Abstract
Little is known about the biology of murine T-cell receptors (TCR) expressed in human cells. We recently observed that a murine anti-human p53 TCR is highly functional when expressed in human lymphocytes. Herein, we compare human and mouse TCR function and expression to delineate the molecular basis for the apparent superior biological activity of murine receptors in human T lymphocytes. To this end, we created hybrid TCRs where we swapped the original constant regions with either human or mouse ones, respectively. We showed that murine or “murinized” receptors were overex-pressed on the surface of human lymphocytes compared with their human/humanized counterparts and were able to mediate higher levels of cytokine secretion when cocultured with peptide-pulsed antigen-presenting cells. Preferential pairing of murine constant regions and improved CD3 stability seemed to be responsible for these observations. These enhanced biological properties translated into significantly greater antitumor response mediated by TCR with mouse constant regions. Furthermore, we were able to circumvent the natural low avidity of class I MHC TCR in CD4+ cells by introducing the murinized TCR into CD4+ lymphocytes, giving them the ability to recognize melanoma tumors. These findings have implications for human TCR gene transfer therapy and may provide new insights into the biology of the TCR/CD3 complex.
Introduction
The T-cell receptor (TCR)/CD3 complex is an elaborate structure that is designed to recognize antigens and convey activation signals to lymphocytes. T-cell activation is critical not only in antigen recognition but also for the differentiation of T cells into memory or effector populations and in T-cell development (1). Signaling through the TCR depends on the interaction of the highly variable TCR α and β chains with the invariant CD3γ/δ/ε and CD3ζ chains. This association is mediated by charged residues located in the transmembrane region of the constant part of the TCR α and β chains that interact noncovalently with CD3γ/ε and CD3δ/ε heterodimers and the CD3ζ chain homodimer (2). The TCR α and β chains, linked by a disulfide bond, form the TCR that binds antigenic peptides presented by MHC molecules on antigen-presenting cells and dictates the specificity of the T cells.
Several studies have shown that it is feasible to transduce TCR genes into human lymphocytes to redirect the specificity of transduced populations to antigens of interest (reviewed in refs. 3, 4). Of particular interest is the reprogramming of human lymphocytes for cancer treatment because cellular adoptive immunotherapy has been shown to mediate the regression of large solid tumors in patients with metastatic melanoma (5). However, a limitation of adoptive immunotherapy is the need to isolate and expand tumor-reactive lymphocytes that preexist in the patient. Therefore, TCR transfer procedures to human lymphocytes may overcome the requirement for preexisting tumor-specific immunity and the need to laboriously identify and isolate tumor-reactive T cells from each patient. In this regard, several groups, including ours, have shown that it is possible to engineer lymphocytes to express human TCRs that confer novel antitumor activity (6–10).
Recently, we and others showed the promising potential of a murine TCR that recognized the human epitope 264 to 272 derived from p53 (11, 12), a tumor-associated antigen known to be overexpressed in ~50% of common epithelial cancers (13). After transduction of human lymphocytes with this p53-specific murine TCR, we observed enhanced biological activity especially when compared with other human TCR we have characterized (6–8).
Although this might be a sole property of this particular anti-p53 TCR, we sought to examine in this report if the molecular basis for this apparent superior performance is the result of a different biological behavior of TCR with murine constant regions expressed in human lymphocytes. To that end, we constructed hybrid TCRs in which we replaced the original constant regions with either murine or human ones, leaving the variable domains intact. Importantly, we show that TCR with mouse constant regions functions better in human cells than its human counterpart, leading to an increased sensitivity to tumor cells. Biochemical analysis suggested that part of this enhanced activity was due to preferential pairing of murine constant regions with themselves and less mispairing with the endogenous human TCR chains and to increased stability of the TCR/CD3ζ complex. These results could have significant implications for the translation of TCR gene therapy to the clinical setting.
Materials and Methods
Patient peripheral blood mononuclear cells and cell lines
All of the peripheral blood mononuclear cells (PBMC) used in this study were from metastatic melanoma patients treated at the Surgery Branch, National Cancer Institute (NCI), NIH (Bethesda, MD). Jurkat RT3-T3.5 is a radiation-induced Jurkat mutant that is surface TCR negative (ATCC/TIB-153). Melanoma cell lines 526 (HLA-A2+), 624 (HLA-A2+), 624.38 (HLA-A2+), 888 (HLA-A2−), and 938 (HLA-A2−) were generated at the Surgery Branch as described previously (14). p53+/HLA-A2+ cell lines were H2087 (ATCC/CRL-5922), MDA-MB-231 (ATCC/HTB-26), and p53−/HLA-A2+ Saos-2 (ATCC/HTB-85). T2 cells are a lymphoblastoid cell line deficient in TAP function whose HLA/A2 protein can be easily loaded with exogenous peptides (15).
All cells were cultured in R10 medium consisting of RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (Biofluids, Rockville, MD) and maintained in a 37°C and 5% CO2 incubator. Lymphocytes were cultured in AIM-V medium (Invitrogen, Carlsbad CA) supplemented with 5% human AB serum (Valley Biomedical, Winchester, VA) and 300 IU/mL interleukin-2 (IL-2) at 37°C and 5% CO2.
Peptide synthesis
The sequences of the peptide used in this study are as follows: p53264-272 (LLGRNSFEV), MART-1/27L26-35 (ELAGIGILTV), gp100 210 M209-217 (IMDQVPFSV), gp100280-288 (YLEPGPVTA), HBVc 23Y18-27 (FLPSDYFPSV), Flu-MP58-66 (GILGFVFTL), and NY-ESO-1161-180 (WITQCFLPVFLAQPPSGQRA).
Hybrid TCR generation and cloning
The α and β chains from the previously characterized TCRs specific for p53264-272 (11) and MART-1/27L (7) were subcloned into the pGEM-4Z/64A vector as described previously (16). In addition, we created two mutant TCRs in which we swapped the original constant regions by either human or murine ones using a megaprimer-based approach (Fig. 1). Briefly, we humanized the anti-p53 TCR by replacing the murine human α and β domains by those from the original anti-MART-1 TCR. The primers used to amplify the p53 variable α domain were p53vA-RNAF ATCTAGAGCCGCCATGGCTCCTGGCGCTCCTCCCAG and p53vA-RNAR GGCAGGGTCAGGGTTCTGGATGTCTGGCTTTATAATTAGCTT to generate p53vA megaprimer; for the p53-variable β domain, we used p53vB-RNAF ATCTAGAGCCGCCATGGCTACAAGGCTCCTCTGTTAC and p53vB-RNAR TGGGAACACCTTGTTCAGGTCCTCTACAACTGTGAGTCTGGTTCC to generate p53vB megaprimer. We then used those megaprimers to fuse the human constant regions; for the α chain, we used p53vA-RNAF and p53HcA-RNAR CTAGGCGGCCGCTCAGCTGGACCACAGCCGCAG to generate p53-MHα chain and p53vB-RNAF and p53HcB-RNAR CTAGGCGGCCGCTCAGAAATCCTTTCTCTTGACCATGGC to generate p53-MHβ chain. Both p53-MHα and p53-MHβ products were digested with XbaI and NotI cloned separately into the pGEM-4Z/64A vector.
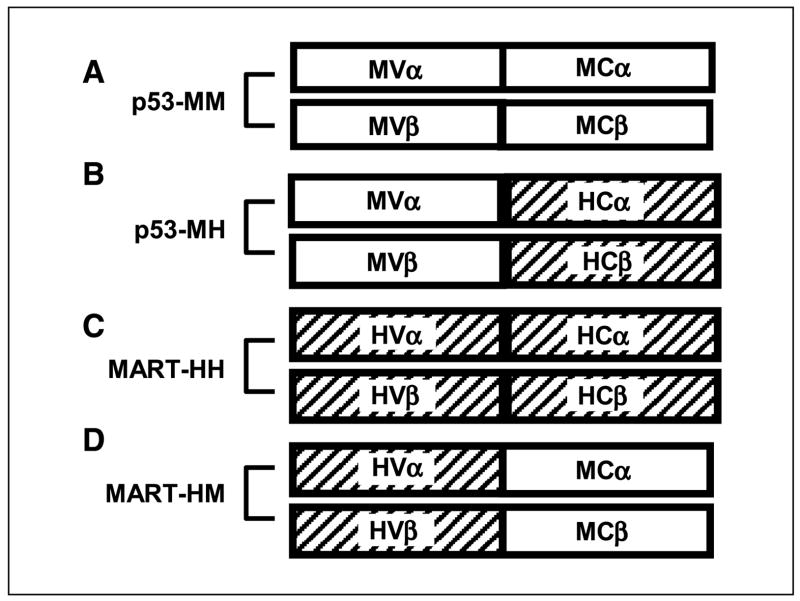
Figure 1.
Schematic representation of the TCR chains and their hybrids. A, murine anti-p53 TCR, p53-MM. B, its humanized version, p53-MH, with mouse variable regions linked to human constant regions. Human anti-MART-1 TCR, MART-HH (C), and its murinized hybrid, MART-HM (D), with human variable regions linked to murine constant regions.
Similarly, to “murinize” the human MART-1 TCR, we first amplified the variable α chain with MARTvA-RNAF GCTCTAGAGCCGCCATGTTGCTTTTGAACATTTATTAATAA and MARTvA-RNAR AGCAGGTTCTGGGTTCTGGATATTTGGAATGACCGTCAAACTTGT to generate MARTvA meg-aprimer. For the variable β chain, we used MARTvB-RNAF GCAAGCTTGCCGCCATGGGCACAAGGTTGTTCTTCTATG and MARTvB-RNAR AGAGTCACATTTCTCAGATCCTCTAGGATGGAGAGTCGAGTCCCAT to generate MARTvB megaprimer. Then, we subjected those megaprimers to a second PCR using the primers MARTvA-RNAF and MART-McA-RNAR CCGCGGCCGCTCAACTGGACCACAGCCTCAGCG to generate MART-HMα and MARTvB-RNAF and MART-McB-RNAR CCGCGGCCGCTCATTGAATTCTTTCTTTTGACCATAGC to generate MART-HMβ.
The MART-HMα product was digested with XbaI and NotI and the MART-HMβ product was digested with HindIII and NotI. Subsequently, both of them were cloned separately into the pGEM-4Z/64A vector.
We constructed a mouse hybrid version of a HLA-DP4-restricted NY-ESO-1 TCR, which we recently isolated (17). Briefly, the human variable α chain was amplified using the primers T7-ESO-II-a TAATACGACTCACTATAGGGAGAGCCGCCATGAACTATTCTCCAGGCTTAG and AV9-2-McA-R CAGCAGGTTCTGGGTTCTGGATATTGGAACTCACTGATAAGGTGGTTC and then linked to the mouse constant α region in a PCR with T7-ESO-II-a and McA-R1 AATGCGGCCGCTCAACTGGACCACAGCCTCAG. For the β chain, we amplified the variable region with the primers T7-ESO-II-b TAATACGACTCACTATAGGGAGAAGCTTGCCGCCATGCTGCTGCTTCTGCTGCTTC and BV20-1-McB-R GGAGTCACATTTCTCAGATCCTCGAGCACCAGGAGCCGCGTG. This product was linked to the mouse β constant region using the primers T7-ESO-II-b and McB-R1 AATGCGGCCGCTCATGAATTCTTTCTTTTGACCATAG.
Electroporation of peripheral blood lymphocytes
This technique has been extensively described in our previous report (16). Briefly, in vitro transcribed mRNA for both α and β TCR chains was generated using mMESSAGE mMACHINE (Ambion, Austin, TX) and purified using RNeasy Mini kit (Qiagen, Valencia, CA). Peripheral blood lymphocytes (PBL) were collected by leukopheresis, and lymphocytes were separated by centrifugation on a Ficoll-Hypaque cushion, washed in HBSS, and resuspended at a concentration of 1 × 106 cells/mL in AIM-V supplemented with 5% human serum, 50 ng/mL OKT-3, and 300 IU/mL IL-2. The lymphocytes were then plated at 1 × 106 cells/mL in 24-well plates (Costar, Cambridge, MA) and cultured for ~1 week with the addition of new medium (without OKT-3) as needed to maintain density of 106 cells/mL. Electroporation was done as follows: the lymphocytes were washed in Opti-MEM (Invitrogen) and resuspended at 2.5 × 107 cells/mL. Cells were transferred in 2 mm cuvettes chilled on ice and then electroporated at 500 V/500 μs using an Electro-Square Porator ECM 830 (BTX, San Diego, CA). The amount of in vitro transcribed mRNA for each chain was 2 μg/106 PBMCs unless indicated otherwise. Wherever needed, the amount of electroporated mRNA was normalized using nonspecific mRNA. Following electroporation, cells were transferred to six-well plates containing fresh medium and cultured at 37°C.
Fluorescence-activated cell sorting analysis
Cell surface expression of human CD3, CD4, and CD8 molecules on PBL was determined by antibody staining (FITC-, phycoerythrin (PE)–, or antigen-presenting cell–conjugated antibodies; BD Biosciences, San Jose, CA). Human Vβ12 antibody PE-conjugated was supplied by Immunotech (Westbrook, ME). Antigen-presenting cell–labeled MART-1/27L tetramer was purchased from Beckman Coulter (San Jose, CA). Antigen-presenting cell–labeled p53264-272/HLA-A2 Pro5 pentamer was supplied by ProImmune (Oxford, United Kingdom). Immunofluorescence analyzed as the relative log fluorescence of live cells was measured using a FACSCalibur flow cytometer (Becton Dickinson). Approximately 1 × 105 cells were analyzed. Cells were stained in a fluorescence-activated cell sorting (FACS) buffer made of PBS, 0.5% bovine serum albumin, and 0.02% sodium azide.
Cytokine release assays
PBL cultures were tested for reactivity in cytokine release assays using commercially available ELISA kits [IFN-γ, IL-2, and granulocyte macrophage colony-stimulating factor (GM-CSF); Endogen, Cambridge, MA]. T2 cells were pulsed with peptide (1 μg/mL) in medium for 2 hours at 37°C followed by washing before initiation of cocultures. For these assays, 1 × 105 responder cells (PBL) and 1 × 105 stimulator cells (T2 or tumor lines) were incubated in a 0.2-mL culture volume in individual wells of 96-well plates. Stimulator cells and responder cells were cocultured for 24 hours. Cytokine secretion was measured in culture supernatants diluted to be in the linear range of the assay.
Immunoprecipitation and immunoblotting
TCR/CD3 stability studies were done as follows: Jurkat RT3-T3.5 cells were electroporated with either MART-HH or MART-HM. After 24 hours, the cells were washed once with PBS and placed in lysis buffer containing 1% NP40 or 1% Brij96, 10 mmol/L Tris-HCl (pH 7.2), 140 mmol/L NaCl, 2 mmol/L EDTA, 5 mmol/L iodoacetamide, 1 mmol/L Na3VO4, and complete protease inhibitor cocktail (Roche, Indianapolis, IN) as described previously (18). After removal of nuclear debris by centrifugation, the resultant supernatants were subjected to immunoprecipitation with anti-TCR human Vβ12 antibody for the MART TCRs or anti-TCR mouse Vβ3 antibody (clone KJ25, BD Biosciences) for the p53 TCRs and analyzed for CD3ζ by immunoblotting with a CD3ζ-specific antibody (6B10.2, Santa Cruz Biotechnology, Santa Cruz, CA). Controls for sample loading consisted of blotting for total CD3ζ in cell lysates.
51Cr release assay
The ability of the transduced PBL to lyse HLA-A2+ melanoma targets was measured using a Cr51 release assay as described (14). Briefly, 1 × 105 tumor cells were labeled for 1 hour at 37°C with 50 μCi 51Cr (Amersham, Arlington Heights, IL). Labeled target cells (2 × 103) were incubated with effector cells at the ratios indicated in the text for 3 hours at 37°C in 0.2 mL medium. Harvested supernatants were counted using a Wallac 1470 Wizard automatic γ-counter (Wallac, Gaithersburg, MD). Total and spontaneous Cr51 releases were determined by incubating 2 × 103 labeled targets in either 2% SDS or medium for 3 hours at 37°C, respectively. Each data point was done as an average of quadruplicate wells. The percentage of specific lysis was calculated as follows: % specific lysis = (specific release − spontaneous release) / (total release − spontaneous release) × 100.
CD4/CD8 separation
CD4+ and CD8+ populations were separated using a magnetic bead–based approach for both negative and positive selection of those subsets (Dynal Biotech, Brown Deer, WI and Miltenyi Biotech, Auburn, CA, respectively).
Results
Hybrid TCR generation and enhanced expression in human PBLs
We designed two hybrid TCRs in which we replaced the original constant regions by either mouse or human sequences to generate a murine p53 TCR with human constant regions (p53-MH) and human MART-1 TCR with murine constant regions (MART-HM; Fig. 1A–D). The hybrid TCR genes were cloned in the pGEM-4Z/64A vector and used as templates to produce mRNA for electroporation. To compare the relative levels of surface expression of hybrid TCRs, we electroporated OKT-3-stimulated human PBLs with the TCR α and β chains from the original p53 TCR (p53-MM), the original MART-1 TCR (MART-HH), or their respective hybrids. Twenty-four hours after electroporation, we stained with p53 pentamer for the p53 TCR electroporated cells or with MART-1 tetramer for the MART-1 TCR electroporated populations.
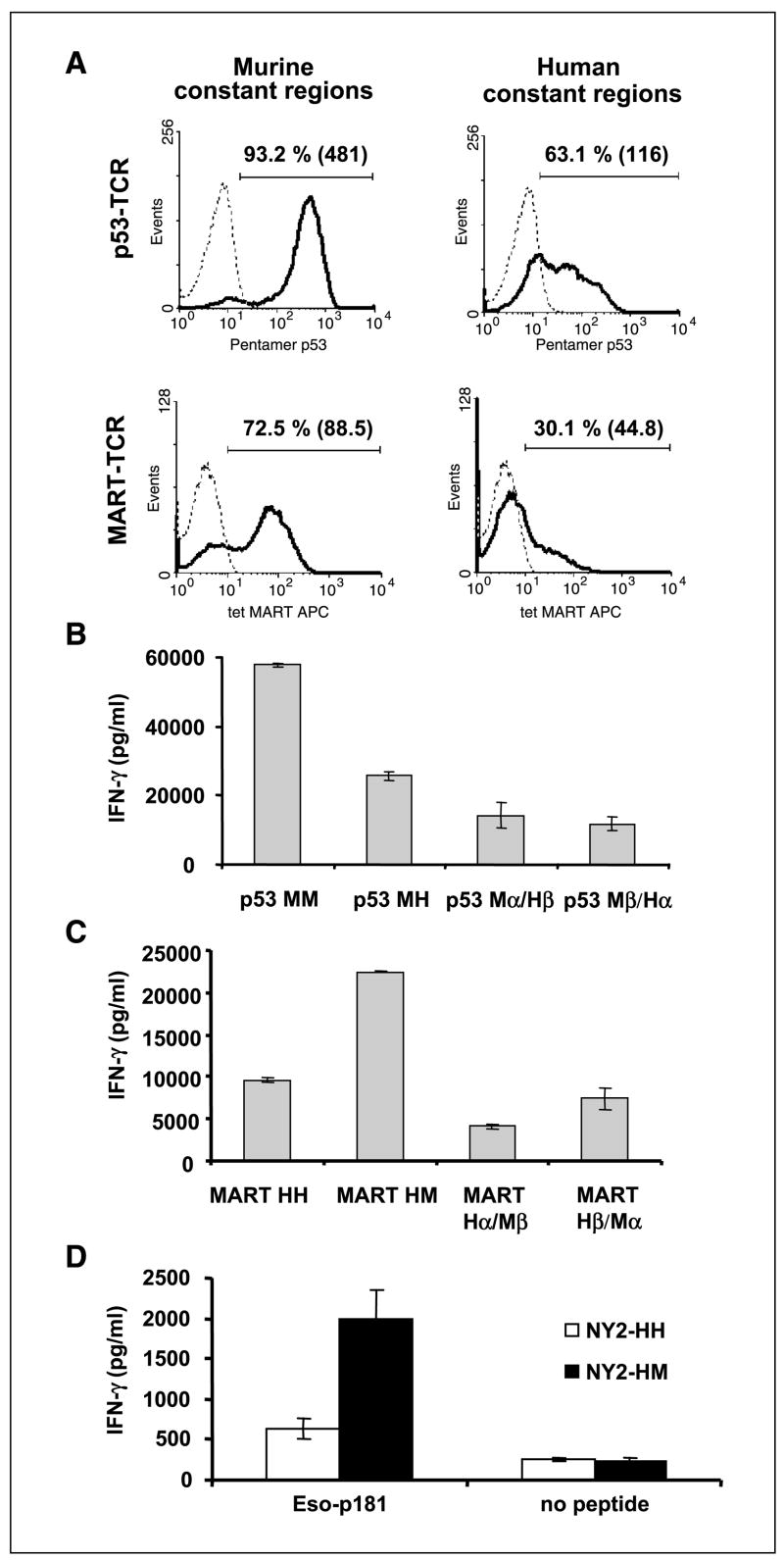
As seen in Fig. 2A, the original p53-MM was expressed at a higher level [mean fluorescence intensity (MFI), 481] on the surface of most of the electroporated human lymphocytes (93.2%), whereas only 63.1% were positive for the hybrid TCR with human constant regions p53-MH, and expression was lower (MFI, 116). Similarly, the cells that expressed the MART-HM stained a higher proportion (72.5%) and had a greater MFI (88.5) than the lymphocytes that expressed the original MART-HH (30.1%; MFI, 44.8). Those results were representative of 12 independent experiments done with 10 different donors (P < 0.001). The duration of cell surface staining for the hybrid MART-1 TCR was also longer than the wild-type TCR (Table 1). We calculated the average percentage of tetramer-stained cells expressing MART-HH or MART-HM based on independent experiments with five different donors. At 2 days after electroporation, MART-HH electroporated cells expressed lower amounts of TCR (4.2 ± 2.1%) than the MART-HM (40 ± 10.4%), and the MART-HH was almost undetectable (2.6 ± 1.4%) on day 3, whereas 20% of MART-HM-expressing lymphocytes were tetramer positive. Those differences were found to be statistically significant (P < 0.001) based on Student’s t test.
Figure 2.
A, comparison of the surface expression of the original TCRs and their hybrids. OKT-3-stimulated PBLs were electroporated with mRNA encoding the different TCR chains and assessed by FACS. Twenty-four hours after electroporation, we assessed p53 pentamer binding for p53-MM or p53-MH and MART-1 tetramer binding for MART-HM or MART-HH. Percentage of positive cells as well as the relative MFI (inside parentheses). B to D, recognition of peptide pulsed cells by original and hybrid TCRs. B, human PBLs were electroporated with the p53-MM, the p53-MH, the α chain of the p53-MM-Mα + the β chain of the p53-MH-Hβ (Mα/Hβ), or the opposite noted as Mβ/Hα. The electroporated cells were then cocultured for 16 hours with T2 cells that were pulsed at 1 μmol/L with specific peptide (p53264-272) or nonspecific peptides (control; data not shown). The concentration of IFN-γ secreted in the medium was measured using an ELISA procedure. C, PBLs were electroporated with the MART-HH, MART-HM, MART-Hα/Mβ, and MART-Hβ/Mα and then cocultured with cultured for 16 hours with T2 cells that were pulsed at 1 μmol/L with specific peptide (MART-1/27L26-35) or nonspecific peptides (control; data not shown). IFN-γ secretion in cultures with T2 pulsed with control peptides (gp100-209, + gp100-280, p53149-157, and HBVc) was ≤200 pg/mL (data not shown). D, CD4 enriched cells were electroporated with mRNA encoding the α and β chains of NY2-HH, a HLA-DP4-restricted NY-ESO-1 TCR, or its mouse hybrid, NY2-HM. NY-ESO-1161-180 peptide-pulsed HLA-DP4+ EBV-B line (DK-EBV-B) as well as nonpulsed cells as a control were cocultured overnight with TCR RNA electroporated CD4 T cells. IFN-γ secretion was detected by ELISA.
Table 1.
Comparison of expression of MART-HH and MART-HM
| Day 1 | Day 2 | Day 3 | |
|---|---|---|---|
| MART-HH | 21.1 ± 5.3 (17.4) | 4.2 ± 2.1 (12.7) | 2.6 ± 1.4 (9.6) |
| MART-HM | 61 ± 7 (40.2) | 40 ± 10.4 (21.7) | 20 ± 5.1 (11.5) |
| No. experiments | 12 | 5 | 5 |
| P | <0.001 | <0.001 | <0.001 |
NOTE: Human PBLs were electroporated with either MART-HH or MART-HM and stained at the indicated day with MART-1 tetramer. The average ± SD of the percentage of positive cells as well as the average MFI (%) are shown at days 1 to 3 after electroporation. We also indicate the number of independent experiments done and the statistical significance of the difference between MART-HH and MART-HM staining based on a Student’s t test.
Cytokine secretion mediated by hybrid TCRs
We sought to determine if the enhanced surface expression of TCRs with murine constant regions was correlated with a higher biological activity. OKT-3-stimulated human PBLs were electroporated with the TCR α and β chains from the original and hybrid forms of the anti-p53 TCR or the anti-MART-1 TCR and cocultured overnight with T2 cells that were pulsed with specific (p53264-272 or MART-1/27L26-35 peptide, respectively) and nonspecific peptides. Although both forms of the p53 or MART TCR mediated antigen-specific IFN-γ release, the original p53-MM mediated secretion of more than twice the amount of IFN-γ compared with the humanized p53-MH (57,800 versus 25,600 pg/mL). Similarly, the murinized MART-HM mediated increased IFN-γ secretion compared with the full human TCR, MART-HH (22,450 versus 9,600 pg/mL for MART-1 TCRs; Fig. 2B and C). Little or no IFN-γ secretion was detected when each chain was electroporated alone or when the T2 cells were pulsed with nonspecific peptides (data not shown). Correspondingly, we observed higher levels of GM-CSF secreted by PBLs expressing TCR with murine constant regions (original p53 TCR or MART TCR hybrid) in cocultures with peptide-pulsed T2 cells (p53-MM, 24,274 versus p53-MH, 12,317 pg/mL and 31,376 versus 15,023 pg/mL for MART-HM and MART-HH, respectively).
We also tested the function of different combinations of TCR chains [e.g., the humanized p53 TCR α chain (Hα) with the original full mouse p53 TCR β chain (Mβ)]. Both combinations Hα/Mβ and Mα/Hβ for anti-p53 and anti-MART-1 TCRs were able to mediate antigen-specific secretion of IFN-γ in cocultures with peptide-pulsed T2 cells. However, these concentrations were always lower than either the human combination (Hα/Hβ) or the murine one (Mα/Mβ; Fig. 2B and C). These data suggest that mouse and human constant regions can pair, but this heterodimer formation results in lower biological activity.
To investigate the generality of these results, we compared the activity of a class II/HLA-DP4-restricted NY-ESO-1 TCR, NY2-HH, to its murinized form, NY2-HM. CD4 purified cells, electroporated with the mRNA encoding either NY2-HH and NY2-HM, were cocultured overnight with HLA-DP4+ EBV-B line pulsed or not pulsed with the specific epitope (NY-ESO-1161-180). We observed higher levels of IFN-γ secreted by PBLs expressing NY2-HM than NY2-HH (1,996 versus 642 pg/mL, respectively), although there was no significant difference in coculture with nonpulsed target cells (Fig. 2D). This was associated with a higher tetramer staining of cells expressing NY2-HM versus NY2-HH (data not shown). In addition, this TCR constant region replacement strategy was also proven beneficial in two other class I MHC-restricted human TCR directed against the melanoma antigens gp100 and MART-1 (data not shown).
Preferential pairing of mouse TCR chains with their counterparts
A potential hurdle in TCR gene transfer approaches is the pairing of the introduced TCR subunits with endogenous TCR chains. The immediate effect of this competition between exogenous and endogenous TCR subunits may result in the reduction of the cell surface density of the exogenous TCR (9, 19, 20). In contrast, we showed an increased proportion of MART-1 tetramer-positive cells that expressed the murinized form of the anti-MART-1 TCR (HM) rather than the original human version (HH), which may suggest a preferential pairing of the mouse constant regions with themselves (Fig. 2A).
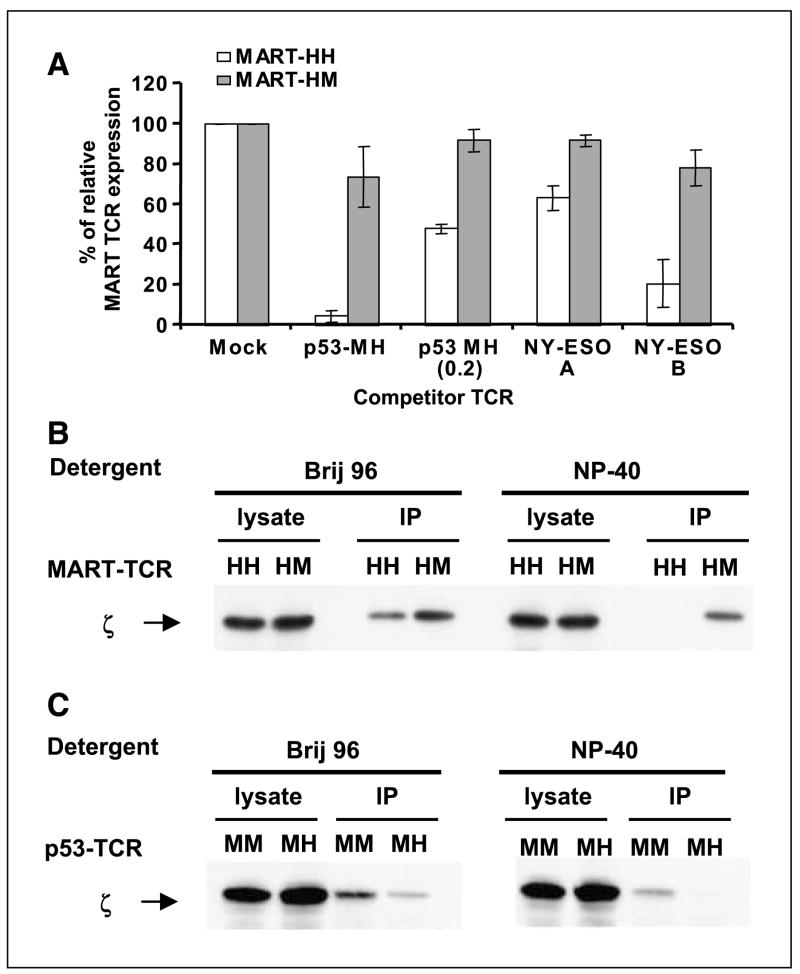
To test this hypothesis, we did a competition experiment by electroporating the TCR-deficient cell line Jurkat RT3-T3.5 with either the original MART-HH ( full human) or its mouse hybrid MART-HM and added a second mRNA encoding another TCR as a competitor (p53-MH and two different NY-ESO-1-specific TCR, NY-ESO-A and NY-ESO-B; a kind gift from Dr. Paul Robbins, Surgery Branch, NCI). Twenty-four hours after electroporation, we stained the cells with MART-1 tetramer to compare the different levels of MART-1 TCR expression relative to controls without competitor TCR.
As shown in Fig. 3A, the MART-1 TCR with mouse constant regions (MART-HM) was relatively insensitive to the addition of competitive TCR. In contrast, the expression of the native MART-1 TCR (MART-HH) was significantly reduced by the presence of competitive TCR. We stained the electroporated cells for Vβ12 surface expression and observed similar levels for both MART-1 TCRs (data not shown), suggesting that the decrease in tetramer staining of MART-HH was not due to a lower TCR chain expression but more likely to nonspecific pairing with the competitor chains. Competition seemed to be dose dependent because we observed an increase in fluorescence intensity for both MART-HH and MART-HM when we lowered the amount of the p53-MH competitor by five times to 0.2 μg. Additionally, we noted diverse levels of competition by different competitor TCRs, which may reflect preferential interactions of certain TCRs with the MART-1 TCR variable regions leading to different expression efficiencies (21, 22) or a “functional allelic exclusion” at the protein level (23).
Figure 3.
A, TCR competition assay. TCR-deficient Jurkat RT3-T3.5 cells were electroporated with 1 μg of each chain of the MART-HH (white columns) or MART-HM (gray columns) along with 1 μg of each chain of the competitor TCR [for p53-MH, we also used an additional amount (i.e., 0.2 μg) indicated as p53-MH (0.2)]. Twenty-four hours after electroporation, the cells were stained with MART-1 tetramer and the percentage of MART-1 TCR relative expression was calculated by dividing percentage of tetramer-positive cells of a given sample by that of the control sample (without competitor TCR). All the differences were statistically significant based on Student’s t test (P < 0.005). B, enhanced CD3/TCR stability mediated by MART-HM. TCR-deficient Jurkat RT3-T3.5 cells were electroporated with the MART-HH or MART-HM. Twenty-four hours after electroporation, cells were lysed in two different detergents (Brij96, mild detergent; NP40, strong detergent). The TCR was immunoprecipitated with a Vβ12 and the precipitate was subjected to a Western blot analysis for CD3ζ. As a control for protein loading (CD3ζ), we used the unprecipitated cell lysate. Representative of one of three independent experiments. C, enhanced CD3/TCR stability mediated by p53-MM. Similarly, we electroporated Jurkat RT3-T3.5 cells with either p53-MM or p53-MH and immunoprecipitated the TCR with an anti-murine Vβ3 antibody under different detergent conditions. We then subjected the precipitated to a Western blot analysis for CD3ζ and used the unprecipitated lysate as control.
Increased stability of the CD3ζ/TCR complex mediated by mouse constant regions
Due to its relatively short intracellular tail, the TCR heterodimer cannot signal by itself. Rather, the TCR recognition signal is conveyed by the CD3 complex, which is bound noncovalently to the TCR α and β chains (2). We sought to examine if there was a difference in the nature of the interaction of TCR human or mouse constant regions with the human CD3 complex.
To that end, we electroporated Jurkat RT3-T3.5 cells with either the original MART-HH or its mouse hybrid MART-HM. Twenty-four hours after the electroporation, we stained those cells with MART-1 tetramer and detected similar levels of surface expression for both TCRs (data not shown). We then solubilized the cells with two different detergents, Brij96 and NP40. Brij96 is a mild detergent that does not dissociate the TCR/CD3 complex, whereas NP40 is known to disrupt human TCR-CD3 interactions (24, 25). We subsequently immunoprecipitated the MART-1 TCR complexes using a Vβ12-specific antibody followed by Western blot analysis for CD3ζ.
As seen in Fig. 3B, both human and mouse hybrid TCR retained their interaction with the CD3ζ chain under mild detergent conditions (Brij96). However, when we used a stronger detergent NP40, CD3ζ association with human constant regions was lost, whereas a clear CD3ζ band was detected for the cells that were electroporated with MART-HM. In addition, we did a similar immunoprecipitation experiment using p53-MM and its humanized form, p53-MH. As was observed with the MART-HM construct, we detected CD3ζ association only with p53-MM but not with p53-MH under stringent detergent conditions (Fig. 3C).
Preliminary results indicate that both the murine α and β regions are needed to achieve enhanced TCR/CD3 stability because neither the combination Hα/Mβ nor Mα/Hβ was able to associate with the human CD3ζ in a similar immunoprecipitation (data not shown).
Mouse TCR constant regions increase tumor recognition and killing
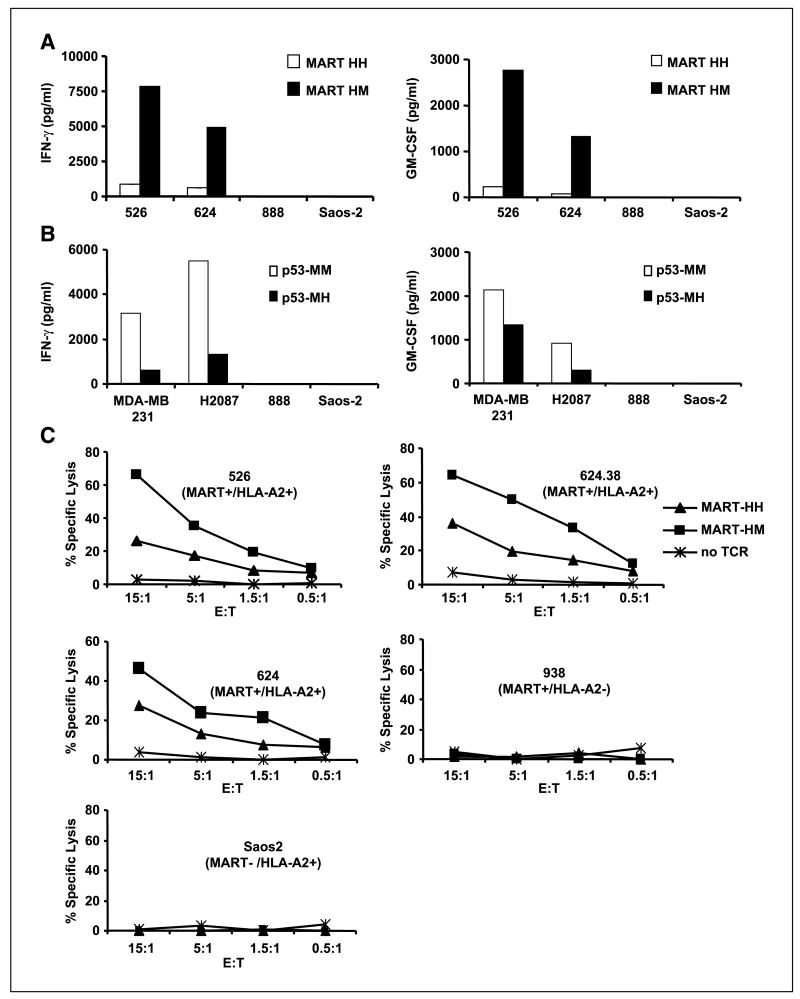
Because TCRs harboring mouse constant regions displayed a higher biological activity against peptide-pulsed T2 cells, we decided to examine the possible clinical relevance of these observations by testing tumor cell recognition. We cocultured PBLs expressing the original or the hybrid anti-MART-1 TCR with different human tumors. HLA-A2+ melanoma tumors (526 and 624) specifically stimulated MART-1 TCR-expressing T cells to secrete cytokines IFN-γ and GM-CSF (Fig. 4A). The MART-1 TCR murine hybrid (HM) was able to trigger up to 10-fold more cytokine secretion than its human counterpart (HH). No significant cytokine secretion was noted in cocultures with HLA-A2+/nonmelanoma tumor (Saos-2) or HLA-A2− melanoma lines (888). This was observed in independent experiments for 10 different donors and the difference between MART-HH and MART-HM was proven to be statistically significant (P < 0.001, Student’s t test).
Figure 4.
Enhanced recognition of tumor lines. A, human PBLs expressing either the MART-HH (white columns) or the MART-HM (black columns) were cocultured with the HLA-A2+ 526 and 624, the HLA-A2− 888 melanoma cell lines, and the HLA-A2+ Saos-2 osteosarcoma line. Twenty-four hours after the beginning of the coculture, the concentration of IFN-γ and GM-CSF secreted in the medium was measured using an ELISA procedure. Representative of 1 of 10 independent experiments each done with different donors. B, similarly, human PBLs expressing either the p53-MM (white columns) or the p53-MH (black columns) were cocultured with the p53+/HLA-A2+ MDA-MB-231, H2087 tumors, and the p53−/HLA-A2+ Saos-2 and HLA-A2− 888 control cell lines. Twenty-four hours after the beginning of the coculture, the concentration of IFN-γ and GM-CSF secreted in the medium was determined by ELISA. C, specific killing of tumor cell lines. CD8+ purified human PBLs expressing the MART-HH (triangles), the MART-HM (squares), or mock electroporated (stars) were cocultured for 3 hours with the indicated tumor cell lines labeled previously with 51Cr. Specific lysis was measured at the E:T ratios (specific release − spontaneous release) / (total release − spontaneous release). We used the following HLA-A2+/melanoma cell lines: 526, 624.38, and 624 as well as the HLA-A2−/melanoma 938 or HLA-A2+/osteosarcoma Saos-2 as control lines.
Correspondingly, we cocultured PBLs expressing the original murine anti-p53 TCR (MM) or its humanized version (MH) with HLA-A2+/p53+ tumor cells and noted a decrease in the level of both IFN-γ and GM-CSF secreted by the humanized TCR-expressing T cells compared with the full mouse TCR (Fig. 4B). There was little or no significant cytokine secretion in control cocultures with HLA-A2− or p53− cell lines.
Additionally, cell-mediated cytotoxicity of human PBLs expressing either the MART-HH or its MART-HM hybrid TCR was compared in a 3-hour 51Cr release assay. CD8+ PBL cultures were electroporated with mRNA encoding both anti-MART-1 TCRs and cocultured with Cr51-labeled tumors. Both anti-MART-1 TCRs were able to mediate specific lysis of HLA-A2+ melanoma tumor lines as seen in Fig. 4C. However, the lymphocytes expressing the murinized TCR MART-HM showed higher lysis compared with the original human TCR MART-HH (e.g., 65.9% versus 26.1% of specific lysis for the 526 target cell line at 15:1 E:T ratio, respectively). No significant lysis was observed by mock electroporated PBLs or of tumors that were HLA-A2− or non-melanoma.
MART-HM TCR can mediate class I MHC-restricted tumor recognition by CD4+ cells
Whereas a high-affinity TCR may be less dependent on the participation of a coreceptor (26), ordinary class I MHC-restricted receptors require CD8 molecules to stabilize binding (27). Because the avidity of a T cell is dictated by a combination of the affinity of its TCR for a defined MHC/peptide complex and the number of TCR molecules expressed on the surface, it might be possible to overcome the need for a coreceptor by augmenting the density of the transferred TCR. As the MART-HM is expressed at a higher density on the cell surface (Fig. 2A), we therefore postulated that this hybrid TCR might be biologically active in CD4+ cells.
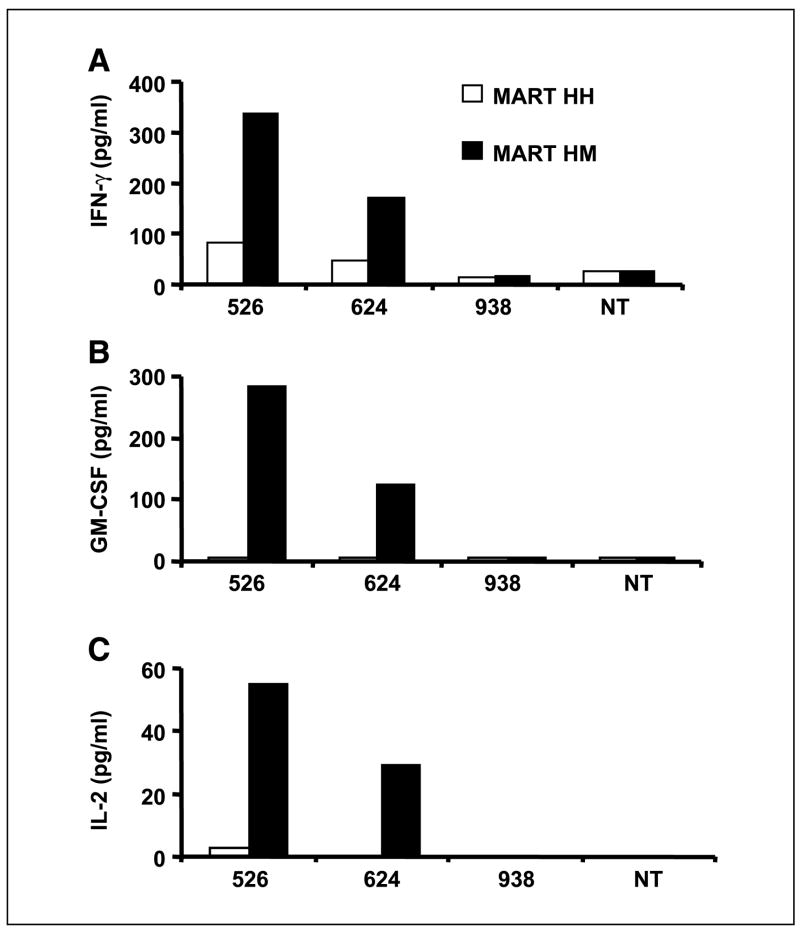
CD4+ purified OKT-3-stimulated PBLs (>95% purity) were electroporated with both the original (HH) and murine hybrid (HM) anti-MART-1 TCRs and cocultured with different melanoma tumor lines. MART-HM was able to mediate higher IFN-γ secretion by the CD4+ effector cells compared with MART-HH (Fig. 5A). GM-CSF and IL-2 secretion was only detected by MART-HM electroporated PBLs (Fig. 5B and C). No significant cytokine secretion was detected when the cells were electroporated with control mRNA (green fluorescent protein) or in the absence of targets.
Figure 5.
Increased antigen specificity in CD4+ cells expressing MART-HM. Purified CD4+ human PBLs expressing either the MART-HH (white columns) or the MART-HM (black columns) were cocultured with the HLA-A2+/melanoma cell lines 526 and 624, the HLA-A2−/melanoma 938, or without target (NT). The concentration of IFN-γ (A), GM-CSF (B), and IL-2 (C) secreted in the medium was measured using an ELISA procedure.
Discussion
In the present work, we showed that TCRs containing murine constant regions exhibit superior expression and biological activity in human lymphocytes compared with TCRs with human constant regions. We created two sets of TCRs by swapping the constant regions of mouse and human TCRs (i.e., a murine anti-p53 TCR containing human constant regions and a human anti-MART-1 TCR with murine constant regions). Those constructs were in vitro transcribed and the mRNA encoding those chains was used for electroporation into lymphocytes. This methodology is efficient in generating potent lymphocytes with redirected specificity (16, 28). Moreover, this approach enables the use of normalized levels of mRNA and is, unlike other common gene transfer methods, not dependent on retroviral insertion sites or the influence of diverse transcription mechanisms.
TCRs harboring mouse constant regions that are expressed in human PBLs showed a higher surface density as well as enhanced cytokine secretion compared with TCRs with human constant regions. We reproducibly achieved in 12 independent experiments with 10 different donors, higher MFI values (i.e., more TCR per cell surface), and higher numbers of TCR-expressing cells (e.g., 61% versus 21.1%; Table 1) when we used a MART-1 TCR with mouse constant regions in comparison with the original human TCR. To molecularly characterize this phenomenon, we did TCR competition experiments in the TCR-deficient Jurkat RT3-T3.5 cell line. We discovered that the murinized anti-MART-1 TCR (MART-HM) was less influenced by the presence of other human/humanized competitor TCRs than the original full human anti-MART-1 TCR (MART-HH), although we noted similar levels of Vβ12 surface expression for both MART-1 TCRs (data not shown), implying that the decreased density of human MART-1 TCR was not the result of a lower protein expression but mispairing with the competitor chains. Nevertheless, we did notice that the expression of the murinized anti-MART-1 TCR was slightly decreased compared with control experiments without any competitor, which indicated that the pairing between murinized TCR subunits is preferential but not exclusive. This was also supported by the fact that we observed antigen-specific IFN-γ secretion by PBLs in which we introduced different combinations of TCR chains (e.g., the original human MART-1 TCR α chain, Hα, with the murinized MART-1 TCR β chain, Mβ; Fig. 2B and C). Taken together, these findings indicated that a murine constant region hybrid TCR is less likely to participate in unproductive mispairing with the endogenous TCR in human lymphocytes, which leads to its overrepresentation on the cell surface.
The observation of preferential paring has potential clinical relevance for ongoing cancer gene therapy trials,1,2 as one major limitation of TCR transfer is the dilution of the exogenous TCR due to mispairing. For example, if we assume that the introduced TCR chains represent 20% of the total chains available and that the probability that one α will pair with either β (and vice versa) is the same for endogenous and exogenous TCR components, the introduced TCR will represent only 4% of the total receptors present on the surface. The formation of a correctly paired and functional TCR may be considerably improved by using a TCR with mouse constant regions (as shown in Fig. 2A). Additionally, preferential pairing might decrease the formation of undesirable TCR heterodimers, including those with potential self-reactivity (3, 29). Moreover, a higher density of TCR may help to reach the critical threshold for T-cell activation as was shown previously (30).
In addition to the approach we used in this study, other strategies to increase the specific pairing of exogenous TCR chains may include the introduction of an interchain disulfide bond into the interface between TCR constant domains (31), the swapping of charged residues between chains, the manipulation of the transmembrane associations domains, or the development of other selective pairing strategies (32). It is also possible to use other forms of MHC-restricted receptors as signaling moieties, such as TCR-like antibodies (33, 34) or single-chain TCRs (32, 35), although the latter has been shown less effective than full-length TCRs (36).
We observed superior TCR/CD3 stability mediated by the murine constant regions either grafted on the human MART-1 TCR or naturally present on p53-MM (Fig. 3B and C). This structural enhancement may not result in enhanced TCR signaling, as preliminary experiments did not show statistically significant differences in phosphorylation patterns of pERK (data not shown). It has been reported that most of the interaction between TCR and CD3ζ occurs in the transmembrane region (2). Structure comparison of human and murine TCR constant regions reveals that the transmembrane regions are nearly identical (37), with >95% identity for the amino acid sequence. More specifically, the three basic residues (R, K, and K) in the transmembrane regions the TCR αβ heterodimer are conserved between mouse and human chains. These residues were proposed to drive the associations between TCR and CD3 components by forming pair-wise ionic interactions similar to salt bridges commonly observed in soluble proteins (38). Based on our observations, we hypothesize that the extracellular region of murine TCR constant chains may interact with the CD3 complex, providing the basis for an innovative approach to study CD3/TCR interactions.
Both TCRs with murine constant regions (i.e., the MART-HM and p53-MM) showed enhanced tumor recognition, which was subsequently translated in improved killing of melanoma tumors by the hybrid MART-1-specific TCR (Fig. 4). Our observations are further supported by Stanislawski et al. (39), where in one experiment a humanized murine TCR was less active in human cells than the native mouse receptor. In the present report, we observed up to 10-fold more IFN-γ and GM-CSF secretion by lymphocytes expressing the MART-HM TCR, compared with the original MART-HH TCR, in cocultures with melanoma cell lines. Furthermore, we also observed higher cytokine secretion by MART-HM-expressing cells when we normalized their number to be equal or lower than MART-HH-expressing cells in parallel cocultures (data not shown). In addition, the MART-HM TCR mediated a higher cytotoxic activity, enabling the enhanced lysis of different melanomas at lower E:T ratios (Fig. 4C). Such biological improvement has important implications for the treatment for viral disease and cancer, because TCR gene transfer to reprogram the specificity of patient lymphocytes is currently under clinical investigation.1,2
In addition to their function in cytotoxic lymphocytes, the utilization of TCR with mouse constant regions could help to overcome the inherent low avidity of CD4+ lymphocytes expressing class I MHC-restricted TCRs. CD4+ T lymphocytes showed an increase antitumor activity when electroporated with a “murinized” TCR. The conversion of CD4+ cells into class I MHC-restricted lymphocytes and their recruitment at tumor sites may be especially important because CD4 helper responses can be essential for mediating efficient antitumor activity and in the maintenance of functional CD8+ T-cell memory (40, 41). In addition to their helper functions, CD4+ lymphocytes have also been shown to exhibit cytotoxic activity in several experimental systems (11, 42) and more recently in vivo (43).
The present report is aimed at shedding light on the biology of murine TCR expressed in human lymphocytes. Indeed, such receptors isolated from HLA-A2 transgenic mice have become valuable for antitumor adoptive immunotherapy (11, 12, 39, 44) because they provide a means to circumvent natural tolerance to self-antigens (45). The putative antigenicity of those murine domains (although highly homologous to their human counterparts) when expressed in patient lymphocytes remains to be examined. The robust immunosuppressive nonmyeloablative conditioning in patients treated with allogeneic hematopoietic stem cell transplants (46, 47) or in adoptive cell transfer procedures (5) may moderate or preclude the undesirable rejection of genetically modified lymphocytes expressing murine TCR genes. Nevertheless, it may be possible to fine-mutate TCR subunits and we are currently trying to identify the key residues of the murine sequences that mediate improved pairing and TCR function in human lymphocytes. Several reports indicated that it is also possible to mutate the TCR transmembrane domains to influence lymphocyte function and survival (48, 49).
In conclusion, we have shown that a modified human TCR with murine constant regions is overexpressed and functions better than the full human TCR in human lymphocytes. Beyond its clinical relevance, this work suggests that the biochemistry of murine and human TCRs and their interactions with the human CD3 signaling complex are different, paving the way to potential immunologic studies dealing with lymphocytes function and differentiation.
Acknowledgments
We thank Drs. Nicholas Restifo, Paul Robbins, and James Yang for helpful comments.
Footnotes
References
- 1.Germain RN, Stefanova I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol. 1999;17:467–522. doi: 10.1146/annurev.immunol.17.1.467. [DOI] [PubMed] [Google Scholar]
- 2.Call ME, Wucherpfennig KW. The T cell receptor: critical role of the membrane environment in receptor assembly and function. Annu Rev Immunol. 2005;23:101–25. doi: 10.1146/annurev.immunol.23.021704.115625. [DOI] [PubMed] [Google Scholar]
- 3.Schumacher TN. T-cell-receptor gene therapy. Nat Rev Immunol. 2002;2:512–9. doi: 10.1038/nri841. [DOI] [PubMed] [Google Scholar]
- 4.Kershaw MH, Teng MW, Smyth MJ, Darcy PK. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol. 2005;5:928–40. doi: 10.1038/nri1729. [DOI] [PubMed] [Google Scholar]
- 5.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan RA, Dudley ME, Yu YY, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–95. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes MS, Yu YYL, Dudley ME, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:1–16. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–23. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roszkowski JJ, Lyons GE, Kast WM, Yee C, Van Besien K, Nishimura MI. Simultaneous generation of CD8+ and CD4+ melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor. Cancer Res. 2005;65:1570–6. doi: 10.1158/0008-5472.CAN-04-2076. [DOI] [PubMed] [Google Scholar]
- 10.Engels B, Noessner E, Frankenberger B, Blankenstein T, Schendel DJ, Uckert W. Redirecting human T lymphocytes toward renal cell carcinoma specificity by retroviral transfer of T cell receptor genes. Hum Gene Ther. 2005;16:799–810. doi: 10.1089/hum.2005.16.799. [DOI] [PubMed] [Google Scholar]
- 11.Cohen CJ, Zheng Z, Bray R, et al. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol. 2005;175:5799–808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuball J, Schmitz FW, Voss RH, et al. Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity. 2005;22:117–29. doi: 10.1016/j.immuni.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–6. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 14.Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989;142:3714–25. [PubMed] [Google Scholar]
- 15.Salter RD, Howell DN, Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21:235–46. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Zheng Z, Cohen CJ, et al. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006;13:151–9. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Zheng Z, Kong HT, Rosenberg SA, Morgan RA. Transduction of an HLA-DP4 restricted NY-ESO-1 specific TCR into primary human CD4+ lymphocytes. J Immunother. 2006;29:398–406. doi: 10.1097/01.cji.0000203082.20365.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dittel BN, Stefanova I, Germain RN, Janeway CA., Jr Cross-antagonism of a T cell clone expressing two distinct T cell receptors. Immunity. 1999;11:289–98. doi: 10.1016/s1074-7613(00)80104-1. [DOI] [PubMed] [Google Scholar]
- 19.Rubinstein MP, Kadima AN, Salem ML, et al. Transfer of TCR genes into mature T cells is accompanied by the maintenance of parental T cell avidity. J Immunol. 2003;170:1209–17. doi: 10.4049/jimmunol.170.3.1209. [DOI] [PubMed] [Google Scholar]
- 20.Blichfeldt E, Munthe LA, Rotnes JS, Bogen B. Dual T cell receptor T cells have a decreased sensitivity to physiological ligands due to reduced density of each T cell receptor. Eur J Immunol. 1996;26:2876–84. doi: 10.1002/eji.1830261211. [DOI] [PubMed] [Google Scholar]
- 21.Saito T, Sussman JL, Ashwell JD, Germain RN. Marked differences in the efficiency of expression of distinct αβ T cell receptor heterodimers. J Immunol. 1989;143:3379–84. [PubMed] [Google Scholar]
- 22.Li ZG, Wu WP, Manolios N. Structural mutations in the constant region of the T-cell antigen receptor (TCR)β chain and their effect on TCR α and β chain interaction. Immunology. 1996;88:524–30. [PMC free article] [PubMed] [Google Scholar]
- 23.Sant’Angelo DB, Cresswell P, Janeway CA, Jr, Denzin LK. Maintenance of TCR clonality in T cells expressing genes for two TCR heterodimers. Proc Natl Acad Sci U S A. 2001;98:6824–9. doi: 10.1073/pnas.121179998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.San Jose E, Sahuquillo AG, Bragado R, Alarcon B. Assembly of the TCR/CD3 complex: CD3 ε/δ and CD3 ε/γ dimers associate indistinctly with both TCR α and TCR β chains. Evidence for a double TCR heterodimer model. Eur J Immunol. 1998;28:12–21. doi: 10.1002/(SICI)1521-4141(199801)28:01<12::AID-IMMU12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Call ME, Pyrdol J, Wucherpfennig KW. Stoichiometry of the T-cell receptor-CD3 complex and key intermediates assembled in the endoplasmic reticulum. EMBO J. 2004;23:2348–57. doi: 10.1038/sj.emboj.7600245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherman LA, Hesse SV, Irwin MJ, La Face D, Peterson P. Selecting T cell receptors with high affinity for self-MHC by decreasing the contribution of CD8. Science. 1992;258:815–8. doi: 10.1126/science.1439792. [DOI] [PubMed] [Google Scholar]
- 27.Wooldridge L, van den Berg HA, Glick M, et al. Interaction between the CD8 coreceptor and major histocompatibility complex class I stabilizes T cell receptor-antigen complexes at the cell surface. J Biol Chem. 2005;280:27491–501. doi: 10.1074/jbc.M500555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaft N, Dorrie J, Muller I, et al. A new way to generate cytolytic tumor-specific T cells: electroporation of RNA coding for a T cell receptor into T lymphocytes. Cancer Immunol Immunother. 2005;55:1132–41. doi: 10.1007/s00262-005-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue S, Gillmore R, Downs A, et al. Exploiting T cell receptor genes for cancer immunotherapy. Clin Exp Immunol. 2005;139:167–72. doi: 10.1111/j.1365-2249.2005.02715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–6. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 31.Boulter JM, Glick M, Todorov PT, et al. Stable, soluble T-cell receptor molecules for crystallization and therapeutics. Protein Eng. 2003;16:707–11. doi: 10.1093/protein/gzg087. [DOI] [PubMed] [Google Scholar]
- 32.Willemsen RA, Weijtens ME, Ronteltap C, et al. Grafting primary human T lymphocytes with cancer-specific chimeric single chain and two chain TCR. Gene Ther. 2000;7:1369–77. doi: 10.1038/sj.gt.3301253. [DOI] [PubMed] [Google Scholar]
- 33.Cohen CJ, Denkberg G, Lev A, Epel M, Reiter Y. Recombinant antibodies with MHC-restricted, peptide-specific, T-cell receptor-like specificity: new tools to study antigen presentation and TCR-peptide-MHC interactions. J Mol Recognit. 2003;16:324–32. doi: 10.1002/jmr.640. [DOI] [PubMed] [Google Scholar]
- 34.Chames P, Willemsen RA, Rojas G, et al. TCR-like human antibodies expressed on human CTLs mediate antibody affinity-dependent cytolytic activity. J Immu-nol. 2002;169:1110–8. doi: 10.4049/jimmunol.169.2.1110. [DOI] [PubMed] [Google Scholar]
- 35.Chung S, Wucherpfennig KW, Friedman SM, Hafler DA, Strominger JL. Functional three-domain single-chain T-cell receptors. Proc Natl Acad Sci U S A. 1994;91:12654–8. doi: 10.1073/pnas.91.26.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang T, He X, Tsang TC, Harris DT. Transgenic TCR expression: comparison of single chain with full-length receptor constructs for T-cell function. Cancer Gene Ther. 2004;11:487–96. doi: 10.1038/sj.cgt.7700703. [DOI] [PubMed] [Google Scholar]
- 37.Garcia KC, Degano M, Pease LR, et al. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science. 1998;279:1166–72. doi: 10.1126/science.279.5354.1166. [DOI] [PubMed] [Google Scholar]
- 38.Cosson P, Lankford SP, Bonifacino JS, Klausner RD. Membrane protein association by potential intramem-brane charge pairs. Nature. 1991;351:414–6. doi: 10.1038/351414a0. [DOI] [PubMed] [Google Scholar]
- 39.Stanislawski T, Voss RH, Lotz C, et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol. 2001;2:962–70. doi: 10.1038/ni1001-962. [DOI] [PubMed] [Google Scholar]
- 40.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–94. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 41.Ho WY, Yee C, Greenberg PD. Adoptive therapy with CD8(+) T cells: it may get by with a little help from its friends. J Clin Invest. 2002;110:1415–7. doi: 10.1172/JCI17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appay V. The physiological role of cytotoxic CD4(+) T-cells: the holy grail? Clin Exp Immunol. 2004;138:10–3. doi: 10.1111/j.1365-2249.2004.02605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corthay A, Skovseth DK, Lundin KU, et al. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–83. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Yu Z, Liu X, McCarty TM, Diamond DJ, Ellenhorn JD. The use of transgenic mice to generate high affinity p53 specific cytolytic T cells. J Surg Res. 1997;69:337–43. doi: 10.1006/jsre.1997.5058. [DOI] [PubMed] [Google Scholar]
- 45.Theobald M, Biggs J, Dittmer D, Levine AJ, Sherman LA. Targeting p53 as a general tumor antigen. Proc Natl Acad Sci U S A. 1995;92:11993–7. doi: 10.1073/pnas.92.26.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 47.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98:3456–64. doi: 10.1182/blood.v98.12.3456. [DOI] [PubMed] [Google Scholar]
- 48.Backstrom BT, Hausmann BT, Palmer E. Signaling efficiency of the T cell receptor controlled by a single amino acid in the β chain constant region. J Exp Med. 1997;186:1933–8. doi: 10.1084/jem.186.11.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teixeiro E, Daniels MA, Hausmann B, et al. T cell division and death are segregated by mutation of TCRβ chain constant domains. Immunity. 2004;21:515–26. doi: 10.1016/j.immuni.2004.08.014. [DOI] [PubMed] [Google Scholar]