Abstract
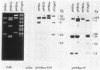
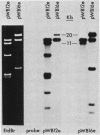
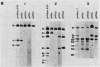
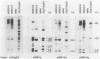
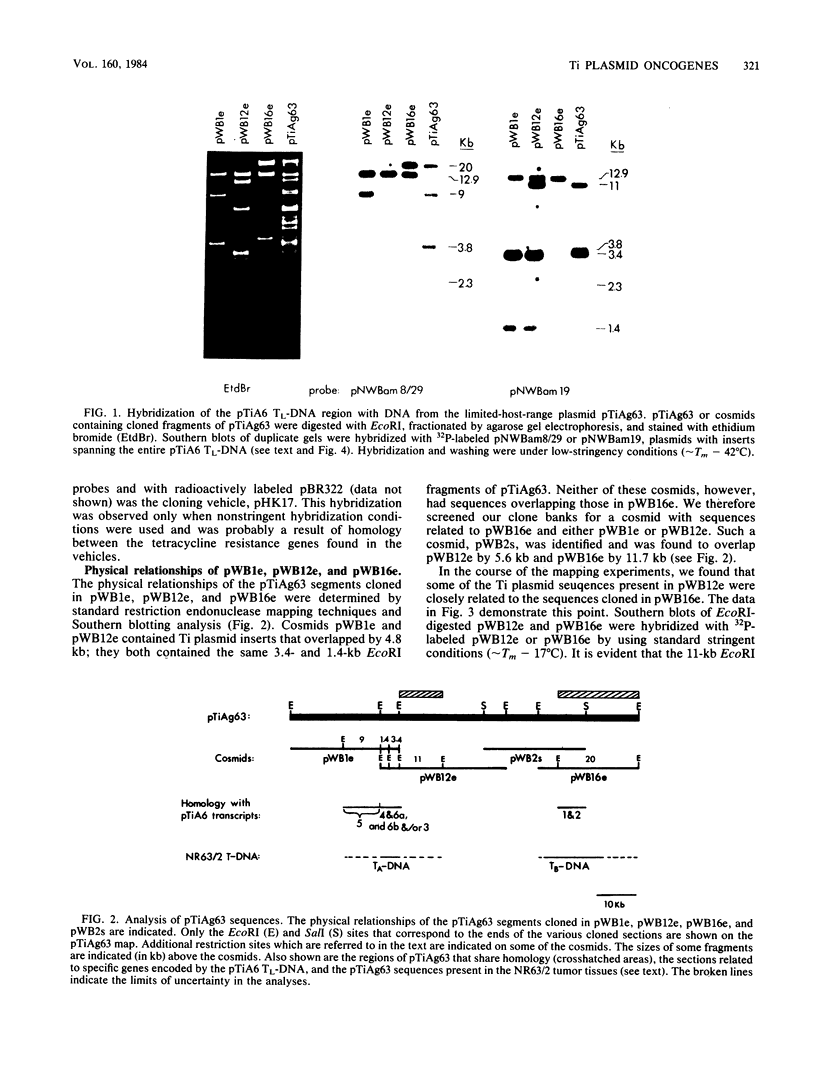
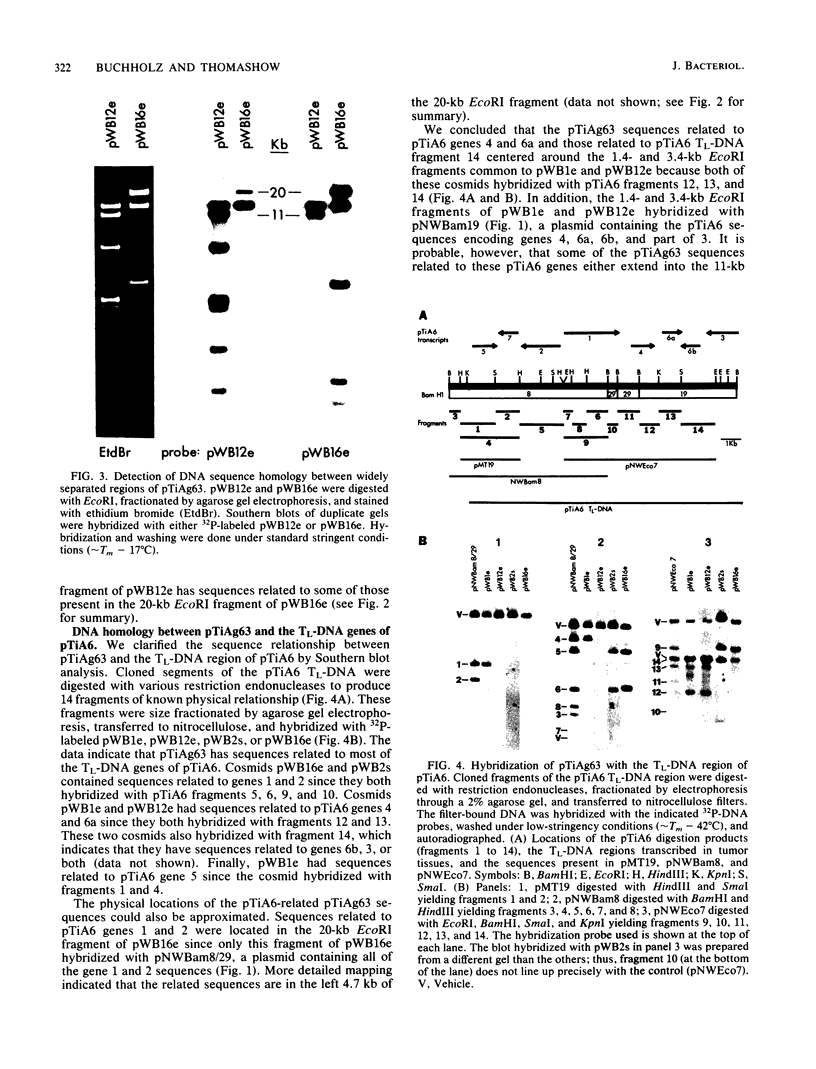
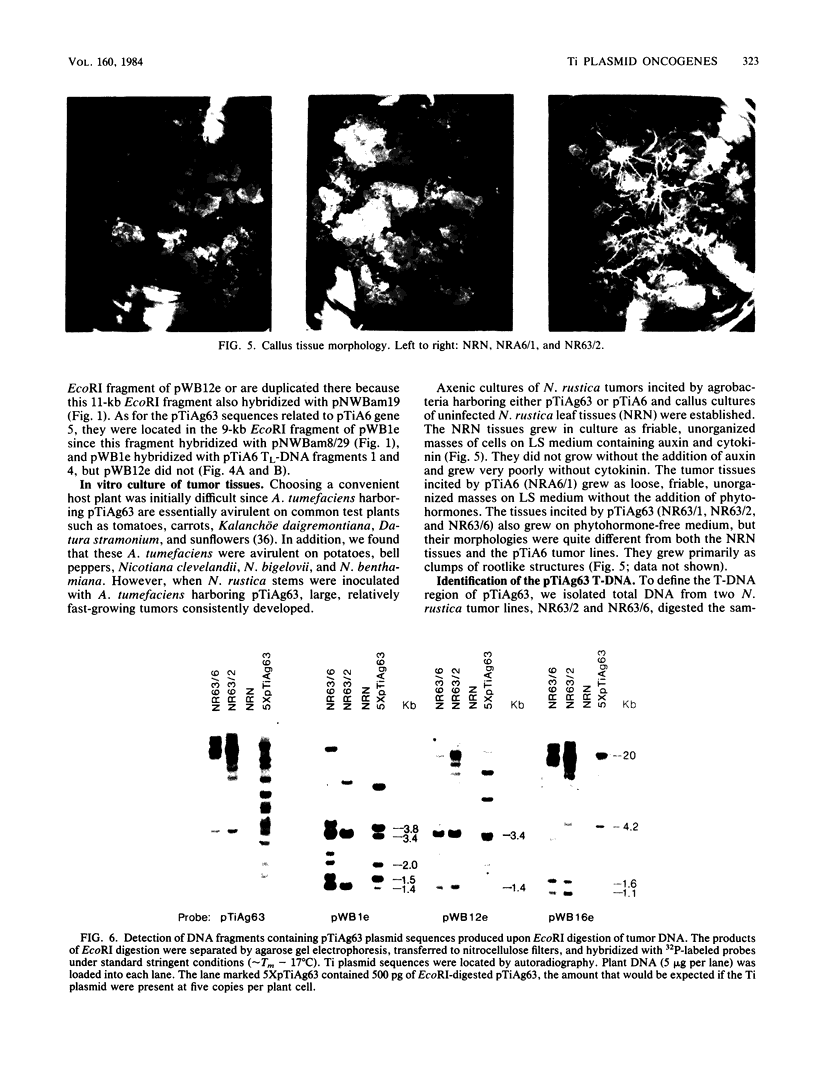
The T-DNA oncogene complements of the limited-host-range tumor-inducing plasmid pTiAg63 and the wide-host-range plasmid pTiA6 were compared. The resulting data indicate that pTiAg63 has DNA sequences related to most of the genes encoded by the oncogene region, the TL-DNA, of pTiA6 and that these sequences are divided between two T-DNA regions, the TA-DNA, which encoded sequences related to pTiA6 genes 4 (the cytokinin independence gene) and 6a, as well as to a pTiA6 TL-DNA fragment that encoded gene 6b and a portion of gene 3, and the TB-DNA, which encoded sequences related to genes 1 and 2 (the auxin independence genes). Tumor tissues of Nicotiana rustica incited by Agrobacterium tumefaciens harboring either pTiA6 or pTiAg63 grew axenically in vitro on phytohormone-free medium. The morphologies of the tissues, however, differed; whereas those incited with pTiA6 grew as loose, friable, unorganized callus, the tumors incited by pTiAg63 grew as clumps of rootlike structures. Thus, the T-DNA oncogene complements of these plasmids were not equivalent. The results are discussed in relation to the A. tumefaciens host range.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi D. E., Morris R. O., Hinz R., Mischke B. S., Kosuge T., Garfinkel D. J., Gordon M. P., Nester E. W. Cytokinin/auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc Natl Acad Sci U S A. 1983 Jan;80(2):407–411. doi: 10.1073/pnas.80.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. W., Chilton M. D. T-DNA of the Agrobacterium Ti and Ri plasmids. Annu Rev Genet. 1982;16:357–384. doi: 10.1146/annurev.ge.16.120182.002041. [DOI] [PubMed] [Google Scholar]
- Binns A. N., Sciaky D., Wood H. N. Variation in hormone autonomy and regenerative potential of cells transformed by strain A66 of Agrobacterium tumefaciens. Cell. 1982 Dec;31(3 Pt 2):605–612. doi: 10.1016/0092-8674(82)90316-6. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Blechl A. E., Denniston-Thompson K., Faber H. E., Richards J. E., Slightom J. L., Tucker P. W., Smithies O. Cloning human fetal gamma globin and mouse alpha-type globin DNA: preparation and screening of shotgun collections. Science. 1978 Dec 22;202(4374):1279–1284. doi: 10.1126/science.725603. [DOI] [PubMed] [Google Scholar]
- Buchholz W. G., Thomashow M. F. Host range encoded by the Agrobacterium tumefaciens tumor-inducing plasmid pTiAg63 can be expanded by modification of its T-DNA oncogene complement. J Bacteriol. 1984 Oct;160(1):327–332. doi: 10.1128/jb.160.1.327-332.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D. J., Simpson R. B., Ream L. W., White F. F., Gordon M. P., Nester E. W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981 Nov;27(1 Pt 2):143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsters M., de Waele D., Depicker A., Messens E., van Montagu M., Schell J. Transfection and transformation of Agrobacterium tumefaciens. Mol Gen Genet. 1978 Jul 11;163(2):181–187. doi: 10.1007/BF00267408. [DOI] [PubMed] [Google Scholar]
- Johnson R., Guderian R. H., Eden F., Chilton M. D., Gordon M. P., Nester E. W. Detection and quantitation of octopine in normal plant tissue and in crown gall tumors. Proc Natl Acad Sci U S A. 1974 Feb;71(2):536–539. doi: 10.1073/pnas.71.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos H., Inzé D., Caplan A., Sormann M., Van Montagu M., Schell J. Genetic analysis of T-DNA transcripts in nopaline crown galls. Cell. 1983 Apr;32(4):1057–1067. doi: 10.1016/0092-8674(83)90290-8. [DOI] [PubMed] [Google Scholar]
- Klee H. J., Gordon M. P., Nester E. W. Complementation analysis of Agrobacterium tumefaciens Ti plasmid mutations affecting oncogenicity. J Bacteriol. 1982 Apr;150(1):327–331. doi: 10.1128/jb.150.1.327-331.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Panagopoulos C. G., Nester E. W. Comparison of Ti plasmids from three different biotypes of Agrobacterium tumefaciens isolated from grapevines. J Bacteriol. 1983 Mar;153(3):1535–1542. doi: 10.1128/jb.153.3.1535-1542.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loper J. E., Kado C. I. Host range conferred by the virulence-specifying plasmid of Agrobacterium tumefaciens. J Bacteriol. 1979 Aug;139(2):591–596. doi: 10.1128/jb.139.2.591-596.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Kosuge T. Plasmids specifying plant hyperplasias. Annu Rev Microbiol. 1981;35:531–565. doi: 10.1146/annurev.mi.35.100181.002531. [DOI] [PubMed] [Google Scholar]
- Ooms G., Hooykaas P. J., Moolenaar G., Schilperoort R. A. Grown gall plant tumors of abnormal morphology, induced by Agrobacterium tumefaciens carrying mutated octopine Ti plasmids; analysis of T-DNA functions. Gene. 1981 Jun-Jul;14(1-2):33–50. doi: 10.1016/0378-1119(81)90146-3. [DOI] [PubMed] [Google Scholar]
- Panagopoulos C. G., Psallidas P. G. Characteristics of Greek Isolates of Agrobacterium tumefaciens (E. F. Smith & Townsend) Conn. J Appl Bacteriol. 1973 Jun;36(2):233–240. doi: 10.1111/j.1365-2672.1973.tb04096.x. [DOI] [PubMed] [Google Scholar]
- Perry K. L., Kado C. I. Characteristics of Ti plasmids from broad-host-range and ecologically specific biotype 2 and 3 strains of Agrobacterium tumefaciens. J Bacteriol. 1982 Jul;151(1):343–350. doi: 10.1128/jb.151.1.343-350.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOOG F., MILLER C. O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–130. [PubMed] [Google Scholar]
- Schröder G., Waffenschmidt S., Weiler E. W., Schröder J. The T-region of Ti plasmids codes for an enzyme synthesizing indole-3-acetic acid. Eur J Biochem. 1984 Jan 16;138(2):387–391. doi: 10.1111/j.1432-1033.1984.tb07927.x. [DOI] [PubMed] [Google Scholar]
- Sciaky D., Montoya A. L., Chilton M. D. Fingerprints of Agrobacterium Ti plasmids. Plasmid. 1978 Feb;1(2):238–253. doi: 10.1016/0147-619x(78)90042-2. [DOI] [PubMed] [Google Scholar]
- Thomashow L. S., Reeves S., Thomashow M. F. Crown gall oncogenesis: evidence that a T-DNA gene from the Agrobacterium Ti plasmid pTiA6 encodes an enzyme that catalyzes synthesis of indoleacetic acid. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5071–5075. doi: 10.1073/pnas.81.16.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Knauf V. C., Nester E. W. Relationship between the limited and wide host range octopine-type Ti plasmids of Agrobacterium tumefaciens. J Bacteriol. 1981 May;146(2):484–493. doi: 10.1128/jb.146.2.484-493.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Willmitzer L., Dhaese P., Schreier P. H., Schmalenbach W., Van Montagu M., Schell J. Size, location and polarity of T-DNA-encoded transcripts in nopaline crown gall tumors; common transcripts in octopine and nopaline tumors. Cell. 1983 Apr;32(4):1045–1056. doi: 10.1016/0092-8674(83)90289-1. [DOI] [PubMed] [Google Scholar]
- Willmitzer L., Simons G., Schell J. The TL-DNA in octopine crown-gall tumours codes for seven well-defined polyadenylated transcripts. EMBO J. 1982;1(1):139–146. doi: 10.1002/j.1460-2075.1982.tb01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]