Abstract
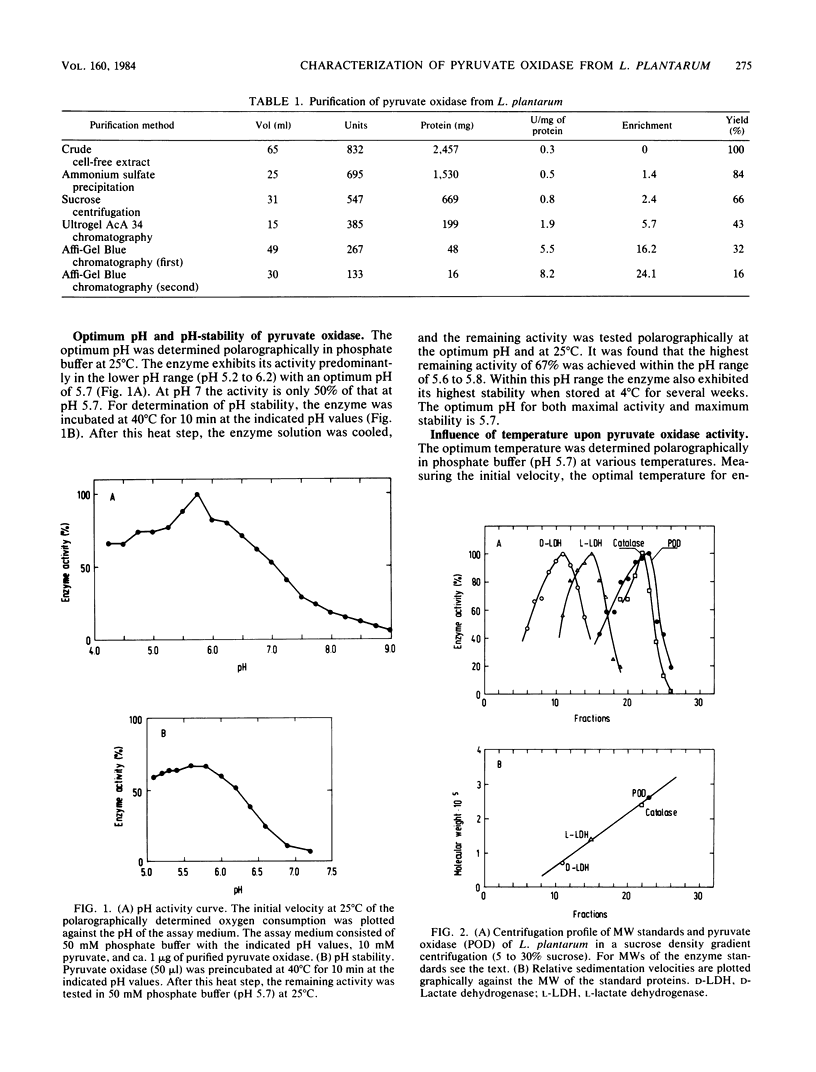
Pyruvate oxidase (EC 1.2.3.3) was isolated and characterized from Lactobacillus plantarum. The enzyme catalyzes the oxidative decarboxylation of pyruvate in the presence of phosphate and oxygen, yielding acetyl phosphate, carbon dioxide, and hydrogen peroxide. This pyruvate oxidase is a flavoprotein, with the relatively tightly bound cofactors flavin adenine dinucleotide, thiamine pyrophosphate, and a divalent metal ion, with Mn2+ being the most effective. The enzyme is only slightly inhibited by EDTA, implying that the enzyme-bound metal ion is poorly accessible to EDTA. Only under relatively drastic conditions, such as acid ammonium sulfate precipitation, could a colorless and entirely inactive apoenzyme be obtained. A partial reactivation of the enzyme was only possible by the combined addition of flavin adenine dinucleotide, thiamine pyrophosphate, and MnSO4. The enzyme has a molecular weight of ca. 260,000 and consists of four subunits with apparently identical molecular weights of 68,000. For catalytic activity the optimum pH is 5.7, and the optimum temperature is 30 degrees C. The Km values for pyruvate, phosphate, and arsenate are 0.4, 2.3, and 1.2 mM, respectively. The substrate specificity revealed that the enzyme reacts also with certain aldehydes and that phosphate can be replaced by arsenate. In addition to oxygen, several artificial compounds can function as electron acceptors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S., Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981 Jan;145(1):442–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badwey J. A., Robinson J. M., Karnovsky M. J., Karnovsky M. L. Superoxide production by an unusual aldehyde oxidase in guinea pig granulocytes. Characterization and cytochemical localization. J Biol Chem. 1981 Apr 10;256(7):3479–3486. [PubMed] [Google Scholar]
- Cunningham C. C., Hager L. P. Reactivation of the lipid-depleted pyruvate oxidase system from Escherichia coli with cell envelope neutral lipids. J Biol Chem. 1975 Sep 25;250(18):7139–7146. [PubMed] [Google Scholar]
- Donoghue N. A., Norris D. B., Trudgill P. W. The purification and properties of cyclohexanone oxygenase from Nocardia globerula CL1 and Acinetobacter NCIB 9871. Eur J Biochem. 1976 Mar 16;63(1):175–192. doi: 10.1111/j.1432-1033.1976.tb10220.x. [DOI] [PubMed] [Google Scholar]
- Götz F., Elstner E. F., Sedewitz B., Lengfelder E. Oxygen utilization by Lactobacillus plantarum. II. Superoxide and superoxide dismutation. Arch Microbiol. 1980 Apr;125(3):215–220. doi: 10.1007/BF00446879. [DOI] [PubMed] [Google Scholar]
- Götz F., Sedewitz B., Elstner E. F. Oxygen utilization by Lactobacillus plantarum. I. Oxygen consuming reactions. Arch Microbiol. 1980 Apr;125(3):209–214. doi: 10.1007/BF00446878. [DOI] [PubMed] [Google Scholar]
- HAGER L. P., GELLER D. M., LIPMANN F. Flavoprotein-catalyzed pyruvate oxidation in Lactobacillus delbrueckii. Fed Proc. 1954 Sep;13(3):734–738. [PubMed] [Google Scholar]
- Keevil T., Mason H. S. Molecular oxygen in biological oxidations--an overview. Methods Enzymol. 1978;52:3–40. doi: 10.1016/s0076-6879(78)52003-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Malmström B. G. Enzymology of oxygen. Annu Rev Biochem. 1982;51:21–59. doi: 10.1146/annurev.bi.51.070182.000321. [DOI] [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- STRITTMATTER C. F. Flavin-linked oxidative enzymes of Lactobacillus casei. J Biol Chem. 1959 Oct;234:2794–2800. [PubMed] [Google Scholar]
- Sedewitz B., Schleifer K. H., Götz F. Physiological role of pyruvate oxidase in the aerobic metabolism of Lactobacillus plantarum. J Bacteriol. 1984 Oct;160(1):462–465. doi: 10.1128/jb.160.1.462-465.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell E. E., Strong F. M., Peterson W. H. Growth factors for bacteria: Fractionation and properties of an accessory factor for lactic acid bacteria. Biochem J. 1937 Oct;31(10):1789–1799. doi: 10.1042/bj0311789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]