Abstract
Recent delayed matching studies have demonstrated that maintaining trial-unique stimuli in working memory modulates activity in temporal lobe structures. In contrast, most previous studies that focused on the role of the prefrontal cortex (PFC) used familiar stimuli. We combined fMRI with a Delayed-match-to-sample (DMS) task in humans that allowed us to manipulate stimulus pre-exposure (trial-unique vs. familiar objects) and stimulus domain (object vs. location). A visually-guided saccade task was used to localize the frontal eye fields (FEF). We addressed two questions: First, we examined whether delay-period activity within PFC regions was more strongly engaged when stimuli were familiar (pre-exposed) than when they were not seen previously (trial-unique). Second, we examined the role of regions within the PFC in object vs. location working memory. Subjects were instructed to remember one stimulus domain while ignoring the other over an 8-sec delay period. Object-specific delay-period activity was greatest in the posterior orbitofrontal cortex (OFC) bilaterally, and was stronger for familiar than trial-unique objects. In addition, consistent with previous findings, right posterior superior frontal sulcus, and the FEF were specifically active during the delay period of the location DMS task. These activations outside FEF were not related to saccadic eye movements. In contrast to previous reports, object-specific delay activity was more prominent in the posterior OFC than in the ventrolateral PFC, and was found to be greater for familiar than for trial-unique objects. These results suggest a critical role for the orbitofrontal cortex for maintaining object information in working memory.
Keywords: Orbitofrontal cortex, frontal eye fields, dorsolateral prefrontal cortex, superior frontal sulcus, match-to-sample, working memory
INTRODUCTION
Sustained activity in prefrontal (PFC) neurons during delayed match to sample tasks has been regarded as the neural correlate of memory maintenance (Fuster and Alexander 1971, Kubota and Niki 1971, Fuster 1973, Rosenkilde et al. 1981, Quintana et al. 1988, see Fuster 1991, for review). While most fMRI and animal studies investigating the role of the PFC in visual working memory have used a small set of highly familiar stimuli (Courtney et al. 1997, Rao et al. 1997, Postle and D’Esposito 1999a, Postle et al. 2000b, Sala et al. 2003), those studies that used working memory paradigms with novel or trial-unique stimuli have focused on the medial temporal lobes (Gaffan 1974, Zola-Morgan et al. 1989, Gaffan and Murray 1992, Zola-Morgan et al. 1993, Alvarez et al. 1994, Eacott et al. 1994, Ranganath and D’Esposito 2001, Schon et al. 2004). Using fMRI, we have previously shown that 2-back working memory performance with trial-unique visual stimuli recruited the medial temporal lobes, whereas 2-back working memory performance with a small set of familiar visual stimuli recruited the prefrontal cortex (Stern et al. 2001). Based on this finding, it is possible that the PFC may be recruited only when pre-exposed (i.e., highly familiar) objects, but not when trial-unique objects need to be maintained in working memory. Specifically, one candidate may be the dorsolateral PFC (DLPFC) that has been implicated in executive control functions.
Executive functions of the DLPFC include online monitoring and manipulation of information held in working memory regardless of stimulus material (Petrides 1995, Owen et al. 1996, Owen et al. 1999, D’Esposito et al. 1999, Curtis et al. 2000, Stern et al. 2000, Stern et al. 2001, Pochon et al. 2001). Other studies have shown that both DLPFC and ventrolateral PFC (VLPFC) are recruited when executive processes related to short-term storage are needed (Rypma et al. 1999, Stern et al. 2001, Barde and Thompson-Schill 2002, Glahn et al. 2002, Rypma et al. 2002, Veltman et al. 2003).
Another candidate for monitoring task relevant information may be the orbitofrontal cortex (OFC). In the rat, the OFC is necessary for delayed non-matching of odor stimuli drawn from a small stimulus set but not when the stimulus set is large (Otto and Eichenbaum 1992). In monkeys, the OFC, together with the VLPFC, has been shown to be important for selecting behaviorally relevant stimuli (Rushworth et al. 2005). A PET study using a 1-back continuous picture recognition paradigm in humans indicated that while initial learning activated the MTL, posterior medial orbitofrontal cortex activity was evident in subsequent runs in which previously seen pictures increasingly recurred (Schnider et al. 2000). This condition would require close monitoring of the currently relevant stimulus in the face of interference. Successful interference resolution has been shown to activate two cortical networks, one involving the VLPFC, and a secondary one involving the orbitofrontal cortex (Caplan et al. 2006). Thus, another potential candidate within the PFC may be the mid-VLPFC, as recent event-related fMRI (Stern et al. 2001, Henson et al. 2002, Badre and Wagner 2005, Caplan et al, 2006, see Jonides and Nee 2006 for review) and rTMS studies (Feredoes et al. 2006) have shown that the left VLPFC supports interference resolution.
In addition to sustained activity in dorsolateral and ventrolateral prefrontal cortex, delay-dependent activity has also been observed in the frontal eye fields (FEF) in monkeys (Funahashi et al. 1989, Gaymard et al. 1999, Umeno and Goldberg 2001, Sommer and Wurtz 2001), and in humans (Sweeney et al. 1996, Brown et al. 2004, Curtis et al. 2004, Leung et al. 2004, Linden et al. 2003, Postle et al. 2004, Mohr et al., 2006). Delay-period activity in the FEF has been attributed to maintenance of a prospective motor code or maintenance of a saccadic plan (Wurtz et al. 2001, Curtis et al. 2004, Curtis et al. 2005, Curtis and D’Esposito 2006), and to covert spatial attention (Kastner et al. 1999, see Pessoa et al. 2003, for review). Findings from monkey lesion (Sommer and Tehovnik 1997), and human neuropsychological (Pierrot-Deseilligny et al. 1993) studies also demonstrate a role of the FEF in spatial working memory.
Delay-dependent activity in the posterior superior frontal sulcus (SFS) has been observed specifically for short-term maintenance of spatial information in humans (Courtney et al. 1998, Rowe et al. 2000, Rowe and Passingham 2001, Glahn et al. 2002, Sala et al. 2003, Slotnick 2005, but see Postle 2005) and in a homologous region in the monkey (Chafee et al. 1998, Rainer et al. 1998a, Inoue et al. 2004). In both humans and monkeys, this spatial-specific delay-period activity was posterior and superior to the DLPFC, and distinct from and just anterior to the FEF. However, some studies found that activation in this region is not limited to short-term maintenance of spatial information (Jha and McCarthy 2000, Postle et al. 2000a, Zurowski et al. 2002), or that activity in this region is related to saccadic eye movements (Postle et al. 2000a, Brown et al. 2004).
Based on monkey neurophysiological recording data, activity in the mid-DLPFC has also been attributed specifically to the short-term maintenance of spatial information, whereas the ventrolateral PFC has been implicated in the short-term maintenance of nonspatial information (Funahashi et al., 1989, Wilson et al. 1993). However, there is very little support for this domain-specific specialization as most neuroimaging studies (D’Esposito et al. 1998, Owen et al. 1998, Postle and D’Esposito 1999a, Postle et al. 2000b, Nystrom et al. 2000) and single-unit recording studies (Rao et al. 1997, Rainer et al. 1998a, Rainer et al. 1998b, Ferrera et al. 1999) were unable to find such segregation.
This study addressed two questions: First, are there regions within the PFC that are more strongly engaged when stimuli are pre-exposed (i.e., highly familiar) than when they are not previously encountered (i.e., trial-unique)? Possible candidates include DLPFC, OFC, and VLPFC. Second, are the FEF, the posterior SFS, and the DLPFC preferentially recruited for short-term maintenance of locations, and is the VLPFC preferentially recruited for maintenance of objects? If so, can activity in these regions (outside FEF) be distinguished from saccadic eye movement related activity in the FEF? We addressed these questions by combining fMRI with a DMS task that allowed us to manipulate the stimulus type simultaneously on two dimensions: stimulus domain (object vs. its location) and stimulus pre-exposure (trial-unique vs. familiar objects), and added a visually-guided saccadic eye movement task.
MATERIALS AND METHODS
Subjects
Seventeen subjects (8 male and 9 female, mean age 21.29 ± 3.72, age range 18-30) were recruited from the student population at Boston University. All subjects were screened for MR compatibility, and subjects with a history or current condition of neurological or psychiatric illness were excluded. Vision was normal or corrected-to-normal. All subjects gave written informed consent to participate in this study in a manner approved by the Partners Human Research Committee of the Massachusetts General Hospital and by the Boston University Charles River Institutional Review Board.
Stimuli
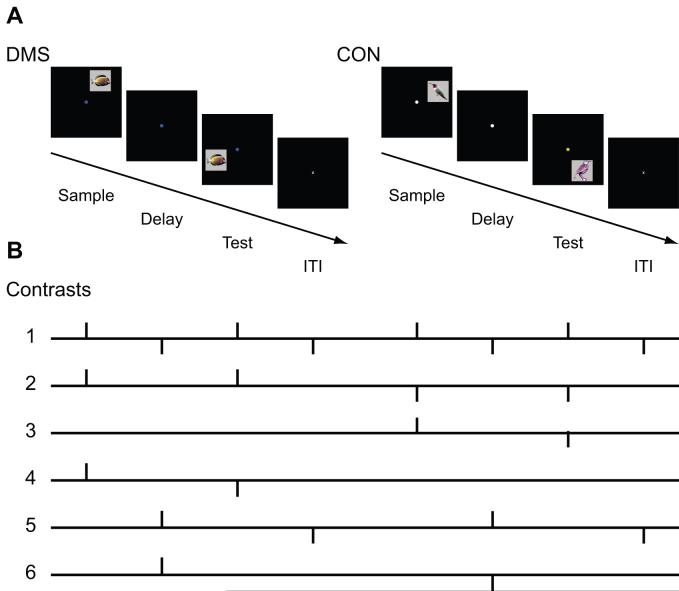
The stimuli for this experiment consisted of 3.8 cm × 3.8 cm digital color pictures of birds and fish on a uniform gray background, and 10 spatial locations around a central fixation dot (see Fig. 1A).
Figure 1.
Tasks and experimental contrasts. A. Delayed match-to-sample (DMS) task, and visual-motor control task (CON) without memory requirements. Subjects were instructed to either attend object or location information. In both tasks, the sample stimulus was presented for 2 s, and followed by an 8-s delay period, which, in turn was followed by a 2-s probe presentation (test). Each trial ended with a 10-s inter-trial interval (ITI). B. Contrasts were modeled after Courtney et al. (1998). Following the methods of Postle et al. (2000c) vertical bars represent positioning of the hemodynamic response function (HRF). Contrasts that were convolved with the HRF constituted the regressors for multiple regression analysis. Please see methods section for details.
Familiar objects
Approximately 20 minutes before the beginning of the functional scans, subjects saw a set of 5 birds and 5 fish on the center of a computer screen five times for 4 seconds each in a randomized order to familiarize themselves with the familiar stimulus set.
Trial-unique objects
All other stimuli (birds and fish) were not pre-exposed to the subjects and were trial-unique. Trial-unique stimuli were randomly selected from a set of 63 pictures of birds and 73 pictures of fish. Across all runs, a stimulus from the trial-unique set would be seen twice only in the case of a DMS match trial.
After stimulus familiarization, all subjects received detailed instructions on a computer screen and practiced the object and location tasks before scanning.
Tasks and Procedures
There were four tasks: spatial DMS, object DMS, spatial control task, and object control task (Fig. 1A). Subjects performed both object and location DMS tasks with either familiar, previously exposed objects or trial-unique, not previously exposed objects. Tasks differed only in instruction. During each DMS trial, subjects saw a picture of a bird or a fish in one of ten possible locations (sample presentation) and a central fixation dot for 2 s. Then, the sample stimulus disappeared and the screen remained black except for a central fixation dot for 8 s. At the end of the delay, the subject saw a stimulus in one of the ten locations for 2 s (probe presentation). The DMS tasks were designed such that attending to the irrelevant stimulus domain did not facilitate performance.
During the object DMS task (ODMS), subjects indicated via button press whether the test object was the same as that seen during sample, regardless of its location. To prevent interference among the tasks, during object trials, the probe location never matched the sample location. During the location DMS task (LDMS), subjects indicated via button press whether the location in which the object appeared during sample was identical to that at probe, regardless of the identity of the test object. To prevent interference among the tasks, during location trials probe objects never matched sample objects.
In addition to the DMS tasks, subjects also performed two different control tasks designed to control for visual input, decision-making, and motor response. During the control task, subjects were instructed to simply wait until the second stimulus (probe) appeared. For probes during object CON (OCON) trials, subjects indicated via button press whether this second stimulus was a bird or a fish. For probes during location CON (LCON) trials, subjects indicated via button press whether the location of the test stimulus was above or below the fixation dot. The tasks were designed such that maintenance of object or location information was maximized during the DMS delays and minimized during the control task delays.
To minimize brain activation due to task switching, functional runs either consisted of object trials (ODMS and OCON) or location trials (LDMS and LCON). The color of the central fixation dot indicated whether a DMS trial or a CON trial would begin (see Fig. 1A). In addition, before each functional run the subjects received a verbal instruction indicating that the upcoming run was an object or location task. Subjects performed 14 trials in each of eight functional runs (7 DMS and 7 CON trials per run, 4 object runs and 4 location runs). Two runs exclusively used highly familiar objects and two runs used trial-unique objects. Each subject performed a total of 112 trials.
After each 12-s trial, there was an intertrial interval (ITI) of 10 s to allow sufficient time for the hemodynamic response to return to baseline (Postle and D’Esposito 1999a, Postle and D’Esposito 1999b). The time between subsequent trial onsets was 22 s. The length of each run was 308 s.
The order of the eight runs, the order of the 14 trials within a run, and the order of the stimuli (locations, objects) were randomized. Each of the two possible behavioral responses of a given trial type (match vs. nonmatch, bird vs. fish, above vs. below) was correct 50% of the time for the corresponding task. No more than three subjects underwent scanning with exactly the same randomization of runs, trials, and stimuli.
Two runs of a visually guided saccadic eye movement (SEM) task were added to localize the frontal eye fields (FEF). Our SEM task was similar to the saccadic eye-movement task used by Petit et al. (1997). We used a visually-guided rather than a self-paced saccadic eye movement task because it has been suggested that previously observed posterior SFS activity (Courtney et al., 1998) may have been due to visually-guided saccades (Postle et al. 2000a) and not due to maintenance of spatial information or sustained spatial attention per se. Subjects made saccadic eye movements to a white fixation dot that moved with a frequency of two Hz (every 500 ms) randomly between five horizontal locations 5 or 10° to the right or left visual field, and to 0°. Each task started with 500 msec of central fixation (0°). Four 20-s blocks of moving dots alternated with four 20-sec blocks of central fixation within a run (Fig. 4B, bottom). Subjects were instructed to follow the dot with their eyes and not to anticipate its next location while keeping their head still. During each block of moving dots the dot appeared in each of the five locations equally often.
Figure 4.
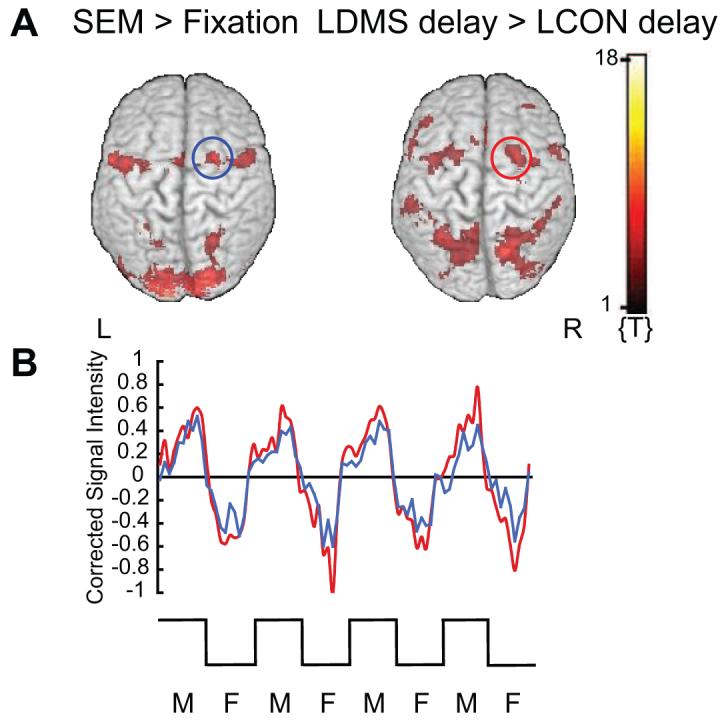
Posterior superior frontal cortex (BA 6), including posterior SFS (BA 6/8) activity during short-term maintenance of spatial locations and during saccadic eye movements. A. Activation related to short-term maintenance of spatial locations [main effect of (location delay > object delay) inclusively masked with one-sample t-contrast (LDMS delay > LCON delay, across stimulus pre-exposure); see Supplementary Materials, Table 1] [red circle, right posterior superior frontal cortex (BA 6), including posterior SFS (BA 6/8) image on right]; and activation related to saccadic eye movements (SEM > fixation)] [FEF; blue circle, right posterior superior frontal cortex (BA 6); image on left]. B. Corrected signal intensity time series during epochs of moving dots (M) and epochs of fixation (F), red corresponds to posterior superior frontal cortex (BA 6) peak from A (short-term maintenance of spatial locations), and blue corresponds to posterior superior frontal cortex (BA 6) peak from A (saccadic eye movement task; FEF). Functional overlays in A were created with MRIcro 1.38 software (Rorden and Brett, 2000) using a statistical threshold of t = 2.88, and were rendered on a canonical brain (ch2bet) provided with the software. For coordinates, Z-scores, and p-values of all brain areas activated during the saccadic eye movement task (SEM > fixation) see Table 4 of the Supplementary Materials online. y-Axes, signal intensity; baseline, overall grand mean. SEM, saccadic eye movements; FEF, frontal eye fields; R, right; L, left.
fMRI data acquisition
Data were acquired on a 3.0 Tesla Siemens MAGNETOM Allegra scanner (Erlangen, Germany) at the Athinoula A. Martinos Center for Biomedical Imaging (Massachusetts General Hospital, Charlestown, MA). Two high-resolution MP-RAGE T1-weighted structural images were acquired for each subject (matrix, 256 × 256; field of view, 256 mm; time to repetition, 6.6 ms; time to echo, 2.9 ms; flip angle, 8°; slice thickness, 1.33 mm). While the subjects performed the DMS and CON tasks, we ran eight functional T2*-weighted gradient-echo echo-planar scans (matrix, 64 × 64; field of view, 200 mm; time to repetition, 2000 ms; time to echo, 30 ms; flip angle, 90°; 21 slices with interleaved excitation order; slice thickness, 5 mm; 1 mm skip between slices; in-plane resolution, 3.125 × 3.125 mm; 154 acquisitions per slice). For the FEF localizer, we ran two functional scans using the same parameters as above, but with 80 acquisitions each. BOLD images were aligned along the AC-PC line to collect data from the whole brain.
fMRI data preprocessing
Using SPM2 (Wellcome Department of Cognitive Neurology, London, UK), we temporally smoothed all functional images by correcting them for differences in slice timing. After slice-time correction, all functional images were realigned to the first image within a series using 4th-degree B-spline interpolation, corrected for movement-by-susceptibility interactions, and were then normalized by warping them into standard MNI305 space (with trilinear interpolation, and resampling to 2 × 2 × 2 mm isotropic voxels). Before statistical analyses, all normalized images were spatially smoothed with a 6 mm full-width half-maximum Gaussian kernel.
Statistical analysis of fMRI data
Because of the time-locked nature of the events that constitute the DMS and CON trials (sample, then delay, then probe, etc.) we used multiple regression analysis with orthogonal regressors. This method has previously been proposed (Zarahn et al. 1997, Postle et al. 2000c) and used (Courtney et al. 1997, Courtney et al. 1998, Postle and D’Esposito 1999a, Postle and D’Esposito 1999b, Schon et al. 2004, Schon et al. 2005) for statistical analysis of time-locked events. With orthogonal regressors, hemodynamic changes to different task components that are segregated in time and that cannot be counterbalanced can be analyzed simultaneously and independently of each other. Similar to Courtney et al. (1998) and Schon et al. (2004, 2005), we created six (nearly) orthogonal contrasts that reflected comparisons of interest and convolved them with a hemodynamic response function (HRF) using a Gamma variate function (Boynton et al. 1996, Josephs et al. 1997) in MATLAB 6.5 (The Mathworks, Inc., Natick, MA). Parameters for the HRF were taken from Boynton et al. (1996).
We created the following six contrasts (Fig. 1B). Following Courtney et al. (1998), contrast 1 assessed activation due to nonspecific visual stimulation, contrast 2 assessed a difference in activation due to the type of stimuli (DMS vs. CON), contrast 3 assessed the difference between the sample stimulus and the probe stimulus for CON trials, contrast 4 assessed the difference between the sample stimulus and the probe stimulus for DMS trials, contrast 5 assessed differences in activity during task delays (across task) versus during the ITI, and contrast 6 assessed the difference in activity during the DMS delay versus activity during the CON delay (Fig. 1B). Following the methods of Postle et al. (2000c), we used “stick” contrasts instead of box-shaped contrasts (Courtney et al. 1998) to position the HRF in order to mitigate collinearity among the convolved regressors (Fig. 1B). Vertical bars represent positioning of the HRFs. Contrasts that were convolved with the HRF constituted the 6 regressors. This approach allowed us to isolate activity that was solely attributable to the delay component of the task. We defined short-term maintenance as differential delay-period activity resulting from regressor 6 (DMS delay activity > CON delay activity). We performed the multiple regression analysis for each subject with SPM2 by entering all six regressors as covariates, scaling the overall grand mean to 100, using proportional threshold masking, and a global calculation of the mean voxel value within per image fullmean/eight mask. This is a standard procedure in SPM that simply scales all data to the same value without altering the statistics. Voxels with less than 1/8 of this mean value are masked out. This procedure generated a statistical parametric t-map (SPM{T}) for each regressor and for each subject.
We entered all 17 SPM{T} maps (one from each subject) for each of the six regressors into second-level random-effects one-sample t-tests and repeated-measures ANOVAs. The ANOVA assessed simple and main effects of stimulus domain (object vs. location), and stimulus pre-exposure (familiar vs. trial-unique), and interactions between the two main factors. Because the current investigation was restricted to positive activations and the single-subject SPM{T} images that were entered into the ANOVA were difference images (DMS delay > CON delay), all covariates of the ANOVA were masked with the corresponding one-sample t-test during results assessment. For example, if one is interested in assessing delay-period activity that is greater for location trials than for object trials, then the main effect would have to be assessed with the contrast ([LDMS delay > LCON delay, across stimulus pre-exposure conditions] > [ODMS delay > OCON delay, across stimulus pre-exposure conditions] (see contrast #12 in Supplementary Materials Table 1). Since this activity compares a difference (DMS delay > CON delay for location trials) with another difference (DMS delay > CON delay for object trials), the resulting statistical parametric map (SPM) needs to be inclusively masked with the corresponding one-sample t-contrast(s). In this case, this is the contrast (LDMS delay > LCON delay, across stimulus pre-exposure conditions) (see one-sample t-contrasts lettered C) and D) in Supplementary Materials Table 1). Inclusive masking of one t-contrast with another one ensures that only voxels for which both contrasts are statistically significant will be displayed in the SPM. In this example, the resulting SPM will display only those voxels that show significantly greater activity during the delay period for location than for object trials and that also show significantly greater activity during the DMS delay than during the CON delay for location trials, across stimulus pre-exposure conditions. A list of all effects assessed by the ANOVA can be viewed online (see Table 1 of the Supplemental Materials). All second-level analyses treated subject as a random factor to allow generalization of results. In addition, statistical estimation in SPM2 included non-sphericity correction with replications over four repetitions (1: ODMS vs. OCON with trial-unique objects, 2: LDMS vs. LCON with trial-unique objects, 3: ODMS vs. OCON with familiar objects, 4: LDMS vs. LCON with familiar objects). For the DMS and CON tasks, the significance level to identify suprathreshold voxels was p ≤ 0.001 uncorrected for multiple voxel-wise comparisons. For the SEM task, the significance level to identify suprathreshold voxels was p ≤ 0.05 with FDR correction (Genovese et al. 2002) across the whole brain. The statistical threshold for the SEM task was set higher than that for the DMS and CON tasks, because the first is a blocked design with high estimation efficiency whereas the latter tasks used event-related analysis procedures with lower estimation power. In all cases the threshold extent was 5 voxels.
Table 1.
Delay period activity related to the short-term maintenance of visual nonspatial objects. Main effect (Object DMS delay > Location DMS delay, across stimulus pre-exposure) inclusively masked with one-sample t-tests (Object DMS delay > Object CON Delay, across stimulus pre-exposure). Note that inclusively masking two contrasts reveals activity that is present for both contrasts (conjunction), whereas exclusively masking two contrasts reveals activity that is present for the first but not for the second contrast. Highlighted in bold are functional blobs that are significant at p ≤ 0.001
| Object Delay Period Activity > Location Delay Period Activity | Coordinates (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Brain Areas | BA | T-score | Z-score | p(unc) | x | y | z |
| Inclusively masked with (ODMS delay > OCON delay) one-sample t-contrast | |||||||
| R Posterior orbitofrontal cortex | 11,47 | 7.11 | 6.08 | <0.001* | 24 | 34 | -16 |
| L Ventral anterior cingulate | 32,10 | 5.25 | 4.77 | <0.001* | -2 | 50 | -6 |
| R Putamen | 4.94 | 4.53 | <0.001* | 18 | 16 | -12 | |
| L Posterior orbitofrontal cortex | 11,47 | 5.54 | 4.99 | <0.001* | -34 | 42 | -14 |
| L Posterior orbitofrontal cortex | 11,47 | 5.17 | 4.71 | <0.001* | -26 | 36 | -14 |
| L Putamen | 3.51 | 3.34 | <0.001* | -18 | 20 | -6 | |
| R Cerebellum | 5.22 | 4.75 | <0.001* | 34 | -48 | -32 | |
| L Frontal pole | 10 | 5.07 | 4.63 | <0.001* | -14 | 58 | 4 |
| L Cerebellum | 4.62 | 4.28 | <0.001* | -34 | -36 | -32 | |
| Cerebellar Vermis | 4.45 | 4.14 | <0.001* | -2 | -54 | -12 | |
| R Precentral gyrus | 4,6 | 4.08 | 3.83 | <0.001* | 24 | -28 | 70 |
| L Frontomarginal sulcus (anterior VLPFC) | 10,47 | 4.07 | 3.83 | <0.001* | -26 | 58 | -4 |
| L Middle occipital gyrus | 18,19 | 3.86 | 3.65 | <0.001* | -38 | -88 | 10 |
| L Hippocampal head (uncus) | 3.78 | 3.58 | <0.001* | -10 | -6 | -18 | |
| L Perirhinal / entorhinal cortex | 28/34 | 3.66 | 3.47 | <0.001* | -30 | -10 | -26 |
| L Anterior inferior temporal gyrus | 20 | 3.51 | 3.34 | <0.001* | -40 | -12 | -32 |
| L Caudate | 3.64 | 3.46 | <0.001* | -10 | -2 | 16 | |
| L Inferior frontal sulcus (anterior DLPFC) | 46 | 3.61 | 3.43 | <0.001* | -22 | 50 | 20 |
| L Inferior frontal gyrus (mid-VLPFC) | 45 | 3.56 | 3.39 | <0.001* | -40 | 32 | 2 |
| R Temporal pole | 38 | 3.56 | 3.39 | <0.001* | 34 | 10 | -24 |
| R Hippocampal tail | 3.47 | 3.31 | <0.001* | 12 | -34 | 10 | |
| R Lat. post. fusiform gyrus | 37 | 3.44 | 3.28 | 0.001 | 34 | -64 | -14 |
| Pre-Supplementary Motor Area | 6 | 3.38 | 3.23 | 0.001 | 2 | 4 | 60 |
| Pre-Supplementary Motor Area | 6 | 3.32 | 3.18 | 0.001 | 6 | 16 | 60 |
| Pre-Supplementary Motor Area | 6 | 3.01 | 2.9 | 0.002 | 8 | 6 | 68 |
| L Cerebellum | 3.15 | 3.03 | 0.001 | -10 | -46 | -40 | |
| R Insula | 3 | 2.89 | 0.002 | 40 | 12 | -2 | |
| L Insula | 2.99 | 2.88 | 0.002 | -44 | 10 | 0 | |
| R Precuneus | 5,7 | 2.91 | 2.81 | 0.002 | 10 | -44 | 56 |
(denotes peak is also statistically significant at Pfdr < 0.05 across whole brain). L. left; R, right; BA, Brodmann area; p(unc), p-value, uncorrected for multiple voxel-wise comparisons. Note that Brodmann areas are approximate and are derived from AAL atlas provided with MRIcro (Rorden and Brett, 2000) using definitions from the Braininfo database (http://braininfo.rprc.washington.edu/menumain.html).
Time series extraction
We extracted signal intensities from functionally defined spheres with a radius of 5 mm. The center coordinates of the spheres were taken from statistical peaks from the repeated-measures ANOVA using the VOI tool in SPM2, from the one-sample t-test when appropriate, and from the moving dot versus fixation dot contrast (SEM > Fixation) of the SEM task. The signal-intensity time series (Y) were adjusted for all effects of interest. Signal intensities extracted from DMS and CON trials were sorted selectively by task (DMS vs. CON), stimulus pre-exposure (trial-unique vs. familiar objects), stimulus domain (objects vs. locations), and event (sample vs. delay vs. probe).
Data analysis was focused on regions of interest (ROIs) in the frontal lobe. These ROIs included the VLPFC, the DLPFC, the OFC, the posterior SFS, and the FEFs.
Interpretation of bar graphs
Bar graphs (Figures 2-4) demonstrate signal intensities as a function of task, stimulus pre-exposure, stimulus domain, and event, such that the baseline is the overall grand mean of the data. Because the global mean was scaled to 100, the height of the bars in the figures displays % signal change with respect to the global mean intensity of the scaled images. The tasks were designed such that the CON task serves as the baseline. Thus, significant positive activations will be evident for events where DMS > CON is true, and no difference between DMS and CON events demonstrate that activity was not different from baseline. In addition, negative activations (deactivations) will be evident for events where DMS < CON is true. Note that for the delay period signal intensities of four TRs were averaged.
Figure 2.
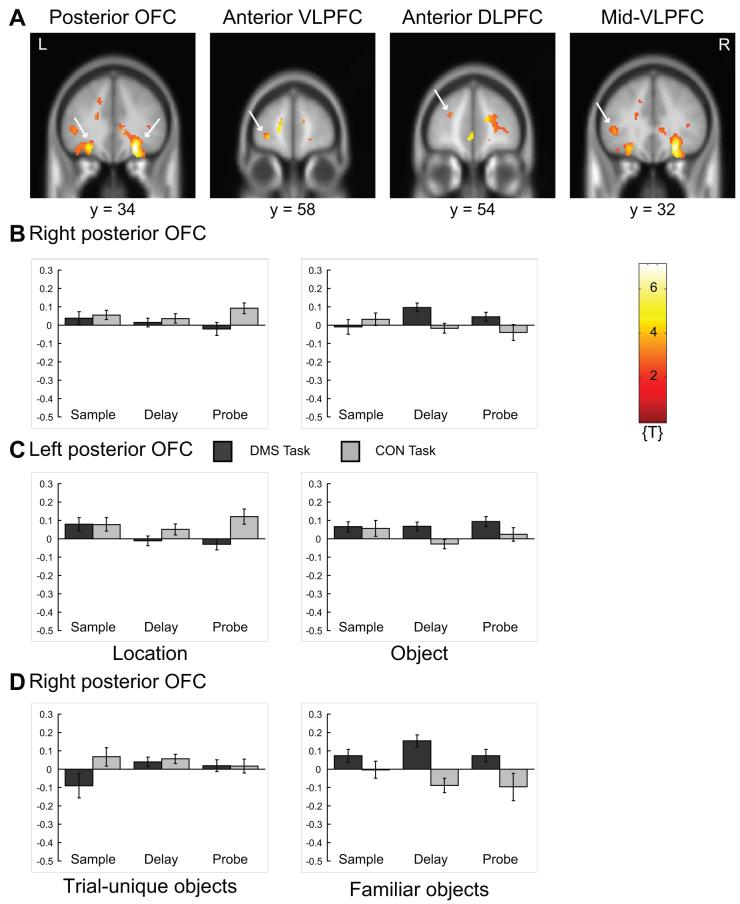
Activity related to short-term maintenance of objects in prefrontal cortex. Please note that activation is displayed on canonical average T1-weighted ICBM/MNI brain using p < 0.01, uncorrected. A. Activations of posterior OFC (BA 11, 47), left frontomarginal sulcus (anterior VLPFC; BA 10, 47), left inferior frontal sulcus (anterior DLPFC; BA 46), and left inferior frontal gyrus (mid-VLPFC; BA 45) correspond to Table 1 [main effect of (object delay > location delay) inclusively masked with one-sample t-contrast (ODMS delay > OCON delay, across stimulus pre-exposure); see Supplementary Materials, Table 1]. B. Corrected signal intensities from right posterior orbitofrontal cortex (BA 11, 47) ([x y z] = [24 34 -16]) during sample presentation, delay-period, and probe presentation of delayed match-to-sample (DMS) and control (CON) tasks for object trials (graph on right) and location trials (graph on left). C. Same as in B but for left posterior orbitofrontal cortex (BA 11, 47) ([x y z] = [-34 42 -14]). D. Same as in B for object trials with familiar objects (graph on right) and for object trials with trial-unique objects (graph on left). y-Axes, signal intensity; baseline, overall grand mean. Light gray bars, CON trial; dark gray bars, DMS trial; OFC, orbitofrontal cortex; VLPFC, ventrolateral prefrontal cortex; DLPFC, dorsolateral prefrontal cortex; R, right; L, left.
Statistical analysis of behavioral data
Median reaction times (RTs), and proportion of correct responses were calculated separately for each subject and then averaged by task (DMS vs. CON), stimulus domain (object vs. spatial), and stimulus pre-exposure (familiar vs. trial-unique objects) by calculating means and SE. Repeated-measures ANOVAs assessed the main effects and interactions of these factors separately for mean RTs and proportion of correct responses. Repeated-measures ANOVAs with these within-subject factors used the three-way interaction as the between-subject error term.
RESULTS
Behavioral performance
A repeated-measures ANOVA on the mean RTs (N = 16) revealed a significant main effect of stimulus domain (F(1,120) = 9.20, p = 0.003). Subjects were faster on location trials than on object trials (Mean RT ± SE; LDMS with trial-unique objects, 1001 ± 57; LDMS with familiar objects, 989 ± 80; ODMS with trial-unique objects, 1214 ± 110; ODMS with familiar objects, 1173 ± 104). No other main effects or interactions reached statistical significance (p > 0.05). With regard to the proportion of correct responses, the ANOVA did not reveal any significant main effects or interactions, indicating that the subjects performed similarly on DMS and CON tasks (Mean Prop. Correct ± SE; LDMS with trial-unique objects, 0.93 ± 0.02; LDMS with familiar objects, 0.95 ± 0.02; ODMS with trial-unique objects, 0.95 ± 0.02; ODMS with familiar objects, 0.92 ± 0.03). We were unable to record behavioral responses from one subject.
fMRI Results
We were interested in investigating the role of the PFC in short-term maintenance of visual-spatial and trial-unique and familiar visual nonspatial information. Therefore, results reported in this section refer only to delay-period activity.
Role of the PFC in short-term maintenance of visual objects
Short-term maintenance of nonspatial information recruits the posterior orbitofrontal cortex within the ventral PFC
The repeated-measures ANOVA (inclusively masked with the corresponding one-sample t-test) revealed a main effect of stimulus domain (object > location) bilaterally in the ventral PFC (posterior orbitofrontal cortex; OFC; BA 11, 47), the left frontomarginal sulcus (anterior VLPFC; BA 10, 47), the left inferior frontal sulcus (anterior DLPFC; BA 46), and the left inferior frontal gyrus (mid-VLPFC; BA 45) (Table 1 and Table 2A). Within the prefrontal cortex, this main effect of stimulus domain during the delay period (object > location), was most prominent in the OFC.Time-series of selectively averaged signal intensities show that this effect was greater in the right posterior OFC when objects were highly familiar, than when objects were trial-unique (Fig. 2D). Activity in left and right posterior OFC including signal-intensity time series is illustrated in Fig. 2. Outside the PFC, short-term maintenance of objects recruited posterior ventral and motor-related brain areas (Table 1).
Table 2.
Delay period activity related to the short-term maintenance of visual nonspatial objects as a function stimulus pre-exposure (trial-unique vs. familiar). A. Simple effect (Object DMS delay > Object CON delay, familiar objects) > (Object DMS delay > Object CON delay, trial-unique objects) inclusively masked with one-sample t-test (Object DMS delay > Object CON delay, familiar objects). B. One-sample t-contrast (ODMS delay > OCON delay for trials with familiar objects). C. Simple effect (Object DMS delay > Object CON delay, trial-unique objects) > (Object DMS delay > Object CON delay, familiar objects) exclusively masked with one-sample t-test (Object DMS delay > Object CON delay, trial-unique objects). Note that inclusively masking two contrasts reveals activity that is present for both contrasts (conjunction), whereas exclusively masking two contrasts reveals activity that is present for the first but not for the second contrast. Highlighted in bold are functional blobs that are significant at p ≤ 0.001. L. left; R, right; BA, Brodmann area; p(unc), p-value, uncorrected for multiple voxel-wise comparisons. Note that Brodmann areas are approximate and are derived from AAL atlas provided with MRIcro (Rorden and Brett, 2000) using definitions from the Braininfo database (http://braininfo.rprc.washington.edu/menumain.html).
| Delay period activity: Familiar Objects | Coordinates (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Brain Areas | BA | T-score | Z-score | p(unc) | x | y | z |
| nA. Familiar Objects > Novel Objects: Inclusively masked with (ODMS delay > OCON delay, familiar objects) one-sample t-contrast | |||||||
| R Superior temporal gyrus | 22 | 4.3 | 4.01 | <0.001 | 46 | -16 | -2 |
| R Superior temporal gyrus | 22 | 4.08 | 3.83 | <0.001 | 46 | -8 | -4 |
| R Posterior orbitofrontal cortex | 11,47 | 3.98 | 3.75 | <0.001 | 24 | 26 | -26 |
| R Central sulcus | 43 | 3.05 | 2.93 | 0.002 | 62 | 2 | 24 |
| L Anterior dorsomedial PFC | 10 | 2.72 | 2.63 | 0.004 | -8 | 58 | 38 |
| B. One-sample t-contrast (ODMS delay > OCON delay, familiar objects) | |||||||
| R Precentral gyrus | 6 | 5.18 | 4.71 | <0.001 | 24 | -24 | 74 |
| R Precentral gyrus | 6 | 4.22 | 3.95 | <0.001 | 26 | -32 | 70 |
| R Central sulcus | 4 | 3.53 | 3.36 | <0.001 | 42 | -26 | 64 |
| L Cerebellum | 5 | 4.58 | <0.001 | -32 | -48 | -26 | |
| L Lateral posterior fusiform gyrus | 37 | 4.74 | 4.37 | <0.001 | -40 | -60 | -16 |
| L Lateral posterior fusiform gyrus | 37 | 4.1 | 3.85 | <0.001 | -46 | -66 | -20 |
| R Dorsal anterior cingulate cortex | 32 | 4.79 | 4.41 | <0.001 | 10 | 16 | 42 |
| L Dorsal anterior cingulate cortex | 32 | 4.44 | 4.13 | <0.001 | -4 | 14 | 42 |
| R Pre-supplementary motor area | 6 | 4.17 | 3.91 | <0.001 | 4 | 12 | 52 |
| R Posterior orbitofrontal cortex | 11,47 | 4.53 | 4.21 | <0.001 | 24 | 26 | -26 |
| R Posterior orbitofrontal cortex | 11,47 | 4.18 | 3.92 | <0.001 | 32 | 30 | -26 |
| R Head of caudate | 3.97 | 3.74 | <0.001 | 20 | 28 | -2 | |
| L Posterior intraparietal sulcus | 5,7 | 4.07 | 3.83 | <0.001 | -12 | -70 | 62 |
| L Posterior intraparietal sulcus | 5,7 | 3.78 | 3.58 | <0.001 | -16 | -76 | 42 |
| L Posterior intraparietal sulcus | 5,7 | 3.73 | 3.54 | <0.001 | -18 | -74 | 52 |
| L Precentral gyrus | 6 | 4.06 | 3.81 | <0.001 | -28 | -4 | 46 |
| L Precentral gyrus | 6 | 3.97 | 3.74 | <0.001 | -32 | 0 | 52 |
| R lateral occipitotemporal sulcus | 37 | 3.96 | 3.73 | <0.001 | 50 | -60 | -12 |
| L Caudate | 3.84 | 3.62 | <0.001 | -16 | 0 | 26 | |
| L Caudate | 3.38 | 3.23 | 0.001 | -10 | 0 | 16 | |
| L Precentral gyrus | 6 | 3.7 | 3.5 | <0.001 | -44 | -4 | 30 |
| L Lateral occipitotemporal sulcus | 20 | 3.67 | 3.48 | <0.001 | -40 | -24 | -24 |
| R Middle frontal gyrus (DLPFC) | 46 | 3.62 | 3.44 | <0.001 | 48 | 34 | 34 |
| R Inferior frontal gyrus (VLPFC) | 45 | 3.56 | 3.39 | <0.001 | 42 | 36 | 8 |
| L Inferior frontal gyrus (VLPFC) | 45 | 3.53 | 3.37 | <0.001 | -52 | 34 | 2 |
| L Precuneus | 7 | 3.48 | 3.32 | <0.001 | -6 | -46 | 58 |
| R Superior temporal gyrus | 22 | 3.41 | 3.26 | 0.001 | 60 | -10 | -6 |
| R Middle occipital gyrus | 19 | 3.4 | 3.25 | 0.001 | 48 | -76 | 12 |
| L Precentral gyrus | 6 | 3.31 | 3.17 | 0.001 | 30 | -2 | 50 |
| R Lateral posterior fusiform gyrus | 37 | 3.31 | 3.16 | 0.001 | 34 | -62 | -18 |
| R Cerebellum | 3.18 | 3.05 | 0.001 | 38 | -42 | -28 | |
| R Precuneus | 7 | 3.16 | 3.04 | 0.001 | 10 | -48 | 54 |
| L Cerebellum | 3.15 | 3.02 | 0.001 | -6 | -74 | -40 | |
| Delay period activity (novel objects) > delay period activity (familiar objects) | |||||||
| C Object, Inclusively masked with (ODMS delay > OCON delay, low) one-sample t contrast | |||||||
| R Cerebellum | 3.63 | 3.45 | <0.001 | 36 | -82 | -24 | |
Inclusive masking of the ANOVA contrast image with that from the SEM task (moving dot > fixation dot) revealed that the FEFs were not active during short-term maintenance of nonspatial objects (Supplementary Materials online, Table 2A). Exclusive masking of the ANOVA contrast image with that from the SEM task did not reveal any activity in the posterior SFS (BA 6/8) (Supplemental Materials online, Table 2B).
Short-term maintenance of a small set of highly familiar visual objects recruits the posterior OFC
The repeated-measures ANOVA (inclusively masked with the corresponding one-sample t-test) revealed a simple effect of object pre-exposure (familiar > trial-unique) in the right posterior OFC (BA 11, 47) for object trials (Table 2A). The only additional prefrontal area that showed a statistical trend was in the anterior dorsomedial PFC (BA 10). Time-series of selectively averaged signal intensities illustrate this effect (Fig. 2D). Additional areas active for this contrast are listed in table 2A. While the ANOVA did not reveal any activity in the DLPFC or VLPFC for this contrast, the one-sample t-test contrasting ODMS delays with OCON delays for trials with highly familiar objects showed activity bilaterally in the inferior frontal gyrus (IFG; VLPFC; BA 45) and in the middle frontal gyrus (MFG; DLPFC; BA 46) (Table 2B).
The simple effect of object pre-exposure (trial-unique > familiar) for object trials revealed no suprashreshold voxels at p < 0.001, uncorrected, except for one in the right cerebellum (Table 2C).
Delay-related PFC activity in short-term maintenance of locations
Short-term maintenance of locations recruits the posterior SFS
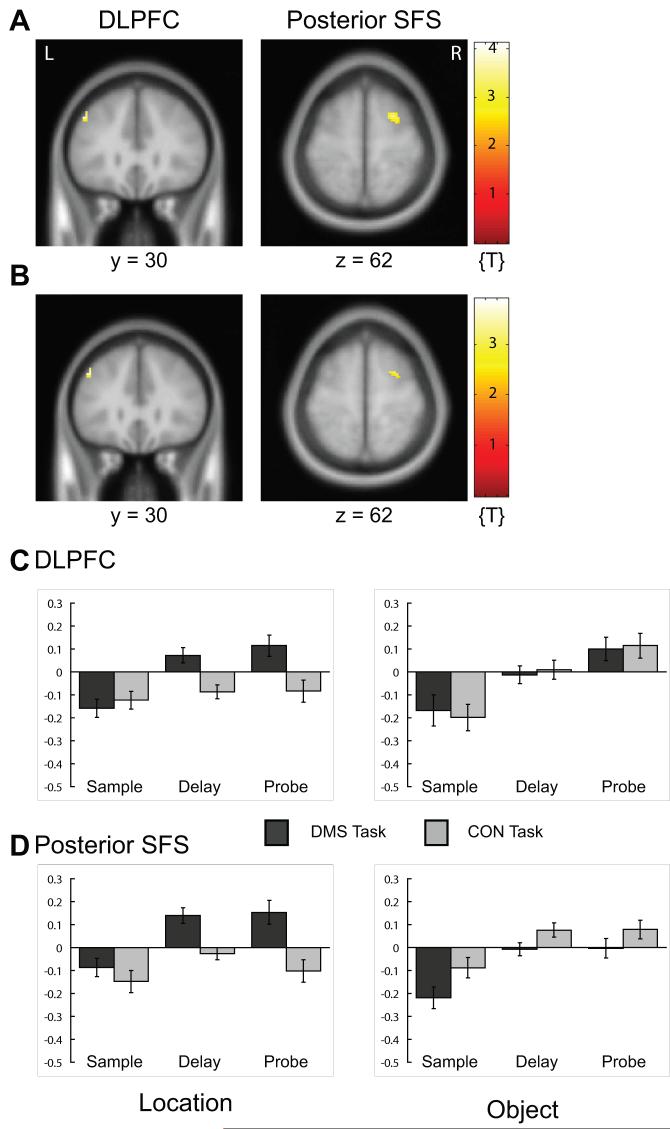
The repeated-measures ANOVA (inclusively masked with the corresponding one-sample t-test) revealed a main effect of stimulus domain (spatial > object) in the left middle frontal gyrus (DLPFC; BA 46), in the right posterior SFS (BA 6/8), the bilateral posterior superior frontal cortex (BA 6), and in posterior regions including the right posterior (BA 7), and the right anterior intraparietal sulcus (IPS) (BA 39) (Table 3A). Note that the one-sample t-test contrasting LDMS delays with LCON delays (across both stimulus pre-exposure conditions) showed identical results. Activity in the left middle frontal gyrus (DLPFC) and the right posterior SFS, including signal-intensity time series are illustrated in Fig. 3. Inclusive masking of the ANOVA contrast image with that from the SEM task (SEM > fixation) revealed FEF activity bilaterally during short-term maintenance of locations (Table 3B). Exclusive masking of the ANOVA contrast image with that from the SEM task demonstrated that the posterior SFS activity was not related to visually-guided saccadic eye movements ([x y z] = [24 6 62], Table 3C). Posterior SFS delay-related activity was related to short-term maintenance of locations. Note that the biggest effect was not located in the PFC but in the right posterior IPS, an area that was also active when subjects performed saccadic eye movements.
Table 3.
Delay period activity related to the short-term maintenance of locations. A. Main effect of (Location DMS delay > Object DMS delay, across stimulus pre-exposure) inclusively masked with the (Location DMS delay > Location CON delay, across stimulus pre-exposure) one-sample t-test. B. Same as in A, but inclusively masked with the saccadic eye movement contrast (SEM > fixation). C. Same as in A, but exclusively masked with saccadic eye movement contrast (SEM > fixation). D. Interaction between stimulus domain (object vs. location) and stimulus pre-exposure (trial-unique vs. familiar) for DMS delays, inclusively masked with the (Location DMS delay > Location CON Delay, across object novelty) one-sample t-tests. Note that inclusively masking two contrasts reveals activity that is present for both contrasts (conjunction), whereas exclusively masking two contrasts reveals activity that is present for the first but not for the second contrast. Highlighted in bold are functional blobs that are significant at p ≤ 0.001. L. left; R, right; BA, Brodmann area; p(unc), p-value, uncorrected for multiple voxel-wise comparisons. Note that Brodmann areas are approximate and are derived from AAL atlas provided with MRIcro (Rorden and Brett, 2000) using definitions from the Braininfo database (http://braininfo.rprc.washington.edu/menumain.html).
| Location delay period activity > object delay period activity | Coordinates (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Brain Areas | BA | T-score | Z-score | p(unc) | x | y | z |
| A. Inclusively masked with (LDMS delay > LCON delay) one-sample t-contrast | |||||||
| R posterior intraparietal sulcus | 7 | 4.12 | 3.86 | <0.001 | 20 | -76 | 52 |
| L middle frontal gyrus (DLPFC) | 46 | 3.91 | 3.69 | <0.001 | -44 | 30 | 38 |
| R posterior superior frontal sulcus | 6/8 | 3.43 | 3.27 | 0.001 | 24 | 6 | 62 |
| R posterior superior frontal cortex (FEF) | 6 | 2.82 | 2.73 | 0.003 | 24 | -2 | 54 |
| R anterior intraparietal sulcus | 39 | 3.15 | 3.02 | 0.001 | 42 | -48 | 42 |
| R anterior intraparietal sulcus | 39 | 3.03 | 2.92 | 0.002 | 46 | -54 | 56 |
| L posterior superior frontal cortex (FEF) | 6 | 2.96 | 2.86 | 0.002 | -20 | 0 | 52 |
| L supramarginal gyrus | 40 | 2.96 | 2.85 | 0.002 | -58 | -34 | 40 |
| R supramarginal gyrus | 40 | 2.94 | 2.84 | 0.002 | 60 | -34 | 48 |
| B. Inclusively masked with saccadic eye movement one-sample t-contrast | |||||||
| R posterior intraparietal sulcus | 7 | 4.12 | 3.86 | <0.001 | 20 | -76 | 52 |
| R posterior superior frontal cortex (FEF) | 6,8 | 3.22 | 3.09 | 0.001 | 26 | 4 | 62 |
| R posterior superior frontal cortex (FEF) | 6 | 2.82 | 2.73 | 0.003 | 24 | -2 | 54 |
| R anterior intraparietal sulcus | 39 | 3.01 | 2.9 | 0.002 | 40 | -48 | 44 |
| L posterior superior frontal cortex (FEF) | 6 | 2.96 | 2.86 | 0.002 | -20 | 0 | 52 |
| L cerebellum | 2.88 | 2.78 | 0.003 | -24 | -86 | -26 | |
| C. Exclusively masked with saccadic eye movement one-sample t-contrast | |||||||
| L middle frontal gyrus (DLPFC) | 46 | 3.91 | 3.69 | <0.001 | -44 | 30 | 38 |
| R posterior superior frontal sulcus | 6/8 | 3.43 | 3.27 | 0.001 | 24 | 6 | 62 |
| R anterior intraparietal sulcus | 39 | 3.15 | 3.02 | 0.001 | 42 | -48 | 42 |
| R precuneus | 7 | 3.07 | 2.95 | 0.002 | 10 | -72 | 48 |
| R anterior intraparietal sulcus | 39 | 3.03 | 2.92 | 0.002 | 46 | -54 | 56 |
| L supramarginal gyrus | 40 | 2.96 | 2.85 | 0.002 | -58 | -34 | 40 |
| R supramarginal gyrus | 40 | 2.94 | 2.84 | 0.002 | 60 | -34 | 48 |
| D. Interaction between stimulus domain and stimulus pre-exposure | |||||||
| L pulvinar | 2.98 | 2.87 | 0.002 | -16 | -28 | 6 | |
| R posterior superior frontal sulcus | 2.94 | 2.83 | 0.002 | 22 | 8 | 56 | |
Figure 3.
Activity related to short-term maintenance of locations in prefrontal cortex. Please note that activation is displayed on canonical average T1-weighted ICBM/MNI brain using p < 0.01, uncorrected. A. Activations of middle frontal gyrus (DLPFC; BA 46) (image on left) and posterior SFS (BA 6/8) (image on right) correspond to Table 3A [main effect of (location delay > object delay) inclusively masked with one-sample t-contrast (LDMS delay > LCON delay, across stimulus pre-exposure); see Supplementary Materials, Table 1]. B. Activations of middle frontal gyrus (DLPFC; BA 46) (image on left) and posterior SFS (BA 6/8) (image on right) correspond to Table 3C (exclusively masked with SEM > Fixation contrast). C. Corrected signal intensities extracted from the left middle frontal gyrus (DLPFC; BA 46) ([x y z] = [-44 30 38]) during sample presentation, delay-period, and probe presentation of delayed match-to-sample (DMS), and control (CON) tasks for object trials (graph on right) and location trials (graph on left). D. Same as in C but for right posterior SFS (BA 6/8) ([x y z] = [24 6 62]). y-Axes, signal intensity; baseline, overall grand mean. Light gray bars, CON trial; dark gray bars, DMS trial; R, right; L, left.
When objects were highly familiar during LDMS trials (i.e., when the irrelevant stimulus domain was the familiar objects), the ANOVA revealed activity in the right posterior SFS (BA 6/8), left MFG (BA 46), and right posterior IPS (BA 7) (Supplementary Materials online, Table 3A). In contrast, when objects were trial-unique, the ANOVA (inclusively masked with the corresponding one-sample t-test) did not reveal any suprathreshold voxels at an uncorrected threshold of p = 0.001 (Supplementary Materials online, Table 3B). Consistent with this result, note that the interaction between stimulus domain and stimulus pre-exposure showed a statistical trend in the posterior SFS (BA 6/8) (Table 3D).
Role of the frontal eye fields in short-term maintenance of locations
We functionally localized the FEF using a visually-guided saccadic eye movement task. A random-effects analysis that compared epochs of moving dots (visually guided saccadic eye movements) with epochs of central fixation (p = 0.05, FDR-corrected; threshold extent, 5 voxels) revealed significant activation bilaterally in the posterior superior frontal cortex, including the superior frontal gyrus and the superior frontal sulcus (BA 6), and in the precentral gyrus (BA 6). These results are illustrated in Fig. 4A (see also Supplementary Materials, Table 4). These results are consistent with previous studies that have reported that the FEF are located in the vicinity of precentral sulcus and the most posterior part of the SFS (Paus 1996, Petit et al. 1997, Koyama et al. 2004), and that their location is posterior to the anterior commissure (y < 0 mm) (Rowe et al. 2000, Rowe and Passingham 2001). In our data, we defined the location of the FEF functionally in the right and left posterior superior frontal cortex (BA 6).
To investigate further whether the FEF were located in the same area that we observed for the short-term maintenance of spatial locations, we extracted signal-intensity time series from the LDMS delay versus LCON delay one-sample t-contrast using the frontal eye field activation peaks ([x y z] = [22 -2 54], and [x y z] = [-26 -8 54]) and sorted them by task (DMS vs. CON), stimulus domain (object vs. location), object pre-exposure (trial-unique vs. familiar objects), and event (sample vs. delay vs. test). We investigated whether the activation peaks in the posterior superior frontal cortex region (BA 6, 8) from the analysis contrasting LDMS delays with LCON delays were the same as those reported above. We extracted signal-intensity time series from the SEM task using peaks from the LDMS delay versus LCON delay one-sample t-contrast ([x y z] = [28 -6 50], [x y z] = [-24 -6 54], and [x y z] = [30 0 64]) and sorted them by epoch (SEM vs. fixation).
Time-series extracted from the right and left posterior superior frontal cortex (right FEF; y = -2, and y = -8, respectively; BA 6) as defined by the FEF localizer scans indicate that the right FEFs were recruited for the short-term maintenance of spatial information (not depicted). In addition, the time-series for the left posterior superior frontal cortex peak (left FEF; BA 6) was identical to that from the left posterior superior frontal cortex (BA 6) peak as defined functionally by the LDMS delay versus LCON delay contrast (not depicted). However, there was no significant activation at the more anterior peak of the posterior SFS as defined by the LDMS delay versus LCON delay contrast of the one-sample t-test when this peak was entered into the moving dot versus fixation dot contrast of the FEF scans (p > 0.02, uncorrected). Therefore, activity in the posterior superior frontal cortex (BA 6) as defined functionally by the LDMS delay versus LCON delay contrast was identical to activity in the posterior superior frontal cortex (BA 6) as defined functionally by the FEF localizer scans, except for the more anterior peak within the posterior SFS (BA 6/8). This latter region is distinct from the FEF and is recruited specifically for the short-term maintenance of spatial locations.
We also extracted VOI time series from the FEF localizer scans using the right and left peaks from the posterior superior frontal cortex (BA 6) that were defined functionally by the LDMS delay versus LCON delay contrast. Figure 4B illustrates that activity in these regions (here illustrated for the right posterior superior frontal cortex; BA 6) is increased during saccadic eye movements, and decreased during fixation (red line). Furthermore, they follow the same pattern as the posterior superior frontal cortex (BA 6) peaks defined functionally by the FEF localizer (blue line).
These results illustrate that the peaks within the posterior superior frontal cortex (BA 6) from the SEM task were not different from the peaks within the same region that were significant for the short-term maintenance of spatial locations. However, a more anterior region within the right posterior SFS (BA 6/8) was preferentially recruited for the active maintenance of spatial locations. In addition, our results demonstrate that the FEF are recruited for the short-term maintenance of spatial locations.
DISCUSSION
Our results demonstrate that the most significant PFC activity related to the short-term maintenance of nonspatial objects across pre-exposure conditions was in the posterior OFC (BA 11, 47), not in the VLPFC, and importantly, OFC activity was greatest when objects to be maintained in short-term memory were familiar. The right DLPFC (right MFG; BA 46) and right and left VLPFC (inferior frontal gyrus; BA 45) were additionally recruited with familiar objects when DMS delays were contrasted with CON delays (Table 2B), however, these areas were not modulated by stimulus pre-exposure (Table 2A). This result suggests that the posterior OFC, may be recruited for the short-term maintenance of visual objects when interference among the stimuli and, hence, monitoring requirements are high, an interpretation consistent with a processing-specificity view of PFC function (Petrides 1995, Owen et al. 1996, Owen et al. 1998). With a small set of highly familiar items, it is much harder to keep track of (monitor) which stimulus is currently the task relevant one than when there is a larger set of familiar items or when the items are trial-unique. Thus, in the condition where a small set of stimuli are repeatedly presented and must be maintained, we propose that the prefrontal cortex, and in particular the OFC, is recruited to monitor which stimulus is currently task relevant.
In contrast, the FEF bilaterally (posterior superior frontal cortex; BA 6), and the right posterior SFS (BA 6/8) were preferentially recruited to maintain spatial information across a delay.
Posterior brain areas were recruited in both stimulus domains regardless of object pre-exposure. Posterior parietal regions (including the posterior IPS) were recruited for the short-term maintenance of locations, and posterior temporal regions (including medial temporal areas) were recuited for the short-term maintenance of nonspatial objects (Tables 3 and 1, respectively).
Does the orbitofrontal cortex play a role in the short-term maintenance of visual nonspatial objects?
While most previous working memory studies have focused on lateral PFC regions, a few previous fMRI studies have also shown that the OFC (BA 11, 47) is active during delayed matching-to-sample and delayed nonmatching-to-sample (Elliott and Dolan 1999, Lamar et al. 2004). The OFC may support the encoding of visual nonspatial information into a short-term memory buffer, so that it can maintain this information during working memory. Evidence for a role of the OFC in encoding comes from several areas of research. Frey and Petrides, using PET in humans, demonstrated that orbitofrontal activity is related to encoding of abstract visual patterns and faces (Frey and Petrides 2000, 2002, 2003), and in nonhuman primates, novel visual stimuli activate neurons in the OFC (Rolls et al. 2005). Anatomically, there are strong connections between the medial temporal areas and the OFC (Insausti and Munoz 2001, Munoz and Insausti 2005). Consistent with this, in the current study, short-term maintenance of objects recruited medial temporal regions in addition to the OFC (Table 1). Specifically, we observed this activity in the left hippocampal head (uncus), in the left perirhinal / entorhinal cortex, and in the right hippocampal tail. This is consistent with previous work by us and others that has demonstrated delay-period activity in medial temporal regions including hippocampus and perirhinal / entorhinal cortex during delayed matching to sample (Stern et al., 2001; Ranganath et al., 2001; Schon et al., 2004). Delay-period activity in the MTL, while not modulated by stimulus pre-exposure in our study, has been shown to support long-term encoding (Schon et al., 2004; Schon et al., 2005), a mechanism that may possibly be responsible also for MTL activity in the current study.
Our OFC activity was evident within the direct contrast of delay-period activity of object trials with delay-period activity of location trials. The object DMS task included an additional requirement that the locations in which the objects were displayed needed to be ignored. Given the role of the OFC in selecting the appropriate behavior in complex settings (Bechara et al. 1997, Barbas et al. 2002), one role of the OFC in the current study may be to monitor which stimulus dimension is performance-relevant in the face of interference. Support for this idea comes from rat studies demonstrating that rats with orbitofrontal lesions are impaired on delayed non-matching to sample when interference is high (Otto and Eichenbaum 1992), and from human neuroimaging studies on interference resolution (Schnider et al. 2000, Caplan et al. 2006). The orbitofrontal cortex may therefore track performance relevant information (Schoenbaum and Setlow 2001) in the face of interference and may send top-down control signals to posterior brain areas. Consistent with this notion, the OFC provides top-down facilitation during object recognition as demonstrated by a combined MEG-fMRI study (Bar et al. 2006). In addition, there are reciprocal connections between OFC and ventral processing stream areas (see Cavada et al. 2000 for review).
Do segregated regions within the prefrontal cortex support the short-term maintenance of objects and locations during delayed matching?
The results demonstrate a functional preference, but not a strong process-specific or domain-specific segregation, within the lateral PFC, such that the right and left ventral PFC, including OFC (BA 11, 47) and VLPFC (right and left IFG; BA 45), are preferentially engaged during the short-term maintenance of nonspatial visual objects while the right posterior SFS (BA 6/8) (anterior to the FEF) together with the FEF (posterior superior frontal cortex; BA 6) are preferentially recruited for the short-term maintenance of spatial information.
Familiar stimuli may require close monitoring via top-down attentional control of task-relevant stimuli in the face of interference. Consistent with this idea, working memory and interference resolution have been shown to share a common neural substrate including the dorsolateral and the ventrolateral PFC (Bunge et al. 2001). In addition, Sakai et al. (2002) found that the dorsolateral PFC actively maintains verbal information during the working memory delay in the face of distraction. The finding that both dorsolateral and ventrolateral PFC are involved in the short-term maintenance of familiar objects (while not modulated by stimulus pre-exposure) is also consistent with the idea of overlapping representations in the lateral PFC (Rao et al. 1997, Rainer et al. 1998a), and with a process-specificity view of prefrontal function (Petrides 1995, Owen et al. 1996, Owen et al. 1998).
An alternative hypothesis, but one that is not incompatible with the idea of interference being higher for familiar stimuli, is that pre-exposure changes the memory strength of stimuli, such that pre-exposed, familiar stimuli have greater memory strength than trial-unique stimuli, which would have no prior representation in the brain. We have suggested in our previous work that stimuli with no previous representation (i.e. trial-unique stimuli) require alternative mechanisms for maintenance than those that are familiar (See Stern et al., 2001; Schon et al., 2004, 2005; Hasselmo and Stern 2006).
Our results also demonstrate that the posterior parietal (together with the DLPFC and posterior SFS) and posterior ventral regions (together with the OFC) play the greatest role in the short-term maintenance of spatial information and object information, respectively. This stimulus domain-specificity in posterior regions during working memory maintenance is consistent with previous neuroimaging studies (Postle and D’Esposito 1999a, Postle et al. 2000b, Prabhakaran et al. 2000). Furthermore, this observation is in line with the idea of a preferential processing of visuospatial information in the dorsal stream in parieto-occipital areas and a preferential processing of visual non-spatial information in the ventral stream in temporal-occipital areas (see Ungerleider and Haxby 1994, for review).
Within the PFC, functional domain specificity is limited to a region within the posterior SFS that is just anterior to the FEF. This region specifically supports the short-term maintenance of visual-spatial information as suggested by Courtney and colleagues (1998). Activity in this posterior PFC area has been interpreted as related to eye movements (Postle et al. 2000a, Brown et al. 2004). Postle and colleagues (2000a) have reported that only when spatial working memory was contrasted with free saccades, but not when it was contrasted with visually guided saccades, was differential activity evident in the posterior superior frontal cortex. This finding is inconsistent with both the Courtney et al. (1998) study and with the current report, which both showed that the posterior SFS is recruited for the short-term maintenance of locations, and is not related to visually-guided saccades. This discrepancy may be due to the fact that the visually-guided saccadic eye movement tasks used by Courtney et al. (1998) and here were blocked design tasks with high signal detection power, whereas the task used by Postle et al. (2000a), while better matched to the spatial working memory task, was event-related and therefore had less signal detection efficiency. It is also possible that the 2-D visually guided saccade task used by Postle et al. (2000a) may recruit the posterior superior frontal cortex region whereas 1-D visually guided saccade tasks to horizontal locations used in Courtney et al. (1998), and in the current study, as suggested by Postle et al. (2000a), may not recruit this area. The more likely explanation is that the spatial mnemonic component recruits the posterior SFS, because activity in this region was present during the delay period of a spatial delayed response task in our study, but not during short-term maintenance of visual nonspatial objects that were displayed in the same spatial locations.
Does delay-period activity related to spatial stimuli reflect maintenance of spatial information in working memory or sustained spatial covert attention?
Activity within the FEF and the posterior parietal cortex has been viewed as being attention-based (Gitelman et al. 1999, Linden et al. 2003, Schall 2004), and these areas are active during overt and covert attentional shifts (Corbetta et al. 1998). Working memory and covert attention recruit a common network of brain areas, including posterior parietal regions and the FEF (LaBar et al. 1999, Pollmann and von Cramon, 2000, Corbetta et al. 2002). Consistent with this, we also demonstrated that the FEF were specifically recruited for the short-term maintenance of spatial locations.
In our study, the FEFs were not the only areas that were jointly recruited for spatial working memory maintenance and saccadic eye movements. Another area was the posterior IPS. Consistent with this observation, previous fMRI studies in humans have demonstrated that posterior parietal regions along the IPS are not only topographically organized by delayed saccades to remembered locations (Sereno et al. 2001, Schluppeck et al. 2005, Swisher et al. 2007), but are also recruited for visual short-term memory (Todd and Marois 2004, Xu and Chun 2006) and visual attention (Wojciulik and Kanwisher 1999, Silver et al. 2005).
Under certain conditions, for example when the spatial working memory load is low (as in the current study), and/or when prospective motor coding is not possible or not required during the delay-period, short-term maintenance of visual-spatial information may be identical to covert spatial attention or retrospective sensory coding (see, e.g., Postle et al. 2004). Recently Postle proposed that working memory may be an emergent property of interactions between brain areas involved in attention, sensory processing, planning of actions, etc. (Postle 2006). This position starkly contrasts that of Courtney (2004), who proposed that attention and cognitive control may be emergent properties of working memory. Either way, attention and working memory, especially in the spatial domain, are intertwined and may, under certain circumstances, describe the same phenomenon from two different perspectives (short-term maintenance of spatial information and sustained spatial covert attention).
CONCLUSIONS
Our data extend previous findings in that they demonstrate a critical role for the orbitofrontal cortex, in concert with the left ventrolateral and the right dorsolateral PFC, for the short-term maintenance of highly familiar stimuli across a working memory delay. Behaviorally, in contrast to trial-unique stimuli, the maintenance of a small set of familiar stimuli requires close monitoring of the currently relevant stimulus in the face of high interference. Our findings are largely consistent with the view that the PFC may be organized in a process-specific rather than a stimulus-specific way (Petrides 1995, Owen et al. 1996, Owen et al. 1998). However, we did observe a stimulus domain-specific specialization within the PFC. Consistent with previous studies (Courtney et al. 1998, Rowe et al. 2000, Rowe and Passingham 2001, Glahn et al. 2002, Sala et al. 2003, Slotnick 2005), the posterior frontal cortex including the FEF and the posterior SFS, that is distinct from and just anterior to the FEF, is recruited specifically for the short-term maintenance of visual spatial information, whereas, in contrast to previous studies, the ventral orbitofrontal cortex supports short-term maintenance of visual nonspatial stimuli. Our data suggest that close monitoring of task-relevant information in the face of interference may be one of the roles the OFC plays during short-term maintenance of visual stimuli.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by NIH Center grants P50 MH071702-01A2 and NCRR P41RR14075, and NSF Science of Learning Center SBE-0354378. We would like to thank Seth Sherman, Ph.D., Courtney Horwitz, M.A., Irina Ostrovskaya, B.A., and Anne Nizenson, B.A., for assistance with stimulus preparation and statistical analyses, and Michael Hasselmo, D.Phil. for discussions about the project. Preliminary data were presented at the Annual Meeting of the Society for Neuroscience in Washington, D.C., Oct. 2005.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alvarez P, Zola-Morgan S, Squire LR. The animal model of human amnesia: long-term memory impaired and short-term memory intact. Proc Natl Acad Sci U S A. 1994;91(12):5637–41. doi: 10.1073/pnas.91.12.5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Frontal lobe mechanisms that resolve proactive interference. Cereb Cortex. 2005 2005 Dec;15(12):2003–12. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Dale AM, Hamalainen MS, Marinkovic K, Schacter DL, Rosen BR, Halgren E. Top-down facilitation of visual recognition. Proc Natl Acad Sci U S A. 2006;103(2):449–54. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei HT, Rempel-Clower NL, Xiao D. Anatomic basis of functional specialization in prefrontal cortices in primates. In: Grafman J, editor. Handbook of Neuropsychology. 2nd edition Vol. 7. 2002. pp. 1–27. [Google Scholar]
- Barde LH, Thompson-Schill SL. Models of functional organization of the lateral prefrontal cortex in verbal working memory: evidence in favor of the process model. J Cogn Neurosci. 2002;14(7):1054–63. doi: 10.1162/089892902320474508. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–5. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16(13):4207–21. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, DeSouza JF, Goltz HC, Ford K, Menon RS, Goodale MA, Everling S. Comparison of memory- and visually guided saccades using event-related fMRI. J Neurophysiol. 2004;91(2):873–89. doi: 10.1152/jn.00382.2003. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124(Pt 10):2074–86. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Caplan JB, McIntosh AR, De Rosa E. Two Distinct Functional Networks for Successful Resolution of Proactive Interference. Cereb Cortex. 2006 2006 Sep 12; doi: 10.1093/cercor/bhl076. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10(3):220–42. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79(6):2919–40. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC. A common network of functional areas for attention and eye movements. Neuron. 1998;21(4):761–73. doi: 10.1016/s0896-6273(00)80593-0. others. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14(3):508–23. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Courtney SM. Attention and cognitive control as emergent properties of information representation in working memory. Cogn Affect Behav Neurosci. 2004;4(4):501–16. doi: 10.3758/cabn.4.4.501. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279(5355):1347–51. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386(6625):608–11. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Selection and maintenance of saccade goals in the human frontal eye fields. J Neurophysiol. 2006;95(6):3923–7. doi: 10.1152/jn.01120.2005. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D’Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. J Neurosci. 2004;24(16):3944–52. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, Sun FT, Miller LM, D’Esposito M. Coherence between fMRI time-series distinguishes two spatial working memory networks. Neuroimage. 2005;26(1):177–83. doi: 10.1016/j.neuroimage.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Zald DH, Pardo JV. Organization of working memory within the human prefrontal cortex: a PET study of self-ordered object working memory. Neuropsychologia. 2000;38(11):1503–10. doi: 10.1016/s0028-3932(00)00062-2. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7(1):1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41(1):66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D, Murray EA. Preserved recognition memory for small sets, and impaired stimulus identification for large sets, following rhinal cortex ablations in monkeys. Eur J Neurosci. 1994;6(9):1466–78. doi: 10.1111/j.1460-9568.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ. Differential neural responses during performance of matching and nonmatching to sample tasks at two delay intervals. J Neurosci. 1999;19(12):5066–73. doi: 10.1523/JNEUROSCI.19-12-05066.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feredoes E, Tononi G, Postle BR. Direct evidence for a prefrontal contribution to the control of proactive interference in verbal working memory. Proc Natl Acad Sci U S A. 2006;103(51):19530–4. doi: 10.1073/pnas.0604509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera VP, Cohen JK, Lee BB. Activity of prefrontal neurons during location and color delayed matching tasks. Neuroreport. 1999;10(6):1315–22. doi: 10.1097/00001756-199904260-00030. [DOI] [PubMed] [Google Scholar]
- Frey S, Petrides M. Orbitofrontal cortex: A key prefrontal region for encoding information. Proc Natl Acad Sci U S A. 2000;97(15):8723–7. doi: 10.1073/pnas.140543497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Petrides M. Orbitofrontal cortex and memory formation. Neuron. 2002;36(1):171–6. doi: 10.1016/s0896-6273(02)00901-7. [DOI] [PubMed] [Google Scholar]
- Frey S, Petrides M. Greater orbitofrontal activity predicts better memory for faces. Eur J Neurosci. 2003;17(12):2755–8. doi: 10.1046/j.1460-9568.2003.02714.x. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61(2):331–49. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J Neurophysiol. 1973;36(1):61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex and its relation to behavior. Prog Brain Res. 1991;87:201–11. doi: 10.1016/s0079-6123(08)63053-8. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173(997):652–4. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Recognition impaired and association intact in the memory of monkeys after transection of the fornix. J Comp Physiol Psychol. 1974;86(6):1100–9. doi: 10.1037/h0037649. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Murray EA. Monkeys (Macaca fascicularis) with rhinal cortex ablations succeed in object discrimination learning despite 24-hr intertrial intervals and fail at matching to sample despite double sample presentations. Behav Neurosci. 1992;106(1):30–8. doi: 10.1037//0735-7044.106.1.30. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud-Pechoux S, Pierrot-Deseilligny C. The frontal eye field is involved in spatial short-term memory but not in reflexive saccade inhibition. Exp Brain Res. 1999;129(2):288–301. doi: 10.1007/s002210050899. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122(Pt 6):1093–106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Kim J, Cohen MS, Poutanen VP, Therman S, Bava S, Van Erp TG, Manninen M, Huttunen M, Lonnqvist J. Maintenance and manipulation in spatial working memory: dissociations in the prefrontal cortex. Neuroimage. 2002;17(1):201–13. doi: 10.1006/nimg.2002.1161. others. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Stern CE. Mechanisms underlying working memory for novel information. Trends Cogn Sci. 2006;10(11):487–93. doi: 10.1016/j.tics.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Josephs O, Dolan RJ. Functional magnetic resonance imaging of proactive interference during spoken cued recall. Neuroimage. 2002;17(2):543–58. [PubMed] [Google Scholar]
- Inoue M, Mikami A, Ando I, Tsukada H. Functional brain mapping of the macaque related to spatial working memory as revealed by PET. Cereb Cortex. 2004;14(1):106–19. doi: 10.1093/cercor/bhg109. [DOI] [PubMed] [Google Scholar]
- Insausti R, Munoz M. Cortical projections of the non-entorhinal hippocampal formation in the cynomolgus monkey (Macaca fascicularis) Eur J Neurosci. 2001;14(3):435–51. doi: 10.1046/j.0953-816x.2001.01662.x. [DOI] [PubMed] [Google Scholar]
- Jha AP, McCarthy G. The influence of memory load upon delay-interval activity in a working-memory task: an event-related functional MRI study. J Cogn Neurosci. 2000;2(12 Suppl):90–105. doi: 10.1162/089892900564091. [DOI] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139(1):181–93. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston K. Event-related fMRI. Hum Brain Mapp. 1997;5(4):243–248. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22(4):751–61. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Koyama M, Hasegawa I, Osada T, Adachi Y, Nakahara K, Miyashita Y. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: comparison of cortical eye fields with humans. Neuron. 2004;41(5):795–807. doi: 10.1016/s0896-6273(04)00047-9. [DOI] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiol. 1971;34(3):337–47. doi: 10.1152/jn.1971.34.3.337. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. Neuroimage. 1999;10(6):695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Lamar M, Yousem DM, Resnick SM. Age differences in orbitofrontal activation: an fMRI investigation of delayed match and nonmatch to sample. Neuroimage. 2004;21(4):1368–76. doi: 10.1016/j.neuroimage.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Leung HC, Seelig D, Gore JC. The effect of memory load on cortical activity in the spatial working memory circuit. Cogn Affect Behav Neurosci. 2004;4(4):553–63. doi: 10.3758/cabn.4.4.553. [DOI] [PubMed] [Google Scholar]
- Linden DE, Bittner RA, Muckli L, Waltz JA, Kriegeskorte N, Goebel R, Singer W, Munk MH. Cortical capacity constraints for visual working memory: dissociation of fMRI load effects in a fronto-parietal network. Neuroimage. 2003;20(3):1518–30. doi: 10.1016/j.neuroimage.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Mohr HM, Goebel R, Linden DE. Content- and task-specific dissociations of frontal activity during maintenance and manipulation in visual working memory. J Neurosci. 2006;26(17):4465–71. doi: 10.1523/JNEUROSCI.5232-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz M, Insausti R. Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (Macaca fascicularis) Eur J Neurosci. 2005;22(6):1368–88. doi: 10.1111/j.1460-9568.2005.04299.x. [DOI] [PubMed] [Google Scholar]
- Nystrom LE, Braver TS, Sabb FW, Delgado MR, Noll DC, Cohen JD. Working memory for letters, shapes, and locations: fMRI evidence against stimulus-based regional organization in human prefrontal cortex. Neuroimage. 2000;11(5 Pt 1):424–46. doi: 10.1006/nimg.2000.0572. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Complementary roles of the orbital prefrontal cortex and the perirhinal-entorhinal cortices in an odor-guided delayed-nonmatching-to-sample task. Behav Neurosci. 1992;106(5):762–75. doi: 10.1037//0735-7044.106.5.762. [DOI] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb Cortex. 1996;6(1):31–8. doi: 10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- Owen AM, Herrod NJ, Menon DK, Clark JC, Downey SP, Carpenter TA, Minhas PS, Turkheimer FE, Williams EJ, Robbins TW. Redefining the functional organization of working memory processes within human lateral prefrontal cortex. Eur J Neurosci. 1999;11(2):567–74. doi: 10.1046/j.1460-9568.1999.00449.x. others. [DOI] [PubMed] [Google Scholar]
- Owen AM, Stern CE, Look RB, Tracey I, Rosen BR, Petrides M. Functional organization of spatial and nonspatial working memory processing within the human lateral frontal cortex. Proc Natl Acad Sci U S A. 1998;95(13):7721–6. doi: 10.1073/pnas.95.13.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia. 1996;34(6):475–83. doi: 10.1016/0028-3932(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23(10):3990–8. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L, Clark VP, Ingeholm J, Haxby JV. Dissociation of saccade-related and pursuit-related activation in human frontal eye fields as revealed by fMRI. J Neurophysiol. 1997;77(6):3386–90. doi: 10.1152/jn.1997.77.6.3386. [DOI] [PubMed] [Google Scholar]
- Petrides M. Impairments on nonspatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. J Neurosci. 1995;15(1 Pt 1):359–75. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Israel I, Berthoz A, Rivaud S, Gaymard B. Role of the different frontal lobe areas in the control of the horizontal component of memory-guided saccades in man. Exp Brain Res. 1993;95(1):166–71. doi: 10.1007/BF00229665. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Poline JB, Crozier S, Lehericy S, Pillon B, Deweer B, Le Bihan D, Dubois B. The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: an fMRI study. Cereb Cortex. 2001;11(3):260–6. doi: 10.1093/cercor/11.3.260. [DOI] [PubMed] [Google Scholar]
- Pollmann S, von Cramon DY. Object working memory and visuospatial processing: functional neuroanatomy analyzed by event-related fMRI. Exp Brain Res. 2000;133(1):12–22. doi: 10.1007/s002210000396. [DOI] [PubMed] [Google Scholar]
- Postle BR. Analysis of fMRI data from tasks containing temporal dependencies: An evaluation of Slotnick (2005) Cognitive Neuropsychology. 2005;22(7):921–924. doi: 10.1080/02643290442000464. [DOI] [PubMed] [Google Scholar]
- Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139(1):23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Awh E, Jonides J, Smith EE, D’Esposito M. The where and how of attention-based rehearsal in spatial working memory. Brain Res Cogn Brain Res. 2004;20(2):194–205. doi: 10.1016/j.cogbrainres.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Postle BR, Berger JS, Taich AM, D’Esposito M. Activity in human frontal cortex associated with spatial working memory and saccadic behavior. J Cogn Neurosci. 2000a;2(12 Suppl):2–14. doi: 10.1162/089892900564028. [DOI] [PubMed] [Google Scholar]
- Postle BR, D’Esposito M. “What”-Then-Where“ in visual working memory: an event-related fMRI study. J Cogn Neurosci. 1999a;11(6):585–97. doi: 10.1162/089892999563652. [DOI] [PubMed] [Google Scholar]
- Postle BR, D’Esposito M. Dissociation of human caudate nucleus activity in spatial and nonspatial working memory: an event-related fMRI study. Brain Res Cogn Brain Res. 1999b;8(2):107–15. doi: 10.1016/s0926-6410(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Postle BR, Stern CE, Rosen BR, Corkin S. An fMRI investigation of cortical contributions to spatial and nonspatial visual working memory. Neuroimage. 2000b;11(5 Pt 1):409–23. doi: 10.1006/nimg.2000.0570. [DOI] [PubMed] [Google Scholar]
- Postle BR, Zarahn E, D’Esposito M. Using event-related fMRI to assess delay-period activity during performance of spatial and nonspatial working memory tasks. Brain Res Brain Res Protoc. 2000c;5(1):57–66. doi: 10.1016/s1385-299x(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Narayanan K, Zhao Z, Gabrieli JD. Integration of diverse information in working memory within the frontal lobe. Nat Neurosci. 2000;3(1):85–90. doi: 10.1038/71156. [DOI] [PubMed] [Google Scholar]
- Quintana J, Yajeya J, Fuster JM. Prefrontal representation of stimulus attributes during delay tasks. I. Unit activity in cross-temporal integration of sensory and sensory-motor information. Brain Res. 1988;474(2):211–21. doi: 10.1016/0006-8993(88)90436-2. [DOI] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK. Memory fields of neurons in the primate prefrontal cortex. Proc Natl Acad Sci U S A. 1998a;95(25):15008–13. doi: 10.1073/pnas.95.25.15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998b;393(6685):577–9. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D’Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31(5):865–73. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Rao SC, Rainer G, Miller EK. Integration of what and where in the primate prefrontal cortex. Science. 1997;276(5313):821–4. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Browning AS, Inoue K, Hernadi I. Novel visual stimuli activate a population of neurons in the primate orbitofrontal cortex. Neurobiol Learn Mem. 2005;84(2):111–23. doi: 10.1016/j.nlm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rosenkilde CE, Bauer RH, Fuster JM. Single cell activity in ventral prefrontal cortex of behaving monkeys. Brain Res. 1981;209(2):375–94. doi: 10.1016/0006-8993(81)90160-8. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Passingham RE. Working memory for location and time: activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage. 2001;14(1 Pt 1):77–86. doi: 10.1006/nimg.2001.0784. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288(5471):1656–60. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Gough PM, Alexander IH, Kyriazis D, McDonald KR, Passingham RE. Attentional selection and action selection in the ventral and orbital prefrontal cortex. J Neurosci. 2005;25(50):11628–36. doi: 10.1523/JNEUROSCI.2765-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D’Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci. 2002;14(5):721–31. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999;9(2):216–26. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Parahippocampal reactivation signal at retrieval after interruption of rehearsal. J Neurosci. 2002;22(15):6315–20. doi: 10.1523/JNEUROSCI.22-15-06315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala JB, Rama P, Courtney SM. Functional topography of a distributed neural system for spatial and nonspatial information maintenance in working memory. Neuropsychologia. 2003;41(3):341–56. doi: 10.1016/s0028-3932(02)00166-5. [DOI] [PubMed] [Google Scholar]
- Schall JD. On the role of frontal eye field in guiding attention and saccades. Vision Res. 2004;44(12):1453–67. doi: 10.1016/j.visres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Schluppeck D, Glimcher P, Heeger DJ. Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol. 2005;94(2):1372–84. doi: 10.1152/jn.01290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnider A, Treyer V, Buck A. Selection of currently relevant memories by the human posterior medial orbitofrontal cortex. J Neurosci. 2000;20(15):5880–4. doi: 10.1523/JNEUROSCI.20-15-05880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Integrating orbitofrontal cortex into prefrontal theory: common processing themes across species and subdivisions. Learn Mem. 2001;8(3):134–47. doi: 10.1101/lm.39901. [DOI] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, LoPresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004;24(49):11088–97. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Atri A, Hasselmo ME, Tricarico MD, LoPresti ML, Stern CE. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. J Neurosci. 2005;25(40):9112–23. doi: 10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294(5545):1350–4. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94(2):1358–71. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD. Spatial working memory specific activity in dorsal prefrontal cortex? Disparate answers from fMRI beta-weight and timecourse analysis. Cognitive Neuropsychology. 2005;22(7):905–920. doi: 10.1080/02643290442000455. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Tehovnik EJ. Reversible inactivation of macaque frontal eye field. Exp Brain Res. 1997;116(2):229–49. doi: 10.1007/pl00005752. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Frontal eye field sends delay activity related to movement, memory, and vision to the superior colliculus. J Neurophysiol. 2001;85(4):1673–85. doi: 10.1152/jn.2001.85.4.1673. [DOI] [PubMed] [Google Scholar]
- Stern CE, Owen AM, Tracey I, Look RB, Rosen BR, Petrides M. Activity in ventrolateral and mid-dorsolateral prefrontal cortex during nonspatial visual working memory processing: evidence from functional magnetic resonance imaging. Neuroimage. 2000;11(5 Pt 1):392–9. doi: 10.1006/nimg.2000.0569. [DOI] [PubMed] [Google Scholar]
- Stern CE, Sherman SJ, Kirchhoff BA, Hasselmo ME. Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus. 2001;11(4):337–46. doi: 10.1002/hipo.1048. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J Neurophysiol. 1996;75(1):454–68. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC. Visual topography of human intraparietal sulcus. J Neurosci. 2007;27(20):5326–37. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428(6984):751–4. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. II. Memory responses. J Neurophysiol. 2001;86(5):2344–52. doi: 10.1152/jn.2001.86.5.2344. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4(2):157–65. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Veltman DJ, Rombouts SA, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. Neuroimage. 2003;18(2):247–56. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260(5116):1955–8. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N. The generality of parietal involvement in visual attention. Neuron. 1999;23(4):747–64. doi: 10.1016/s0896-6273(01)80033-7. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Sommer MA, Pare M, Ferraina S. Signal transformations from cerebral cortex to superior colliculus for the generation of saccades. Vision Res. 2001;41(2526):3399–412. doi: 10.1016/s0042-6989(01)00066-9. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D’Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997;6(2):122–38. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci. 1989;9(12):4355–70. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Clower RP, Rempel NL. Damage to the perirhinal cortex exacerbates memory impairment following lesions to the hippocampal formation. J Neurosci. 1993;13(1):251–65. doi: 10.1523/JNEUROSCI.13-01-00251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurowski B, Gostomzyk J, Gron G, Weller R, Schirrmeister H, Neumeier B, Spitzer M, Reske SN, Walter H. Dissociating a common working memory network from different neural substrates of phonological and spatial stimulus processing. Neuroimage. 2002;15(1):45–57. doi: 10.1006/nimg.2001.0968. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440(7080):91–5. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.