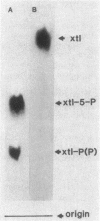
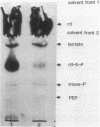
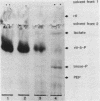
Abstract
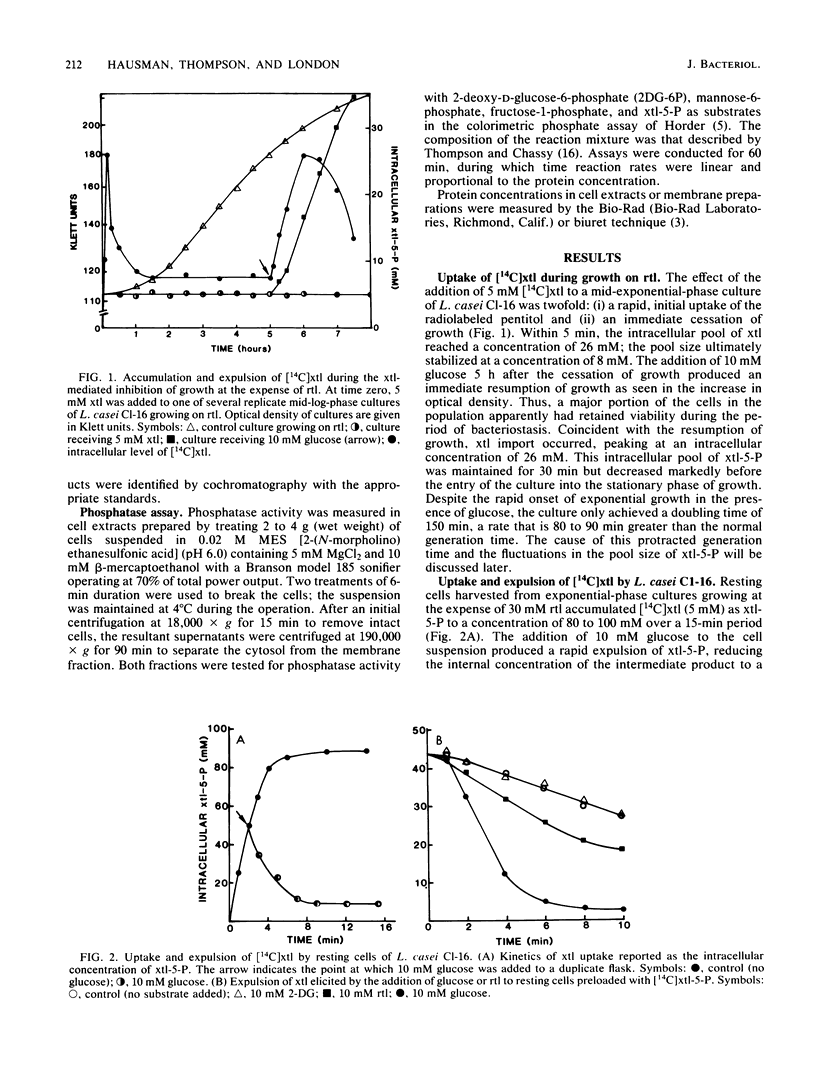
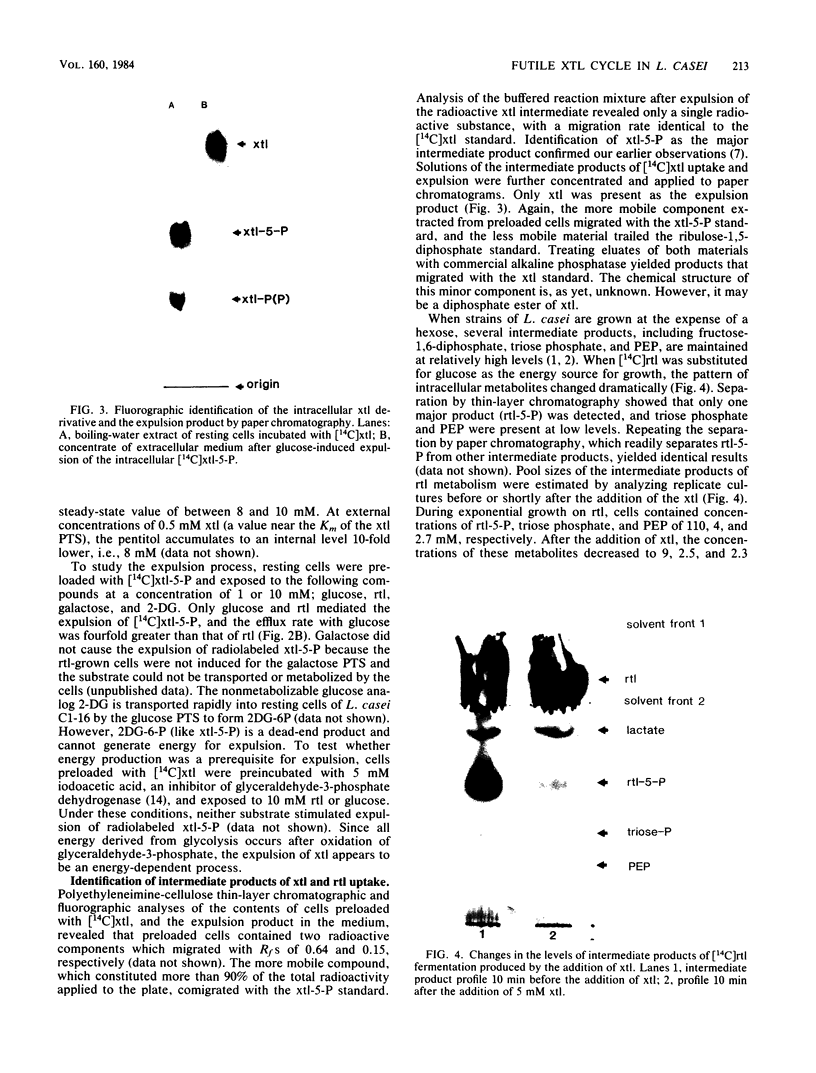
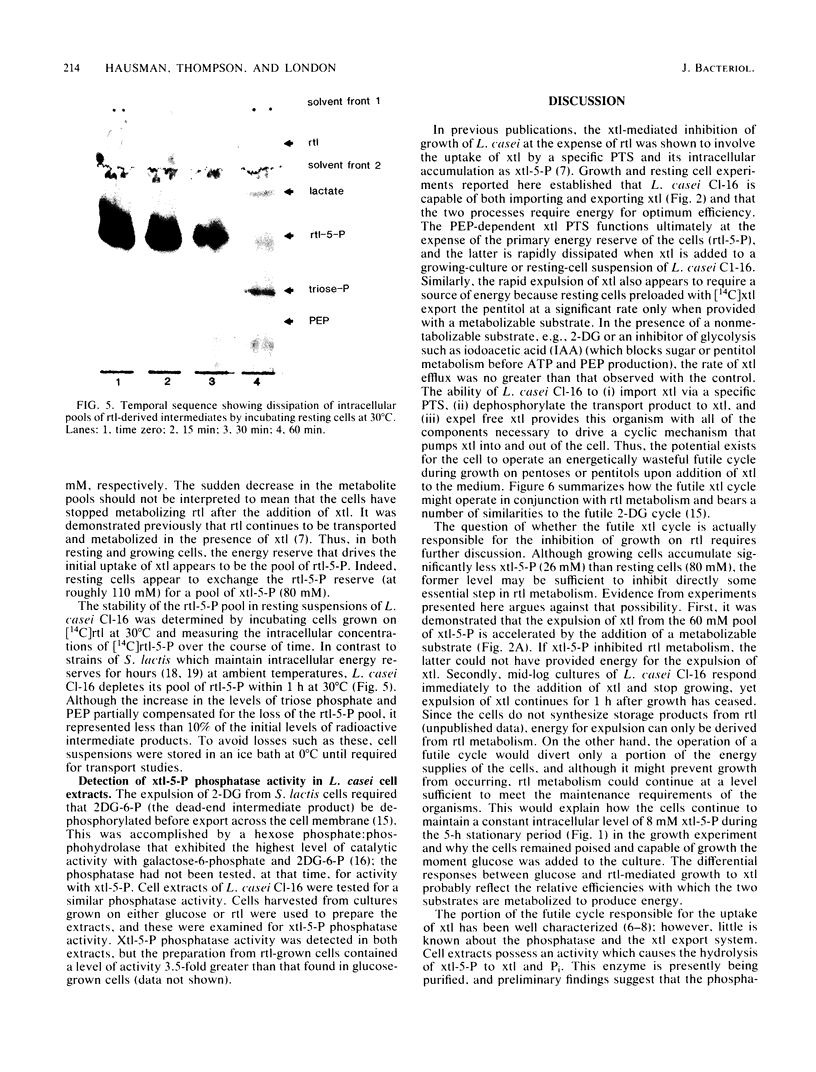
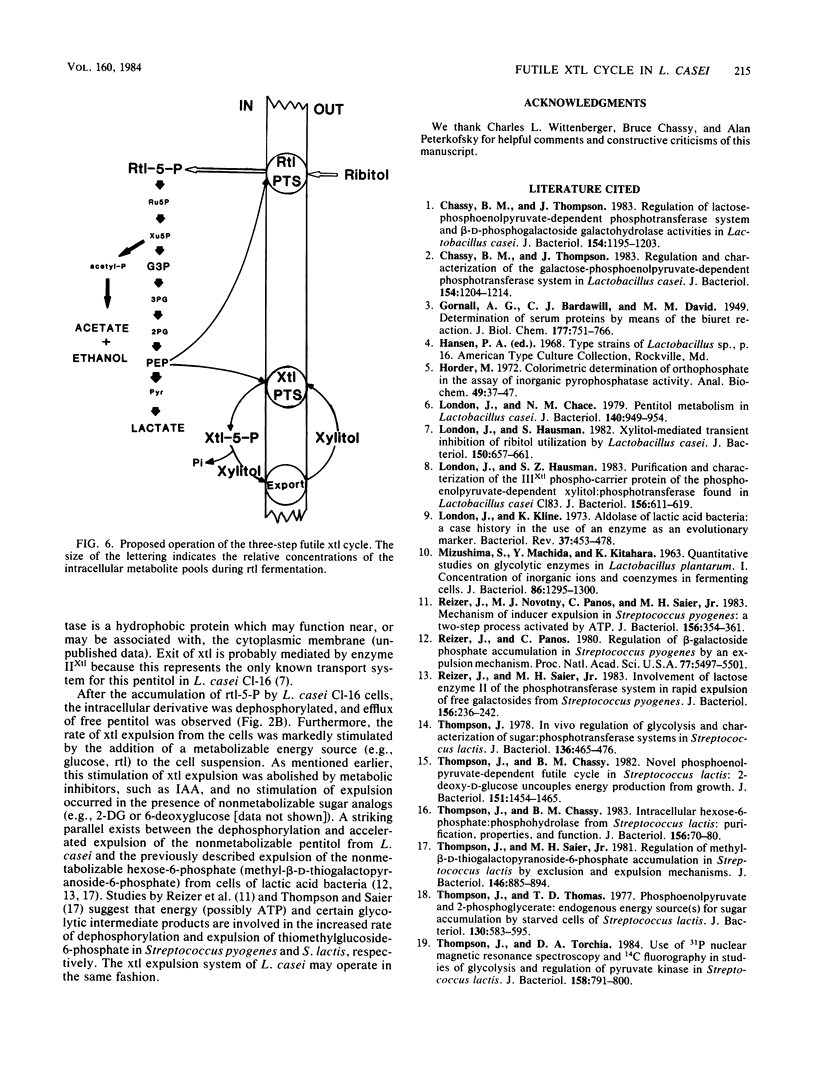
A futile xylitol cycle appears to be responsible for xylitol-mediated inhibition of growth of Lactobacillus casei Cl-16 at the expense of ribitol. The gratuitously induced xylitol-specific phosphoenolpyruvate-dependent phosphotransferase accumulates the pentitol as xylitol-5-phosphate, a phosphatase cleaves the latter, and an export system expels the xylitol. Operation of the cycle rapidly dissipates the ribitol-5-phosphate pool (and ultimately the energy supply of the cell), thereby producing bacteriostasis.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chassy B. M., Thompson J. Regulation and characterization of the galactose-phosphoenolpyruvate-dependent phosphotransferase system in Lactobacillus casei. J Bacteriol. 1983 Jun;154(3):1204–1214. doi: 10.1128/jb.154.3.1204-1214.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Thompson J. Regulation of lactose-phosphoenolpyruvate-dependent phosphotransferase system and beta-D-phosphogalactoside galactohydrolase activities in Lactobacillus casei. J Bacteriol. 1983 Jun;154(3):1195–1203. doi: 10.1128/jb.154.3.1195-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horder M. Colorimetric determination of orthophosphate in the assay of inorganic pyrophosphatase activity. Anal Biochem. 1972 Sep;49(1):37–47. doi: 10.1016/0003-2697(72)90240-0. [DOI] [PubMed] [Google Scholar]
- London J., Chace N. M. Pentitol metabolism in Lactobacillus casei. J Bacteriol. 1979 Dec;140(3):949–954. doi: 10.1128/jb.140.3.949-954.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Hausman S. Z. Purification and characterization of the IIIXtl phospho-carrier protein of the phosphoenolpyruvate-dependent xylitol:phosphotransferase found in Lactobacillus casei C183. J Bacteriol. 1983 Nov;156(2):611–619. doi: 10.1128/jb.156.2.611-619.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Hausman S. Xylitol-mediated transient inhibition of ribitol utilization by Lactobacillus casei. J Bacteriol. 1982 May;150(2):657–661. doi: 10.1128/jb.150.2.657-661.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Kline K. Aldolase of lactic acid bacteria: a case history in the use of an enzyme as an evolutionary marker. Bacteriol Rev. 1973 Dec;37(4):453–478. doi: 10.1128/br.37.4.453-478.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIZUSHIMA S., MACHIDA Y., KITAHARA K. QUANTITATIVE STUDIES ON GLYCOLYTIC ENZYMES IN LACTOBACILLUS PLANTARUM. I. CONCENTRATION OF INORGANIC IONS AND COENZYMES IN FERMENTING CELLS. J Bacteriol. 1963 Dec;86:1295–1300. doi: 10.1128/jb.86.6.1295-1300.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Panos C., Saier M. H., Jr Mechanism of inducer expulsion in Streptococcus pyogenes: a two-step process activated by ATP. J Bacteriol. 1983 Oct;156(1):354–361. doi: 10.1128/jb.156.1.354-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Panos C. Regulation of beta-galactoside phosphate accumulation in Streptococcus pyogenes by an expulsion mechanism. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5497–5501. doi: 10.1073/pnas.77.9.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Saier M. H., Jr Involvement of lactose enzyme II of the phosphotransferase system in rapid expulsion of free galactosides from Streptococcus pyogenes. J Bacteriol. 1983 Oct;156(1):236–242. doi: 10.1128/jb.156.1.236-242.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Intracellular hexose-6-phosphate:phosphohydrolase from Streptococcus lactis: purification, properties, and function. J Bacteriol. 1983 Oct;156(1):70–80. doi: 10.1128/jb.156.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Novel phosphoenolpyruvate-dependent futile cycle in Streptococcus lactis: 2-deoxy-D-glucose uncouples energy production from growth. J Bacteriol. 1982 Sep;151(3):1454–1465. doi: 10.1128/jb.151.3.1454-1465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. In vivo regulation of glycolysis and characterization of sugar: phosphotransferase systems in Streptococcus lactis. J Bacteriol. 1978 Nov;136(2):465–476. doi: 10.1128/jb.136.2.465-476.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Saier M. H., Jr Regulation of methyl-beta-d-thiogalactopyranoside-6-phosphate accumulation in Streptococcus lactis by exclusion and expulsion mechanisms. J Bacteriol. 1981 Jun;146(3):885–894. doi: 10.1128/jb.146.3.885-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Torchia D. A. Use of 31P nuclear magnetic resonance spectroscopy and 14C fluorography in studies of glycolysis and regulation of pyruvate kinase in Streptococcus lactis. J Bacteriol. 1984 Jun;158(3):791–800. doi: 10.1128/jb.158.3.791-800.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]