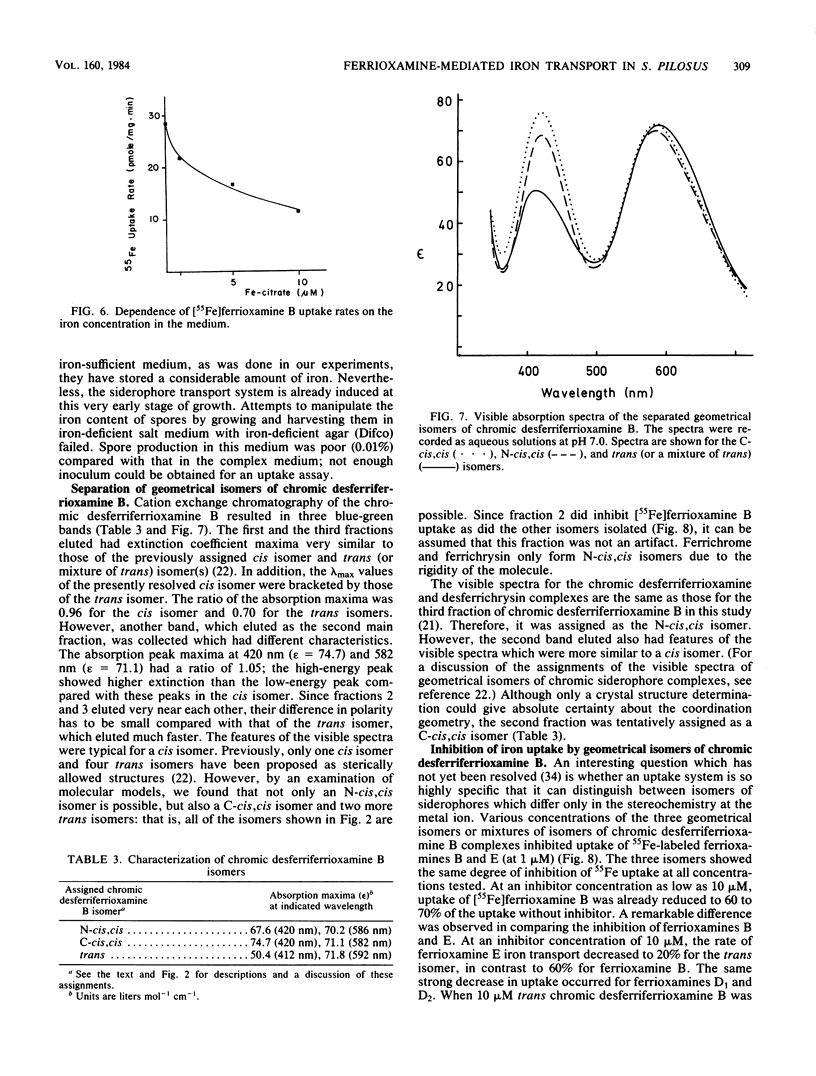
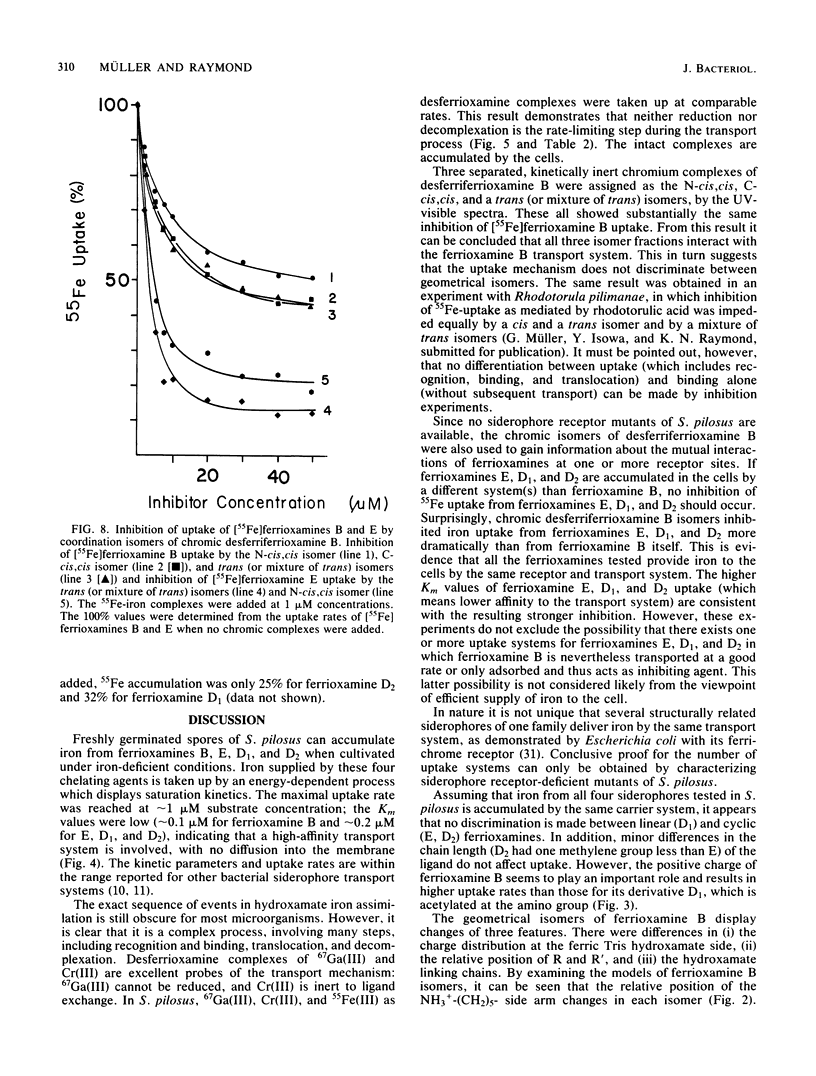
Abstract
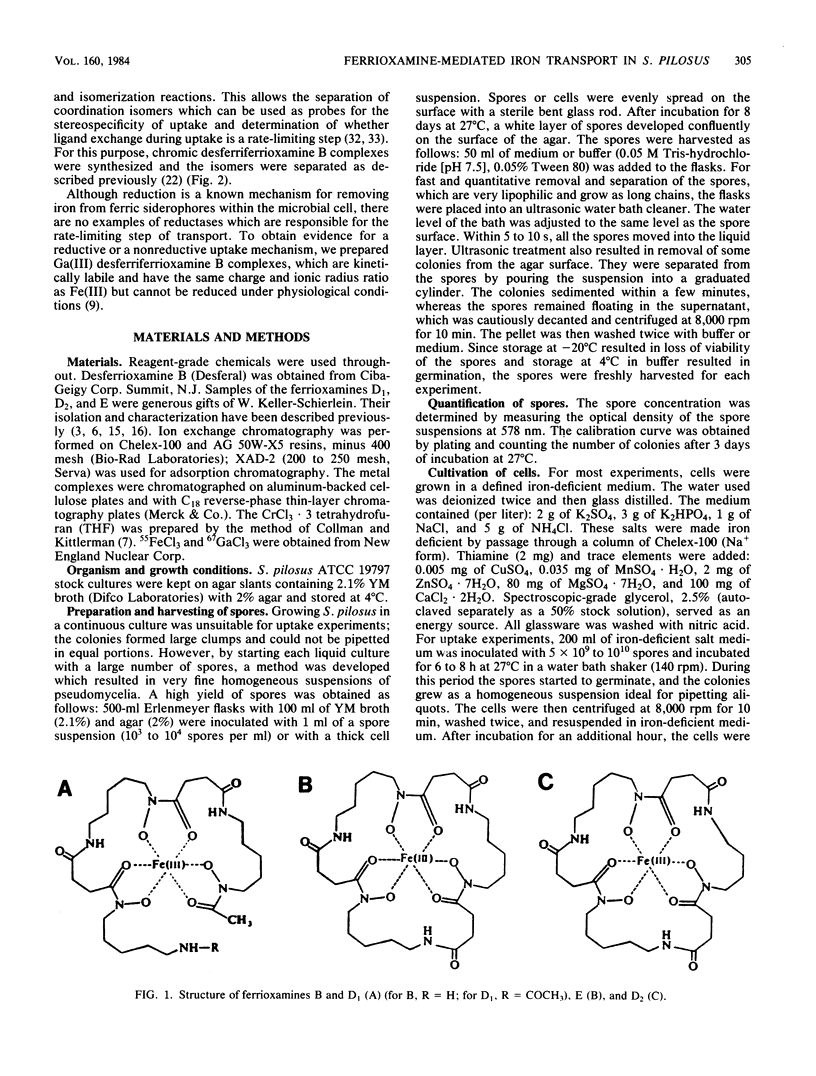
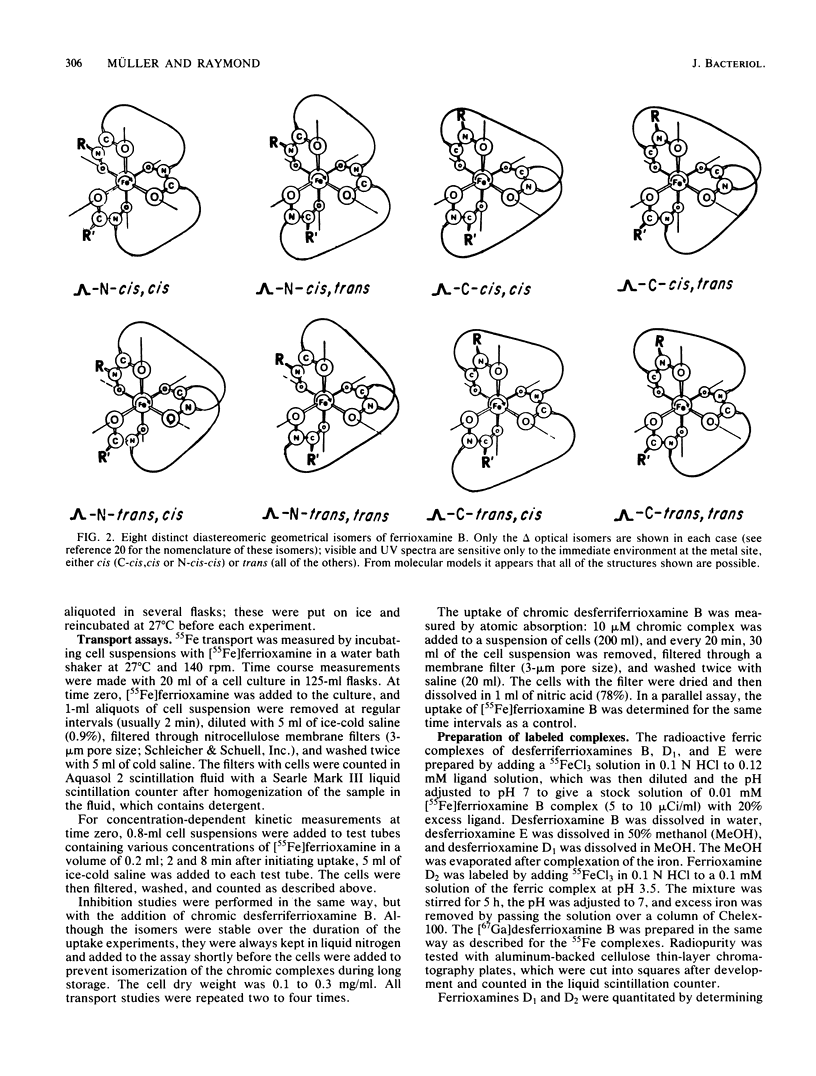
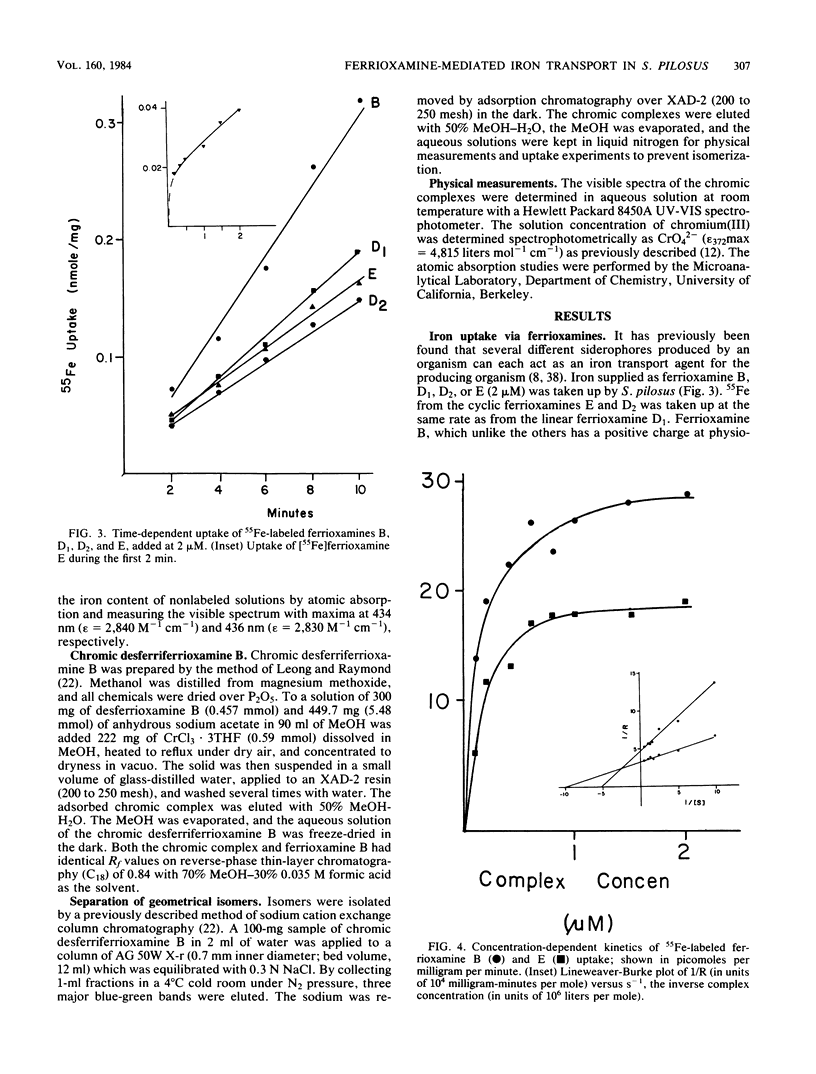
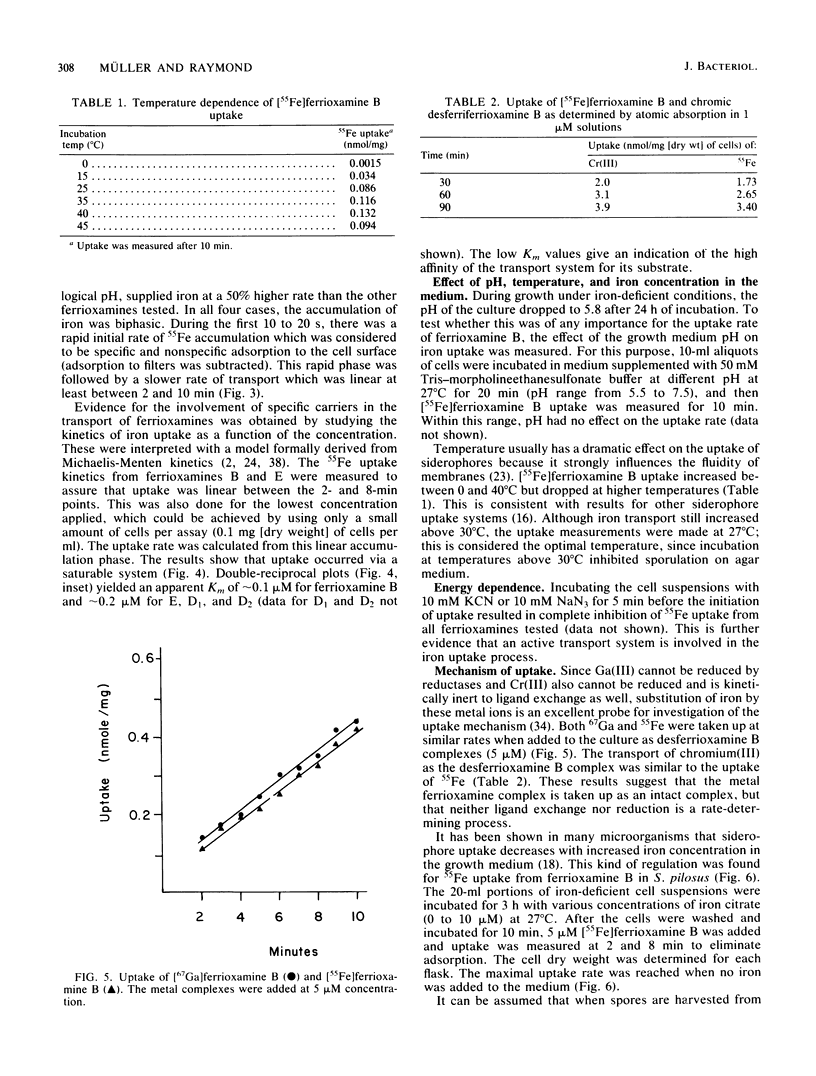
Although the ferrioxamines are an important and well-characterized class of siderophores produced by several species of Nocardia, Streptomyces, Micromonospora, Arthrobacter, Chromobacterium, and Pseudomonas, no studies of the mechanism of ferrioxamine-mediated iron uptake have been performed for an organism which produces the siderophore. This is the first report of metal transport in Streptomyces pilosus mediated by the native ferrioxamines B, D1, D2, and E. 55Fe accumulation in these ferrioxamines was dependent on metabolic energy and was a saturable process with increasing complex concentration. The apparent Km for [55Fe]ferrioxamine B uptake was approximately 0.2 microM. Both chromic desferriferrioxamine B and [67Ga]desferriFerrioxamine B were transported at rates similar to those of the 55Fe complexes: this implies that no decomplexation or reduction of the metal ion is required for transport, since the chromic complexes are kinetically inert and the gallium complexes have no stable divalent state as a possible reduction product. In addition, isomers of inert chromic desferriferrioxamine B complexes were used to probe the stereospecificity of the ferrioxamine uptake system. The chromic complexes were separated into three fractions by cationic exchange chromatography and assigned as two cis and a (mixture of) trans geometrical isomer(s) by their visible spectra. [55Fe]ferrioxamine B uptake was equally inhibited by each isomer, suggesting that no differentiation between cis and trans geometrical isomers occurs. In the presence of chromic desferriferrioxamine B isomers, the uptake rates for 55Fe-labeled ferrioxamines E, D1, and D2 were even more strongly reduced than was that for [55Fe]ferrioxamine B itself. From these results we conclude that all the ferrioxamines tested are transported into the cells by the same uptake system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BICKEL H., GAEUMANN E., KELLER-SCHIERLEIN W., PRELOG V., VISCHER E., WETTSTEIN A., ZAEHNER H. [On iron-containing growth factors, sideramines, and their antagonists, the iron-containing antibiotics, sideromycins]. Experientia. 1960 Apr 15;16:129–133. doi: 10.1007/BF02157712. [DOI] [PubMed] [Google Scholar]
- Ecker D. J., Passavant C. W., Emery T. Role of two siderophores in Ustilago sphaerogena. Regulation of biosynthesis and uptake mechanisms. Biochim Biophys Acta. 1982 Jun 8;720(3):242–249. doi: 10.1016/0167-4889(82)90047-7. [DOI] [PubMed] [Google Scholar]
- Emery T., Hoffer P. B. Siderophore-mediated mechanism of gallium uptake demonstrated in the microorganism Ustilago sphaerogena. J Nucl Med. 1980 Oct;21(10):935–939. [PubMed] [Google Scholar]
- Hancock R. E., Hantke K., Braun V. Iron transport in Escherichia coli K-12. 2,3-Dihydroxybenzoate-promoted iron uptake. Arch Microbiol. 1977 Sep 28;114(3):231–239. doi: 10.1007/BF00446867. [DOI] [PubMed] [Google Scholar]
- Hartmann A., Braun V. Iron transport in Escherichia coli: uptake and modification of ferrichrome. J Bacteriol. 1980 Jul;143(1):246–255. doi: 10.1128/jb.143.1.246-255.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon A. H., Davis W. B., Arceneaux J. E., Byers B. R. Hydroxamate recognition during iron transport from hydroxamate-ion chelates. J Bacteriol. 1973 Sep;115(3):912–918. doi: 10.1128/jb.115.3.912-918.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch C. F., Ensign J. C. Nutritionally defined conditions for germination of Streptomyces viridochromogenes spores. J Bacteriol. 1976 Apr;126(1):13–23. doi: 10.1128/jb.126.1.13-23.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLER-SCHIERLEIN W., MERTERNS P., PRELOG V., WALSER A. STOFFWECHSELPRODUKTE VON MIKROORGANISMEN. 49. DIE FERRIOXAMINE A1, A2, UND D2. Helv Chim Acta. 1965 Jun 1;48:710–723. doi: 10.1002/hlca.19650480407. [DOI] [PubMed] [Google Scholar]
- Leong J., Neilands J. B. Mechanisms of siderophore iron transport in enteric bacteria. J Bacteriol. 1976 May;126(2):823–830. doi: 10.1128/jb.126.2.823-830.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J., Neilands J. B., Raymond K. N. Coordination isomers of biological iron transport compounds. III. (1) Transport of lambda-cis-chromic desferriferrichrome by Ustilago sphaerogena. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1066–1071. doi: 10.1016/0006-291x(74)90421-5. [DOI] [PubMed] [Google Scholar]
- Leong J., Raymond K. N. Coordination isomers of biological iron transport compounds, IV, Giometrical isomers of chromic desferriferrioxamine B. J Am Chem Soc. 1975 Jan 22;97(2):293–296. doi: 10.1021/ja00835a011. [DOI] [PubMed] [Google Scholar]
- Leong J., Raymond K. N. Coordination isomers of biological iron transport compounds. II. The optical isomers of chromic desferriferrichrome and desferriferrichrysin. J Am Chem Soc. 1974 Oct 16;96(21):6628–6630. doi: 10.1021/ja00828a014. [DOI] [PubMed] [Google Scholar]
- Müller A., Zähner H. Stoffwechselprodukte von Mikroorganismen. 65. Ferrioxamine aus Eubacteriales. Arch Mikrobiol. 1968;62(3):257–263. [PubMed] [Google Scholar]
- Müller G., Matzanke B. F., Raymond K. N. Iron transport in Streptomyces pilosus mediated by ferrichrome siderophores, rhodotorulic acid, and enantio-rhodotorulic acid. J Bacteriol. 1984 Oct;160(1):313–318. doi: 10.1128/jb.160.1.313-318.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrin R. S., Neilands J. B. Ferrichrome transport in inner membrane vesicles of Escherichia coli K12. J Biol Chem. 1978 Apr 10;253(7):2339–2342. [PubMed] [Google Scholar]
- Neilands J. B., Erickson T. J., Rastetter W. H. Stereospecificity of the ferric enterobactin receptor of Escherichia coli K-12. J Biol Chem. 1981 Apr 25;256(8):3831–3832. [PubMed] [Google Scholar]
- Winkelmann G. Metabolic products of microorganisms. 132. Uptake of iron by Neurospora crassa. 3. Iron transport studies with ferrichrome-type compounds. Arch Mikrobiol. 1974 Jun 7;98(1):39–50. [PubMed] [Google Scholar]
- Winkelmann G., Zähner H. Stoffwechselprodukte von Mikroorganismen. 115. Eisenaufnahme bei Neurospora crassa. I. Zur Spezifität des Eisentransportes. Arch Mikrobiol. 1973;88(1):49–60. [PubMed] [Google Scholar]
- Yang C. C., Leong J. Production of deferriferrioxamines B and E from a ferroverdin-producing Streptomyces species. J Bacteriol. 1982 Jan;149(1):381–383. doi: 10.1128/jb.149.1.381-383.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAEHNER H., HUETTER R., BACHMANN E. [Metabolites of Actinomycetes. Part 23. On a study of the effect of sideromycin]. Arch Mikrobiol. 1960;36:325–349. [PubMed] [Google Scholar]
- ZAEHNER H., von BACHMANN M., HUETTER R., NUESCH J. [Sideramines, iron-containing growth factors from micro-organisms]. Pathol Microbiol (Basel) 1962;25:708–736. [PubMed] [Google Scholar]



