imposed proton and sodium ion gradients across the cytoplasmic membrane. Biochemistry. 1983 Apr 12;22(8):1959–1965. doi: 10.1021/bi00277a034. [DOI] [PubMed] [Google Scholar]
- Gober J. W., Kashket E. R. Methylammonium uptake by Rhizobium sp. strain 32H1. J Bacteriol. 1983 Mar;153(3):1196–1201. doi: 10.1128/jb.153.3.1196-1201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo J. F., Gibson J. Regulation of phosphate accumulation in the unicellular cyanobacterium Synechococcus. J Bacteriol. 1979 Nov;140(2):508–517. doi: 10.1128/jb.140.2.508-517.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackette S. L., Skye G. E., Burton C., Segel I. H. Characterization of an ammonium transport system in filamentous fungi with methylammonium-14C as the substrate. J Biol Chem. 1970 Sep 10;245(17):4241–4250. [PubMed] [Google Scholar]
- Ihlenfeldt M. J., Gibson J. CO2 fixation and its regulation in Anacystis nidulans (Synechococcus). Arch Microbiol. 1975;102(1):13–21. doi: 10.1007/BF00428339. [DOI] [PubMed] [Google Scholar]
- Ihlenfeldt M. J., Gibson J. Phosphate utilization and alkaline phosphatase activity in Anacystis nidulans (Synechococcus). Arch Microbiol. 1975;102(1):23–28. doi: 10.1007/BF00428340. [DOI] [PubMed] [Google Scholar]
- Kleiner D. Ammonium (methylammonium) transport by Klebsiella pneumoniae. Biochim Biophys Acta. 1982 Jun 28;688(3):702–708. doi: 10.1016/0005-2736(82)90282-6. [DOI] [PubMed] [Google Scholar]
- Kleiner D. Ammonium uptake and metabolism by mitrogen fixing bacteria. II. Klebsiella pneumoniae. Arch Microbiol. 1976 Dec 1;111(1-2):85–91. doi: 10.1007/BF00446553. [DOI] [PubMed] [Google Scholar]
- Kleiner D. Ammonium uptake by nitrogen fixing bacteria I. Azotobacter vinelandii. Arch Microbiol. 1975 Jun 22;104(2):163–169. doi: 10.1007/BF00447319. [DOI] [PubMed] [Google Scholar]
- Kleiner D., Fitzke E. Some properties of a new electrogenic transport system: the ammonium (methylammonium) carrier from Clostridium pasteurianum. Biochim Biophys Acta. 1981 Feb 20;641(1):138–147. doi: 10.1016/0005-2736(81)90577-0. [DOI] [PubMed] [Google Scholar]
- Kleiner D. The transport of NH3 and NH4+ across biological membranes. Biochim Biophys Acta. 1981 Nov 9;639(1):41–52. doi: 10.1016/0304-4173(81)90004-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Padan E., Schuldiner S. Energy transduction in the photosynthetic membranes of the cyanobacterium (blue-green alga) P-lectonema boryanum. J Biol Chem. 1978 May 10;253(9):3281–3286. [PubMed] [Google Scholar]
- Pateman J. A., Kinghorn J. R., Dunn E., Forbes E. Ammonium regulation in Aspergillus nidulans. J Bacteriol. 1973 Jun;114(3):943–950. doi: 10.1128/jb.114.3.943-950.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raboy B., Padan E. Active transport of glucose and alpha-methylglucoside in the cyanobacterium Plectonema boryanum. J Biol Chem. 1978 May 10;253(9):3287–3291. [PubMed] [Google Scholar]
- Roon R. J., Even H. L., Dunlop P., Larimore F. L. Methylamine and ammonia transport in Saccharomyces cerevisiae. J Bacteriol. 1975 May;122(2):502–509. doi: 10.1128/jb.122.2.502-509.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Stevenson R., Silver S. Methylammonium uptake by Escherichia coli: evidence for a bacterial NH4+ transport system. Biochem Biophys Res Commun. 1977 Apr 25;75(4):1133–1139. doi: 10.1016/0006-291x(77)91501-7. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Szalay A. A. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene. 1983 Sep;24(1):37–51. doi: 10.1016/0378-1119(83)90129-4. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Wilson T. H. Counterflow of galactosides in Escherichia coli. Biochim Biophys Acta. 1970;196(2):336–350. doi: 10.1016/0005-2736(70)90021-0. [DOI] [PubMed] [Google Scholar]