Abstract
Hodgkin disease (HD) is characterized by a small number of malignant Hodgkin and Reed-Sternberg (H/RS) cells among a major population of nonmalignant cells. The analysis of H/RS cells has been hampered by their low frequency and fragility. Here, we describe the isolation of viable H/RS cells from HD affected tissues by high gradient magnetic cell sorting (MACS) according to expression of CD30. The cells were enriched to a purity of up to 50%. H/RS cells were distinguished from other CD30+ cells by the expression of CD15, their size and granularity. No CD30/CD15 double-positive cells could be enriched from a lymph node affected by the lymphocyte predominant subtype of HD, activated lymph nodes or peripheral blood of healthy donors. For two cases of HD individual MACS-purified H/RS cells and H/RS cells micromanipulated from tissue sections of the same lymphoma specimens were analyzed for Ig gene rearrangements. In both cases, identical V gene rearrangements were amplified from both sources of H/RS cells, showing that H/RS cells were successfully enriched. Moreover, the finding that in both cases no additional Ig gene rearrangements other than the ones identified in the H/RS cells micromanipulated from tissue sections were amplified from the MACS-purified H/RS cells further supports the monoclonality of these cells throughout the affected lymph nodes. The isolation of viable H/RS cells ex vivo is prerequisite for a direct study of gene expression by those cells and of their interaction with cells in their vicinity.
Keywords: magnetic cell sorting/CD30/single cell PCR/clonality/B cell lineage
The two cell types typical for Hodgkin disease (HD), namely the mononucleated Hodgkin and the bi- or oligonucleated Reed-Sternberg (RS) cells are very rare in the affected tissues. Thus, since their description in 1832 and 1898, respectively (1, 2), their lineage derivation and clonality has been a matter of debate. Most analyses of H/RS cells have been performed by immunohistochemistry (3, 4). Molecular studies of HD have until recently been restricted to DNA extracted from biopsy specimens and to HD-derived cell lines (5). The results obtained from these studies were conflicting and did not give a clear picture as to the derivation and clonality of H/RS cells. This is most likely because of technical problems related to the small number of H/RS cells in the tissue and to the fact that the origin of the cell lines (except for cell line L1236; refs. 6 and 7) is uncertain. Moreover, no specific pattern of antigens expressed by H/RS cells allowed to determine the lineage derivation of the cells (8, 9). H/RS cells in classical (i.e., nodular sclerosis, mixed cellularity and lymphocyte depletion) HD express CD30 and in most cases CD15 as well as CD25 as surface antigens on a fraction or all of the H/RS cells, whereas H/RS cells in lymphocyte predominant HD (HDlp) usually lack expression of these antigens (9, 10).
That H/RS cells derive from B lineage cells was clarified by genotypic analyses of single H/RS cells. Early PCR analyses on single cells derived from HD lymph nodes gave conflicting results (reviewed in ref. 11). However, other studies demonstrated that H/RS cells harbor clonal variable regions of Ig heavy chain (VH) and and light chain (VL) gene rearrangements and thus represent mature B cells (7, 12–16). Furthermore, the pattern of somatic mutations within these rearranged genes identified germinal center B cells as the precursors of H/RS cells in HD (7, 12–16). Besides the lineage derivation of H/RS cells, their clonality within an affected tissue was also debated. In studies describing that H/RS cells in HD patients represent a single clone, the cells were micromanipulated from one or several tissue sections of a biopsy specimen (7, 12–16). It has been argued that these studies do not rule out the possibility that more than one clone may exist in an affected tissue (17–19).
Attempts to isolate H/RS cells by fluorescence-activated cell sorting have so far been largely unsuccessful, presumably because of the fragility of these very large cells. In one analysis, in which H/RS cells were reported to be isolated by flow cytometry, no information is given on the purity and viability of the sorted cells (18). In other analyses describing the isolation of H/RS cells by density centrifugation, a confirmation that the enriched cells indeed represent H/RS cells is missing (20). In another study, single CD3−/CD20− cells with the morphology of H/RS cells were isolated from cell suspensions by micromanipulation and analyzed for the expression of various genes (21). Expression of genes of various hematopoietic cell lineages was observed.
Here we describe the isolation of populations of viable H/RS cells from various human tissues by using high gradient magnetic cell sorting (MACS) that is well suited for the enrichment of rare cell types (22–24). Besides the expression of both CD15 and CD30, the morphology of the cells, i.e., their large size and granularity, was taken as a third criterium for their recognition as H/RS cells. Only from HD-affected tissues cells with this phenotype could be isolated. A molecular analysis of Ig gene rearrangements carried by MACS-enriched CD30+ cells and H/RS cells micromanipulated from the corresponding HD tissue revealed that the enriched cells indeed represent the H/RS cells in the tumor.
MATERIALS AND METHODS
Patients and Tissues.
Twelve lymph nodes, removed for diagnostic purposes, were analyzed (Table 1). Three cases were diagnosed as nodular sclerosis HD (HDns), five as mixed cellularity HD (HDmc), one as HDlp and one as “classical HD, lymphocyte rich” (HDlr) (10). Two cases represented activated lymph nodes. From two other patients, presenting with HDns, cells from bone marrow, and from one patient H/RS cells from pleural and pericardial effusion were analyzed.
Table 1.
Case description and results of CD30+ cell enrichment
| Patient | Age/sex | Tissue | Diagnosis | CD30+ cells
|
CD15 positivity | Phenotype of CD30+ cells on cytospins | |
|---|---|---|---|---|---|---|---|
| Before MACS (%) | After MACS (%) | ||||||
| 2428 | 31, M | LN supracl | HDns | 0.1 | 23.1 | Yes (FACS) | H/RS cells |
| 6104 | 31, M | LN inguin. | HDns | 0.4 | 53.0 | Yes (cyto) | H/RS cells |
| 744 | 22, M | LN supracl | HDns | 0.1 | 3.0 | Yes (FACS) | H/RS cells |
| 1406 | 30, M | BM | HDns | 0.9 | 8.2 | Yes (FACS) | H/RS cells |
| 21, M | BM | HDns | 0.1 | 3.0 | Yes (FACS) | H/RS cells | |
| 15846 | 18, F | pleura | HDns | 0.3 | 20.8 | ND | H/RS cells |
| 15846 | 18, F | pericard | HDns | 0.5 | 47.5 | ND | H/RS cells |
| 3096 | 32, M | LN axill. | HDmc | 0.0 | 6.7 | Yes (cyto) | H/RS cells |
| 6937 | 41, M | LN cerv. | HDmc | 0.7 | 13.0 | ND | H/RS cells |
| 15415 | 72, M | LN cerv. | HDmc | 1.0 | 15.6 | Yes (FACS) | H/RS cells |
| 4964 | 30, M | LN cerv. | HDmc | 0.5 | 32.1 | No (FACS) | H/RS cells |
| 2967 | 77, M | LN cerv. | HDmc | 0.3 | 13.4 | Yes (FACS) | H/RS cells |
| 6444 | 29, M | LN axill. | HDlr | 0.1 | 10.0 | Yes (cyto) | H/RS cells |
| 14740 | 41, M | LN cerv. | HDlp | 0.5 | 41.3 | No (FACS) | Small cells |
| 12232 | ?, M | LN axill. | react. LN | 0.3 | 20.0 | ND | Small cells |
| 12626 | 64, F | LN cerv. | react. LN | 0.8 | 52.4 | ND | Small cells |
The patient data are listed as is the origin of the affected tissues analyzed. Cells positive for CD30 were enriched from lymph nodes (LN) of 10 patients suffering from HD, from two “reactive” lymph nodes, from two HD bone marrow specimens (BM) and from pleural and pericardial effusion of one patient. The frequencies of CD30-positive cells before and after enrichment as determined by flow cytometry are given. Positivity of the enriched cells for CD15 expression was determined either by flow cytometry (fluorescence-activated cell sorter, or FACS) or by immunostaining on cytospins (cyto). The morphology of the sorted cells was determined on cytospins after CD30-specific avidin-biotin-complex staining. ND, not done; HDlr, classical HD, lymphocyte-rich.
Preparation of Cell Suspensions.
Lymph nodes were cut into small pieces using scalpels and suspended in RPMI 1640 medium. The resulting cell suspensions were filtered through a 500-μm pore mesh. In some cases additional cells were obtained from the remaining tissue pieces by enzymatic digestion. For this purpose, the tissue was incubated with 200 units/ml collagenase IV (Worthington) and 10 units/ml DNase I (Promega) for 30 min at 37°C. The cells were then washed in Iscove’s modified Dulbecco’s medium supplemented with 5% fetal calf serum before filtration as described above and pooled with the other cells.
Cells from pleural and pericardial effusion and from bone marrow were first depleted of erythrocytes in a NH4Cl-based buffer (25). In brief, the cell suspension was diluted 1:1 with RPMI 1640 medium and 4 volumes of the lysing buffer were added. The suspensions were incubated for 15 min on ice before washing twice with PBS/0.5%BSA. After a final wash, nearly all erythrocytes were depleted.
High-Gradient Magnetic Cell Sorting.
For cell sorting the VarioMACS system (Miltenyi Biotec, Auburn, CA) was used. Fragment crystalizable receptors on the cells were blocked by incubation with 2 mg/ml of Beriglobin (Behring) for 10 min on ice. The cells were then labeled with a mixture of fluorescein-conjugated and unconjugated anti-human CD30 mAbs (BerH2, Dako), for 10 min on ice in PBS/BSA. After washing, the cells were labeled magnetically with anti-fluorescein isothiocyanate (FITC) Multisort Microbeads (Miltenyi Biotec). Alternatively, cells were magnetically labeled with CD30 Multisort Microbeads and stained using CD30 Phycoerythrin (both Miltenyi Biotec). The labeled cells selectively bound to a VS column (Miltenyi Biotec) using the VarioMACS and were eluted from the column with 5 ml of PBS/BSA after removing it from the magnetic field. Efficiency of the separation was controlled by flow cytometry using a FACScan and FACScan Research or Cellquest software (Becton Dickinson). Dead cells were excluded from the analysis by using propidium iodide staining.
Immunophenotyping.
Fluorescence-labeled cells enriched by MACS were counterstained with a panel of antibodies conjugated to FITC or Phycoerythrin, with specificity for various human cell surface antigens. The CD15 antibodies were purchased from PharMingen and the CD4, CD8, CD14, CD16, CD19 antibodies from Becton Dickinson. The stains were analyzed by flow cytometry (see above) or by fluorescence microscopy. Some cytospin preparations of enriched cells were stained with the avidin biotin complex method and alkaline phosphatase.
Immunohistochemistry and Micromanipulation.
Eight- to 10-μm-thick tissue sections and cytospin preparations were stained using mAbs against CD30 (BerH2, Dako), CD20 (L26, Dako), CD15 (LeuM1, Becton Dickinson), or CD3 (OKT3, Ortho Diagnostic). Staining and micromanipulation were performed as described (26, 27). Bound alkaline phosphatase was visualized by Fast Red (Merck).
Single Cell PCR.
Rearranged V genes were amplified from single cells in two rounds of amplification, using three different sets of primers. In the first round of amplification an Expand High Fidelity enzyme mix (Boehringer Mannheim) and in the second round Taq DNA polymerase was used. Variable region genes of heavy chain (VH) and κ light chain (Vκ) were amplified with a collection of V gene family-specific primers hybridizing to sequences within framework region (FR) I of human VH families 1–7 and Vκ families 1–4, together with nested J gene primers, as described (13, 27). Alternatively, cells were analyzed for VH rearrangements with family-specific VH leader primers together with the same primers for the joining genes of heavy chain (JH) (14). In one experiment, six H/RS cells and the corresponding control samples were analyzed for VH and Vλ gene rearrangements, using the VH FRI and JH primers mentioned above together with primers for eight human Vλ families and Jλ primers (J. Kurth, M.L.H., K.R., and R.K., unpublished data). PCR products were gel-purified and directly sequenced on an Applied Biosystems 377 sequencer (Applied Biosystems).
RESULTS
Magnetic Isolation of CD30-Positive Cells from Different Tissues.
Because CD30 is expressed by H/RS cells in most if not all cases of classical HD (9) this antigen was chosen as target molecule for the enrichment of H/RS cells. However, CD30 is expressed also by other populations of hematopoietic cells (28). We demonstrate below that the large cell size of H/RS cells and the coexpression of CD30 and CD15 on these cells can be used as reliable criteria to discriminate H/RS cells from other CD30-positive cells in the isolation procedure.
We first investigated the expression of CD30 on cells from peripheral blood (PB) and lymph nodes of normal donors. The cells were magnetically labeled using an FITC-coupled CD30 antibody and FITC-specific antibodies conjugated to super-paramagnetic microbeads, or by CD30-specific antibodies directly conjugated to microbeads. In PB of healthy donors, at most 0.2% CD30-expressing cells were detectable before enrichment. These cells were enriched by MACS to up to 30% (Fig. 1). Flow cytometric analysis showed that CD30 is expressed on a subpopulation of CD4- or CD8-positive T cells, as well as on a fraction of CD19+ B cells, which are all CD15-negative. CD30 expression was neither detected on CD16+ natural killer cells or neutrophilic granulocytes, nor on CD15+ eosinophilic granulocytes or CD14+ monocytes (Fig. 1c). In two cases, cells from PB were analyzed after lysis of erythrocytes. In these cell suspensions that contain >50% of CD15-positive granulocytes and monocytes, CD30/CD15 double-positive cells were also not detected (data not shown).
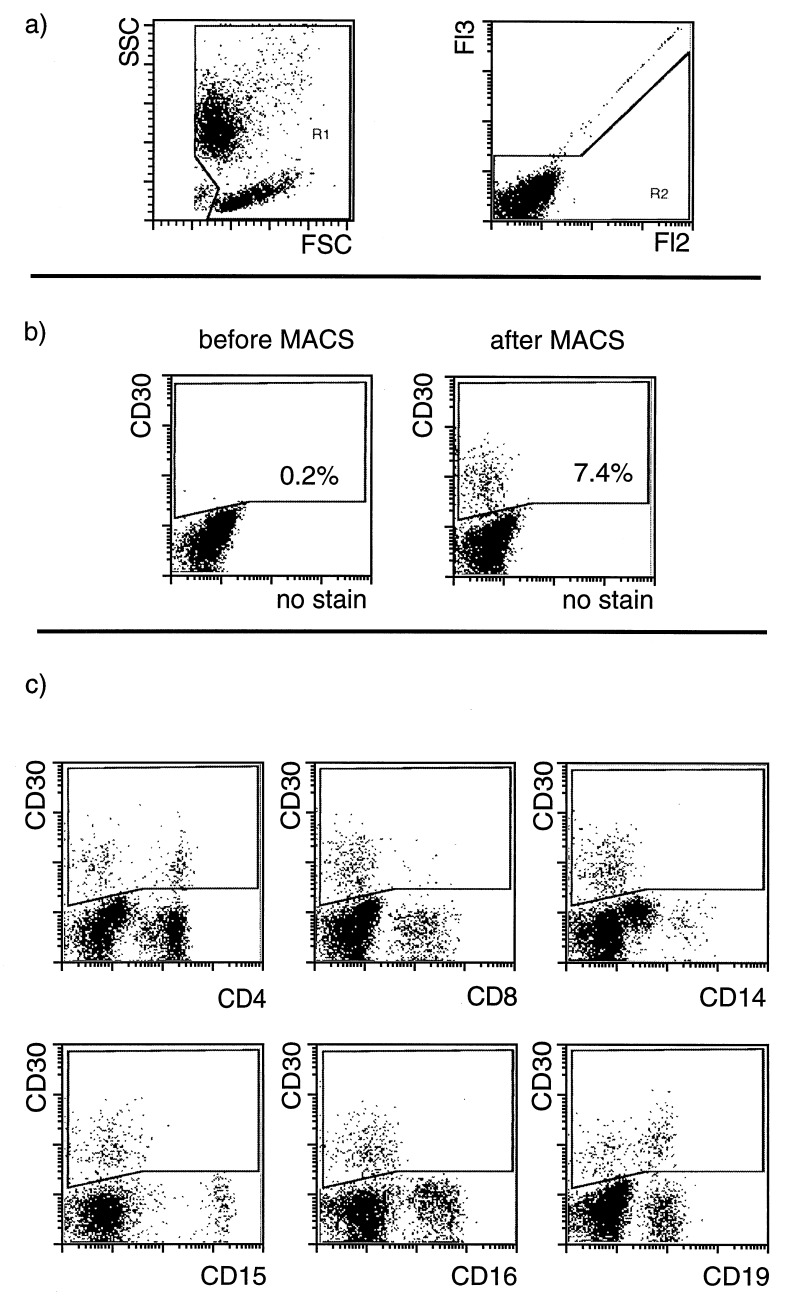
Figure 1.
Enrichment of CD30-positive cells from PB mononuclear cells of a normal donor. (a) Gates used in the analysis are given. Debris and dead cells are excluded. (b) Using CD30 magnetic beads CD30+ cells were enriched from 0.2% to 7.4% purity in one separation step in the given example. The cells were stained with CD30 antibodies conjugated with R-phycoerythrin. (c) Counterstaining with FITC-conjugated antibodies against different cell type specific antigens revealed that a subpopulation of both CD4- or CD8-positive cells as well as of B cells (CD19+) express CD30. No NK cells or neutrophilic granulocytes (CD16+) could be stained. Monocytes (CD14+) and eosinophilic granulocytes (CD15+) do not express CD30.
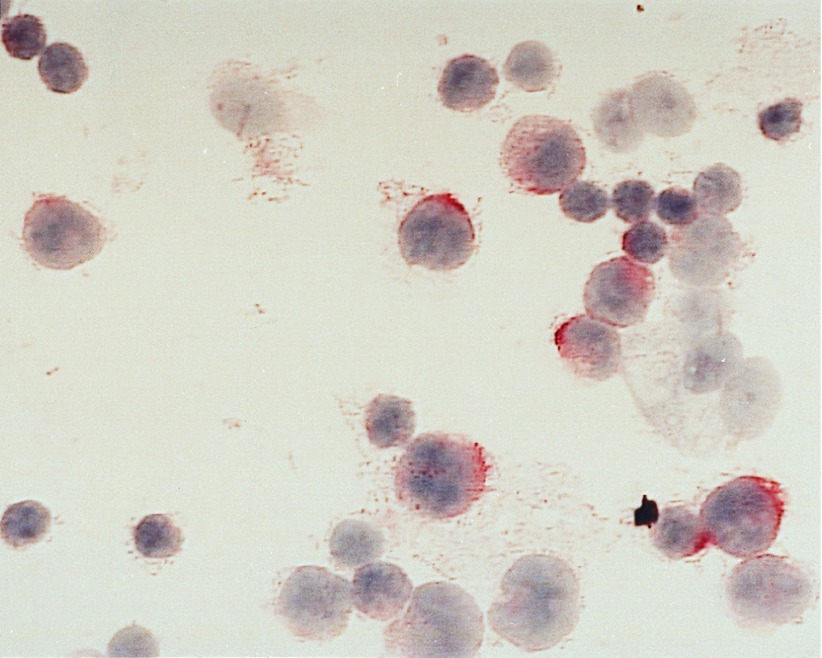
CD30-positive cells were also enriched from two inflamed lymph nodes (Table 1). The enriched CD30-positive cells were small, CD15-negative and presumably represent activated T and B cells (data not shown). From a lymph node of a HDlp case with CD30-negative tumor cells, CD30-positive cells could be enriched to a frequency of 41%. These cells were relatively small, mononucleated and CD15− (Fig. 2).
Figure 2.
Immunohistochemical staining of the CD30+ enriched cells from the lymph node of a HDlp patient (case 14740) on a cytospin slide. The nuclei of the cells are stained with hematoxyline and CD30 expression is visualized by the use of a specific avidin biotin complex staining. The CD30+ cells are smaller than the typical H/RS cells and are less granulated. (×630.)
Isolation and Phenotypic Characterization of CD30-Positive Cells from Cases of Classical HD.
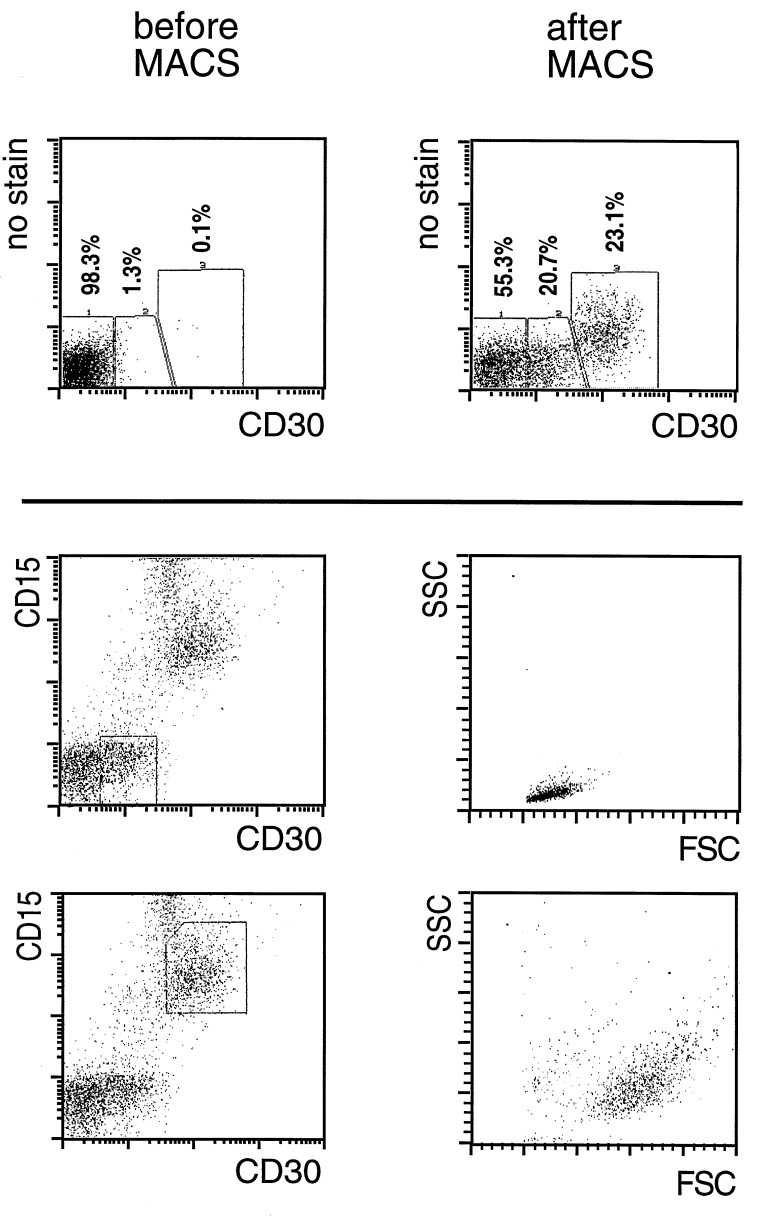
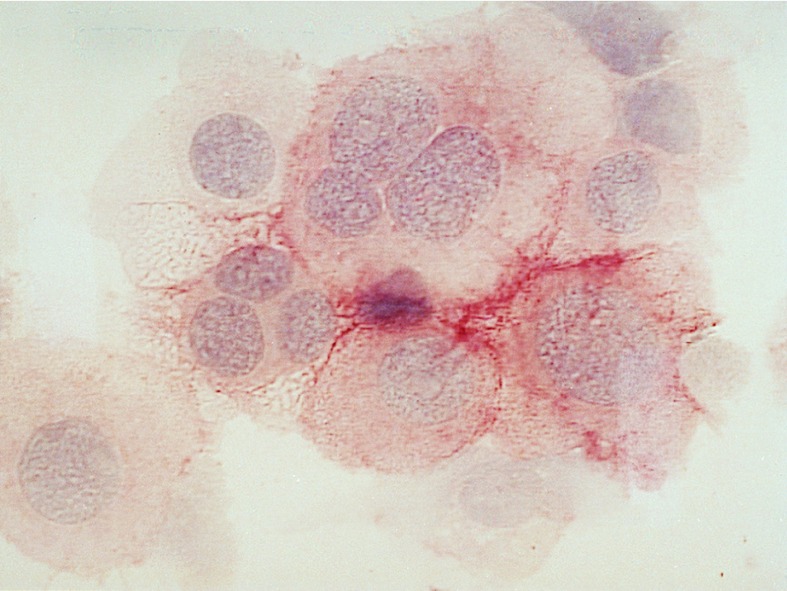
In eight cases of classical HD, CD30-positive cells were enriched from lymph nodes from <0.1–0.7% to more than 50% (Table 1, Fig. 3), with recoveries of up to 40–70%. An immunohistochemical staining of CD30 on cells enriched from a HDmc lymph node is shown in Fig. 4. In two cases, CD30+ cells were enriched from bone marrow of patients suffering from HDns (Table 1). From one patient, CD30-positive cells were isolated from pericardial and pleural effusion. Fig. 3 exemplifies the separation of CD30-positive cells from an affected lymph node (HDns). In this case, as in about half of the cases of classical HD analyzed, two populations of CD30-positive cells were enriched, one brightly stained and the other rather dim. Analysis of the scatter characteristics by electronic gating of these two populations revealed that only the brightly stained population consists of large and granulated cells, whereas the more weakly stained cells are small and not granulated (Fig. 3). Because Hodgkin and especially Reed/Sternberg cells are very large and granulated cells, the scatter profile of the enriched cells indicates that the CD30-bright cells represent the H/RS cell population. Furthermore, only these large and brightly stained cells could be counterstained with antibodies specific for CD15 (Fig. 3). CD30-positive cells isolated from three HDns lymph nodes, from three HDmc lymph nodes, from a case of classical HD, lymphocyte rich, as well as from two bone marrow specimens of HDns cases all expressed CD15 (Table 1). One of four cases of HDmc was negative for CD15. However, in this case also the primary H/RS cells in the tumor tissue did not express this antigen (not shown). Thus, in most cases of classical HD, large CD30+ cells coexpressing CD15 could be isolated, likely representing H/RS cells. The number of cells isolated in this way varied between ≈200 and 104.
Figure 3.
Enrichment of H/RS cells from a HDns affected lymph node (patient 2428). (Upper) Two populations of CD30+ cells are enriched, which are stained differentially bright for this antigen. (Lower) Counterstaining of the enriched cells with CD15-phycoerythrin. Only the CD30-brightly-stained cells are also positive for CD15. The scatter characteristics of the two CD30-positive populations were analyzed by using electronic gating. Only the CD30-bright cells that are also CD15+ display the characteristics of very big and granulated cells. The CD30-dim/CD15− cells show scatter characteristics of small lymphocytes.
Figure 4.
Immunohistochemical staining of enriched H/RS cells (case 6937) on a cytospin slide. The nuclei of the cells are stained with hematoxyline and CD30 expression is visualized by avidin-biotin-complex staining. The giant H/RS cells are clearly visible. Some of the cells are classical RS cells with more than one nucleus. (×630.)
Clonality of Purified H/RS Cells.
Because of their highly variable nature, VH gene rearrangements represent clonal markers for B lineage cells. Because H/RS cells in most if not all cases of HD harbor clonal Ig gene rearrangements (11), amplification of Ig genes from H/RS cells isolated from tissue sections on the one hand and corresponding MACS-enriched H/RS cells on the other is well suited to determine at the molecular level whether the large CD30-positive cells isolated by MACS indeed represent the H/RS cells identifiable in the tumor tissue.
For case 6104, H/RS cells were micromanipulated from tissue sections in two independent experiments. Using VH and Vκ FRI primers together with J gene segment primers, a clonal Vκ1 gene rearrangement was repeatedly amplified in both experiments (Table 2). For the analysis of the MACS-enriched cells isolated from the same lymph node, cells were cytocentrifuged on glass slides and single CD30+ large cells micromanipulated from the cytospins. The same Vκ gene was amplified from five of 16 micromanipulated cells, but not from any of the control samples (Table 2). For 12 cells isolated from the same cytospin a multiplex PCR analysis for three separate segments of the p53 gene was carried out. From eight of 12 cells, no PCR product was amplified, indicating that DNA quality was not optimal in this case. Thus, the low frequency of amplified Vκ1 gene rearrangements from HRS cells micromanipulated from a cytospin (5 of 16 cells positive) may be due to insufficient DNA quality. Sequence analysis of the clonal Vκ1 gene rearrangement showed that it represents an unmutated, out-of-frame rearrangement of the L8 gene to Jκ5 (data not shown).
Table 2.
Molecular analysis of single H/RS cells.
| Patient | Issue | Exp. | Primer set | Cells positive | PCR products | Rearrangements
|
|
|---|---|---|---|---|---|---|---|
| Repeated | Unique | ||||||
| 6104 | Lymph node | 1 | VH, Vκ FRI | 3/18 | 3 | 3 Vκ 1 | |
| Lymph node | 2 | VH, Vκ FRI | 5/12 | 5 | 4 Vκ 1 | 1 Vκ 3 | |
| Cytospin | 1 | VH, Vκ FRI | 5/16 | 6 | 5 Vκ 1 | 1 VH3 | |
| 6444 | Lymph node | 1 | VH, Vκ FRI | 0/6 | |||
| VH, Vκ FRI | 1/6 | 1 | 1 VH3 6 | ||||
| VHL | 4/17 | 4 | 4 VH3 | ||||
| Cytospin | 1 | VHL | 10/18 | 10 | 10 VH3 | ||
| Controls | |||||||
| 6104 | Lymph node | 1 | VH, Vκ FRI | 1/3 B cells | 1 | 1 VH4 | |
| 0/8 neg. controls | |||||||
| Lymph node | 2 | VH, Vκ FRI | 3/3 B cells* | 6 | All 6 | ||
| 0/5 neg. controls | |||||||
| Cytospin | 1 | VH, Vκ FRI | 2/10 CD30 neg. | 2 | 1 Vκ1, 1 VH3 | ||
| 0/4 buffer | |||||||
| 6444 | Lymph node | 1 | VH, Vκ FRI | 0/1 B cells | |||
| 0/2 neg. controls | |||||||
| VH, Vλ FRI | 1/2 B cells | 1 | 1 VH3 | ||||
| 0/3 neg. controls | |||||||
| VHL | 1/4 B cells | 2 | 1 VH3, 1 VH4 | ||||
| 0/8 neg. controls | |||||||
| Cytospin | 1 | VHL | 0/12 neg. controls | ||||
Three different primer sets were used, as indicated: (i) six VH and four Vκ family-specific primers hybridizing to sequences within FRI (VH, VκFRI), (ii) The same six VH primers together with eight Vλ family-specific FRI primers (VH, Vλ FRI), and (iii) five VH leader family-specific primers (VH). Controls consisted of B and T cells micromanipulated on the same day from neighboring sections or independent cytospins. In one experiment, CD30-negative cells were micromanipulated as control from the same cytospin that was used for the isolation of HRS cells. Neg. controls consist of micromanipulated T cells and aliquots of the buffer covering the sections or cytospins. Repeated rearrangements indicates clonally related rearrangements; unique rearrangements are rearrangements obtained only once. The three B cell samples of the second experiment of case 6104 each contained two B cells.
For micromanipulated H/RS cells from case 6444, no PCR product was obtained with the VH and Vκ FRI primers. A single VH3 gene rearrangement was amplified from one of six cells analyzed with VH and Vλ primers. From 17 HRS cells analyzed with VH leader primers, four identical, potentially functional VH3 gene rearrangements were amplified (different from the unique VH3 rearrangement amplified with the VH/Vλ primer set). This rearrangement was also amplifed from 10 of 18 MACS-purified HRS cells of this patient (Table 2). The clonal VH3 gene rearrangement was not amplified from any of 24 controls (Table 2). In comparison to the most homologous germ-line gene (WHG16G, ref. 29), the VH3 gene rearrangement shows 25-point mutations (corresponding to a mutation frequency of 6.2% within the VH segment; sequences not shown). Two of the mutations are located near the 3′ end of the sequence to which the VH3 FRI primer hybridizes. These mutations likely prevented binding of the VH3 FRI primer and thus explain why the rearrangement was not amplified when the VH FRI primer set was used. For case 6444, the three fragments of the p53 gene were successfully amplified from each of 10 cells analyzed, demonstrating a good quality of DNA in this case (not shown).
Taken together, in both cases in which a clonal Ig gene rearrangement was identified in H/RS cells isolated from tissue sections, the same clonal rearrangement (and no others) could also be repeatedly amplified from MACS-purified H/RS cells.
DISCUSSION
Although there is now strong evidence for the clonal nature of H/RS cells and their derivation from GC B cells (reviewed in ref. 11), the molecular basis of the specific physiological properties of H/RS cells is unresolved. A prerequisite for further studies in this direction would be the isolation of populations of viable H/RS cells, if one does not want to resort to the analysis of H/RS cell-derived cell lines. Here we report successful purification of H/RS cells by high-gradient magnetic cell sorting on the basis of CD30 expression. We chose this antigen because it is expressed on H/RS cells of nearly all cases of the most common subtypes of HD (HDns and HDmc) (9, 30). Compared with other methods, MACS has been very efficient in the isolation of large and fragile cells, e.g., megakaryocytes (24).
Magnetic Isolation of CD30+ Cells.
Because CD30 is also expressed on other subsets of lymphoid cells, such cells might be copurified together with the H/RS cells. To characterize CD30-positive non-H/RS cells we first analyzed PB and lymph nodes not affected by classical HD. Moreover, we assessed whether additional characteristics of H/RS cells, namely their large cell size and expression of CD15, represent useful markers to discriminate H/RS cells from other CD30-expressing cells. In PB, subpopulations of CD4 or CD8-positive T cells and CD19+ B cells were found to express CD30, in accordance with the literature (28). These cells had the size of small lymphocytes and were CD15-negative. On the other hand, among CD15-positive PB cells (i.e., neutrophilic and eosinophilic granulocytes), no CD30-positive cells were detectable. Also from inflamed lymph nodes or a lymph node affected by HDlp no large CD30-positive cells coexpressing CD15 could be enriched. Thus, in tissues not affected by classical HD, no CD15/CD30 double-positive large cells were detectable.
In most cases of classical HD, the enriched large CD30-positive cells could also be stained with antibodies specific for CD15, strongly supporting that these cells represent the H/RS cells (Table 1). Only in one of the cases analyzed for CD15 expression, the isolated large CD30+ cells were CD15-negative. In this case also the H/RS cells in the tumor tissue lack CD15 expression. In instances, in which CD30-dim cells were coenriched with CD30-bright cells, the former could be clearly discriminated from the latter by their smaller size as well as the lack of CD15 expression. It should be possible to separate the CD30+ non-H/RS cells from the H/RS cells by performing a second round of MACS separation using CD15 Microboeads after cleaving off the Multisortbeads from the first round of enrichment.
Taken together, by high gradient magnetic cell sorting using anti-CD30 antibodies, CD30/CD15-double positive cells with large forward and side scatter characteristics were enriched from classical HD-infiltrated biopsy specimens. The morphology and phenotype of these cells, and the finding that cells with these features could not be enriched from various other tissues strongly indicates that the enriched cells represent the H/RS tumor cells.
Isolated CD30+ H/RS Cells from Affected Lymph Nodes Are Clonal.
Besides the immunophenotypic and morphological characterization of the enriched CD30+ cells, genotypic analysis of these cells offered an independent approach to determine whether the CD30-positive cells truly represent H/RS cells. Isolated CD30-positive cells micromanipulated from frozen tissue sections as well as cytospins of MACS-enriched cell suspensions were analyzed from two cases of HD for Ig gene rearrangements. In both cases, identical V gene rearrangements were repeatedly amplified from single cells of both sources of cells, showing that H/RS cells were successfully enriched by MACS (Table 2). Although our failure to amplify clonal Ig gene rearrangements from all CD30-positive cells picked from cytospins is likely due to technical problems (like insufficient DNA quality especially in case 6104, see above), we cannot rule out that some of the isolated cells were not H/RS cells. However, the isolation of the tumor-specific clonal Ig gene rearrangements (and no additional others) from the enriched CD30+ cells, together with their flow cytometric features and their morphology strongly argue that the large CD30-positive cells indeed represent the H/RS cells of the patient, and that these cells are a clonal population.
With respect to the latter point it had been argued that picking of H/RS cells from a small area of a lymph node favors the finding of only one clone of cells and that polyclonal Ig gene rearrangements can be amplified from H/RS cells if the cells are isolated from cell suspensions (31). However, in the two cases analyzed in the present study, only the clonal Ig gene rearrangements carried by H/RS cells micromanipulated from tissue sections were detected in H/RS cells isolated from cell suspensions of the same lymph nodes after MACS-enrichment. This further supports the previous conclusion that within a given lymph node the H/RS cells belong to a single clone (13).
Perspectives.
Enrichment of H/RS cells by MACS will allow one not only to monitor the outcome of disease therapy, but also to increase the sensitivity for detection of minimal residual disease. The limit of detection in common PCR protocols is about 1 target cell in 105 cells (32). Assuming that an approximate 100-fold enrichment of H/RS cells as described here is feasible also when the frequency of these cells in the biopsy material is much lower, it should be possible to detect H/RS cells that are present in a biopsy specimen at a frequency below 1 in 106 cells. Moreover, viable H/RS cells can be studies in vitro in a variety of ways. Little is known about the molecular basis of H/RS cell survival in vivo. Nguyen et al. (33) reported the expression of CD95 on H/RS cells as well as high production of bcl-2 protein, speculating that the latter could be responsible for the prevention of apoptosis induced via the former. Moreover, a recent study indicates that constitutive activity of NF-κB-RelA is required for the survival of H/RS cells (34). With the use of ex vivo isolated, viable H/RS cells the signals required to induce apoptosis in H/RS cells can be investigated. This might lead to the development of therapeutic approaches for the elimination of these cells. The isolation of viable H/RS cells will also enable one to analyze the gene expression pattern at the single cell level. Finally, the MACS technique may also be used for purging of residual H/RS cells from stem cell preparations used for autologous transplantations.
Acknowledgments
We thank Michaela Fahrig, Julia Jesdinsky, and Arianne Faβbender for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft through SFB 502.
ABBREVIATIONS
- HD
Hodgkin disease
- H/RS
Hodgkin/Reed-Sternberg
- lymphocyte predominant
lp
- lymphocyte rich
lr
- MACS
magnetic cell sorter
- mc
mixed cellularity
- ns
nodular sclerosis
- VH
variable region of Ig heavy chain
- VL
variable region of Ig light chain
- JH
joining genes of heavy chain
- FITC
fluorescein isothiocyanate
- MACS
magnetic cell sorting
- FRI
framework region I
- PB
peripheral blood
Footnotes
References
- 1.Hodgkin T. Med Chir Trans. 1832;17:68–117. doi: 10.1177/095952873201700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sternberg C. Z Heilkunde. 1898;19:21–90. [Google Scholar]
- 3.Orazi A, Jiang B, Lee C-H, English G W, Cattoretti G, John C, Neiman R. Am J Clin Pathol. 1995;104:413–418. doi: 10.1093/ajcp/104.4.413. [DOI] [PubMed] [Google Scholar]
- 4.Delabie J, Tierens A, Gavrill T, Wu G, Weisenburger D D, Chan W C. Br J Haematol. 1996;94:198–205. doi: 10.1046/j.1365-2141.1996.d01-1780.x. [DOI] [PubMed] [Google Scholar]
- 5.Falk H M, Tesch H, Stein H, Diehl V, Jones D B, Fonatsch C, Bornkamm G W. Int J Cancer. 1987;40:262–269. doi: 10.1002/ijc.2910400223. [DOI] [PubMed] [Google Scholar]
- 6.Wolf J, Kapp U, Bohlen H, Kornacker M, Schoch C, Stahl B, Mücke S, v. Kalle C, Fonatsch C, Schaefer H, Hansmann M-L, Diehl V. Blood. 1996;87:3418–3428. [PubMed] [Google Scholar]
- 7.Kanzler H, Hansmann M L, Kapp U, Wolf J, Diehl V, Rajewsky K, Küppers R. Blood. 1996;87:3429–3436. [PubMed] [Google Scholar]
- 8.Hugh J, Poppema S. Int Rev Exp Pathol. 1992;33:81–114. [PubMed] [Google Scholar]
- 9.Herbst H, Stein H, Niedobitek G. Crit Rev Oncog. 1993;4:191–239. [PubMed] [Google Scholar]
- 10.Harris N L, Jaffe E, Stein H, Banks P M, Chan J K C, Cleary M-L, Delsol G, de Wolf-Peeters C, Falini B, Gatter K C, et al. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 11.Küppers R, Rajewsky K. Annu Rev Immunol. 1998;16:471–493. doi: 10.1146/annurev.immunol.16.1.471. [DOI] [PubMed] [Google Scholar]
- 12.Küppers R, Rajewsky K, Zhao M, Simons G, Laumann R, Fischer R, Hansmann M-L. Proc Natl Acad Sci USA. 1994;91:10962–10966. doi: 10.1073/pnas.91.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanzler H, Küppers R, Hansmann M-L, Rajewsky K. J Exp Med. 1996;184:1495–1505. doi: 10.1084/jem.184.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braeuninger A, Küppers R, Strickler J G, Wacker H H, Rajewsky K, Hansmann M-L. Proc Natl Acad Sci USA. 1997;94:9337–9342. doi: 10.1073/pnas.94.17.9337. . Correction appeared in Proc. Natl. Acad. Sci. USA 94, 14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marafioti T, Hummel M, Anagnostopoulos I, Foss H-D, Falini B, Delsol G, Isaacson P, Pileri S, Stein H. N Engl J Med. 1997;337:453–458. doi: 10.1056/NEJM199708143370703. [DOI] [PubMed] [Google Scholar]
- 16.Ohno T, Stribley J, Wu G, Hinrichs S, Weisenburger D, Chan W. N Engl J Med. 1997;337:459–465. doi: 10.1056/NEJM199708143370704. [DOI] [PubMed] [Google Scholar]
- 17.Delabie J, Tierens A, Wu G, Weisenburger D, Chan W. Blood. 1994;84:3291–3298. [PubMed] [Google Scholar]
- 18.Ohshima K, Suzumiya J, Mukai Y, Tashiro K, Shibata T, Tanaka T, Kato A, Kikuchi M. Hematol Oncol. 1996;14:123–126. doi: 10.1002/(SICI)1099-1069(199609)14:3<123::AID-HON577>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Hummel M, Ziemann K, Lammert H, Pileri S, Sabattini E, Stein H. N Engl J Med. 1995;333:901–905. doi: 10.1056/NEJM199510053331403. [DOI] [PubMed] [Google Scholar]
- 20.Sitar G, Brusanolino E, Bernasconi C, Ascari E. Blood. 1989;73:222–229. [PubMed] [Google Scholar]
- 21.Trümper L H, Brady G, Bagg A, Gray D, Loke S L, Griesser H, Wagman R, Braziel R, Gascoyne R D, Vicini S, et al. Blood. 1993;81:3097–3115. [PubMed] [Google Scholar]
- 22.Irsch J, Irlenbusch S, Radl J, Burrows P D, Cooper M D, Radbruch A. Proc Natl Acad Sci USA. 1994;91:1323–1327. doi: 10.1073/pnas.91.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irsch J, Hunzelmann N, Tesch H, Merk H, Maggi E, Ruffilli A, Radbruch A. Immunotechnology. 1995;1:115–125. doi: 10.1016/1380-2933(95)00012-7. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz B, Radbruch A, Kümel T, Wickenhauser C, Korb H, Hansmann M-L, Thiele J, Fischer R. Eur J Haematol. 1994;52:267–275. doi: 10.1111/j.1600-0609.1994.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 25.Hansel T, De Vries J, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. J Immunol Methods. 1991;145:105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- 26.Küppers R, Zhao M, Hansmann M-L, Rajewsky K. EMBO J. 1993;12:4955–4967. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Küppers R, Hansmann M-L, Rajewsky K. In: Handbook of Experimental Immunology. 5th Ed. Herzenberg L A, Weir D M, Herzenberg L A, Blackwell C, editors. Oxford: Blackwell Scientific; 1997. pp. 206.1–206.4. [Google Scholar]
- 28.Agrawal B, Reddish M, Longenecker B M. J Immunol. 1996;157:3229–3234. [PubMed] [Google Scholar]
- 29.Küppers R, Fischer U, Rajewsky K, Gause A. Immunol Let. 1992;34:57–62. doi: 10.1016/0165-2478(92)90027-l. [DOI] [PubMed] [Google Scholar]
- 30.Durkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Cell. 1992;68:421–427. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- 31.Chan W, Delabie J. Am J Clin Pathol. 1995;104:368–370. doi: 10.1093/ajcp/104.4.368. [DOI] [PubMed] [Google Scholar]
- 32.Chan D W, Liang R, Kwong Y L, Chan V. Am J Hematol. 1996;52:171–177. doi: 10.1002/(SICI)1096-8652(199607)52:3<171::AID-AJH6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen P L, Harris N L, Robertson M J. Am J Pathol. 1996;148:847–853. [PMC free article] [PubMed] [Google Scholar]
- 34.Bargou R C, Emmerich F, Krappmann D, Bommert K, Mapara M Y, Arnold W, Royer H D, Grinstein E, Greiner A, Scheidereit C, et al. J Clin Invest. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]