Abstract
Antioxidants have been proposed to have antiatherogenic potential by their inhibition of low density lipoprotein (LDL) oxidation. Here, we report an antioxidant, BO-653 (2,3-dihydro-5-hydroxy-2,2-dipentyl-4,6-di-tert-butylbenzofuran), designed to exhibit antioxidative potency comparable to that of α-tocopherol, but yet possess a high degree of lipophilicity comparable to that of probucol. BO-653 exhibits a high affinity for LDL and is well distributed in aortic vessels in vivo. In atherosclerosis models of rabbits and mice, BO-653 has been shown to be able to suppress the formation of atherosclerotic lesions without untoward side effects. Specifically, there was no reduction of high density lipoprotein levels. This antioxidant provides additional evidence in support of the oxidized-LDL hypothesis, and itself is a promising candidate antioxidant for clinical use.
Oxidative stress, specifically the oxidation of low density lipoprotein (LDL), has long been suspected of having a critical role in the development of atherosclerosis, in consequence of which antioxidants have been expected to have potential as antiatherogenic agents. Such agents would be able, in theory, to inhibit the oxidative modification of LDL that leads to the accumulation of cholesterol in atherosclerotic lesions (1–4). Unfortunately for those actively working with these potential therapeutics, clinical trials of antioxidants have given rise to a controversy regarding their real clinical benefit (5–7). One well known antioxidant, probucol, simultaneously has the untoward effect of lowering levels of high density lipoprotein (HDL) (8). This was a contrary effect because high HDL levels have been shown to bear an inverse correlation with risks for atherosclerosis (9). Concerning vitamin E, the most active species of the vitamin, α-tocopherol, has a 10- or a 100-fold higher reactivity than probucol (10). The antiatherogenic effect has been reported in various models, though its effect is modest (11, 12), whereas others have reported no effect of vitamin E high intake on spontaneous development of atherosclerosis (13). Although Rimm et al. (14) reported that high intake of vitamin E reduces the risk of coronary heart disease, two large-scale trials failed to demonstrate the effect of α-tocopherol on cardiovascular disease or cerebrovascular mortality (15, 16). One characteristic of α-tocopherol is that it is not able to exert its antioxidative effects in the core of LDL where probucol is active, and thus, despite its apparently greater potency, it has not shown more than a modest antiatherogenic benefit.
In recognition of the situation described above, we set out to design a compound that would be active against oxidation of LDL in the arterial wall in situ but would not lower HDL levels. For this purpose we implemented a screening strategy that led to a promising antioxidant compound, BO-653. This compound is able to significantly suppress the formation of atherosclerotic lesions in several animal models without any reduction of HDL. These results add evidence in support of the oxidized-LDL hypothesis and suggest that this compound is a promising antioxidant for clinical use.
Drug Design.
The design of this antiatherogenic antioxidant was targeted to overcome certain weak points of the available antioxidants. We wanted a unique combination of characteristics: (i) high reactivity against lipoprotein oxidation and (ii) localization inside the hydrophobic core of the LDL particle. The high reactivity is rationally designed by lowering the dissociation energy of the phenolic O—H bond, that is, the oxygen atom located para to the phenolic hydroxyl group can stabilize the phenoxyl radical potently when it belongs to a 5-membered ring, as described by Ingold et al. (17). Thus, we designed a hydroxybenzofuran structure to achieve the former characteristics. Probucol is well known for the latter characteristic (18), and is distinct in this regard from an antioxidant such as α-tocopherol, which is able to interact only with the surface lipids of LDL (19). In addition, we sought a compound that would: (iii) show no side effects, such as HDL reduction (8, 20), and (iv) show efficient absorption and delivery to the arterial wall, a crucial site for LDL oxidation.
Screening Strategy.
From a library of 600 newly synthesized antioxidants, according to the criteria mentioned above, we screened for (i) the inhibition of macrophage degradation of oxidized LDL after incubation with metal ions in the presence of various compounds, (ii) variant effect of oxidative initiators in systems of LDL oxidation, (iii) reactivity with peroxyl radicals after selecting for the first two criteria, and (iv) absorption and distribution properties after oral administration in Watanabe heritable hyperlipidemic (WHHL) rabbits.
METHODS
Experimental Animals.
Male WHHL rabbits, 2–3 months old, were purchased from Kitayama Labes (Ina, Japan). Female C57BL/6J mice, 5 weeks old, were purchased from Clea Japan (Tokyo, Japan). Male and female LDL-receptor knockout mice, 6 weeks old, and JW rabbits, 3 months old, were obtained from CSK Research Park (Tokyo, Japan). Female ICR mice were purchased from Charles River Japan (Atsugi, Japan) for peritoneal macrophage preparations. Peritoneal macrophages were prepared according to the method of Adams (21) by using Brewer’s thioglycolate medium (Difco). Rabbits and mice were housed in animal rooms for at least 1 week before the experiments, with free access to food and water. The animals were treated in accordance with Chugai Pharmaceutical’s ethical guidelines for animal care, handling, and termination. These guidelines meet the generally accepted international criteria of humane treatment, sparing the animals needless pain and suffering and ensuring that the experiments conducted are of actual scientific benefit to humankind.
Chemiluminescence Assay.
Linoleic acid peroxyl radicals were generated by autoxidation and detected by chemiluminescence using a Cypridina luciferin analog [2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo[1,2-a]pyrazin-3-one (MCLA)] (22). A solution of 1 mM linoleic acid in 1-butanol containing 10 μM MCLA was incubated for 10 min at 37°C under air. The chemiluminescence was induced by the fast reaction of MCLA with singlet oxygen generated by linoleic acid peroxyl radicals. The quenching of the chemiluminescence was evaluated as the change in chemiluminescence intensity after addition of the compound.
Oxidation of LDL.
LDL (density 1.019–1.063 g/ml) was isolated by sequential ultracentrifugation from rabbit plasma (23). LDL (200 μg/ml) and the test compound were incubated at 37°C for 24 hr with 10 μM CuSO4 or 40 μg/ml soybean lipoxygenase. Lipid peroxides were determined as thiobarbituric acid-reactive substances (TBARS) (22). For the degradation assays, LDL was labeled with Na125I by using the iodine monochloride procedure (24). 125I-LDL was incubated at 37°C for 24 hr with 10 μM CuSO4, and the degradation was carried out by incubating mouse peritoneal macrophages in RPMI 1640 medium containing 10 μg/ml LDL at 37°C for 5 hr, following which the trichloroacetic acid-soluble non-iodide radioactivity was determined.
Diets and Administration of Compounds.
The high-fat diet (1.25% cholesterol) was prepared according to Nishina et al (25). The diets used in this paper were made by Clea Japan, and the indicated concentration of compound in the diet was confirmed by HPLC as described below. C57BL/6J mice were maintained for 28 weeks on a high-fat diet containing 0.5% of the test compound or cellulose; LDL-receptor knockout mice were maintained for 13 weeks or 21 weeks on the diet. WHHL rabbits were maintained for 6 months on a normal diet (CR3) without added antioxidants or with 1% probucol or 0.2% or 0.5% BO-653.
Measurement of Lipoprotein Profile and Concentration of the Compound.
Plasma lipoprotein profiles were evaluated as cholesterol concentrations of fractions by gel-permeation chromatography (GPC). Ten microliters of blood was obtained from a dorsal metatarsal vein and was quickly diluted into saline. Then the diluted plasma sample was obtained by centrifugation and analyzed by GPC for lipoprotein profile and by auto-analyzer (COBAS FARAII, Hoffmann–La Roche) for lipid composition. The concentrations in diets, plasma, and tissues were determined by reverse-phase HPLC of the methanol-soluble fraction of diet, plasma, and tissue homogenate samples.
Evaluation of Atherosclerotic Lesions and Xanthoma Score.
The atherosclerotic lesion area of WHHL rabbits was evaluated as the area of fatty region on aortic inner surfaces. Frozen cross sections of aortic valves or aortic arches were stained with Sudan IV and counterstained with hematoxylin. The atherosclerotic lesions were evaluated as the mean area of Sudan IV-positive regions in the aortic valve cross sections (26) or aortic cross sections (four sections of 6-mm thickness at 1-mm intervals). All areas were determined by SigmaScan Pro software (Jandel Scientific Software) scanned on the photos of aortic inner surface, aortic valve, or aortic arch. Xanthomas, observed mainly in forelimbs and eyelids, were evaluated by scoring. The xanthoma score was calculated as sum of both forelimb scores (0 = no lesions, 1 = mild swelling, 2 = severe swelling) and eyelid score (0 = no lesions, 1 = lesions).
Statistical Analysis.
Statistical significance versus control group was analyzed by Dunnett’s method of ANOVA.
RESULTS
In Vitro Effects.
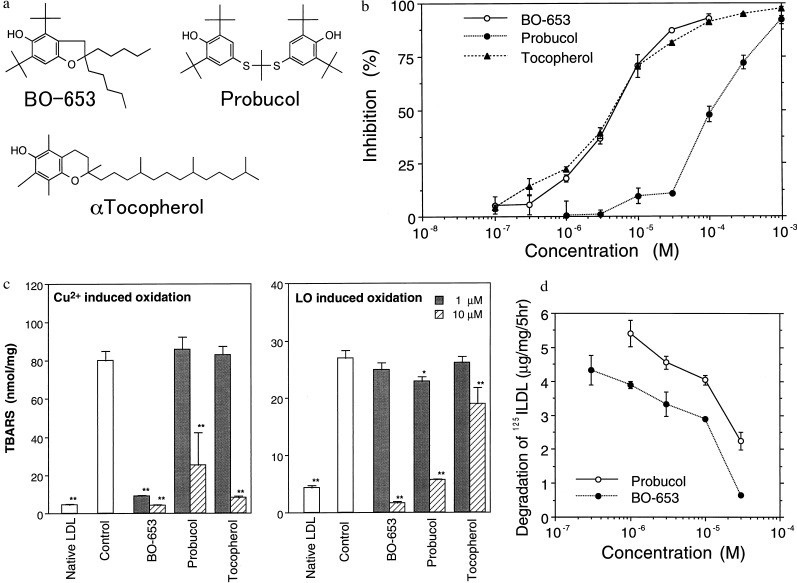
BO-653 was selected as the optimal compound, as it possess the following structural features (Fig. 1a): (i) a benzofuran structure with potent activity as a hydrogen donor; (ii) a di-tert-butyl structure surrounding an antioxidant functional site, thus able to exert effects on oxidation even in the hydrophobic core; and (iii) side chains able to modulate mobility and hydrophobicity, enhancing absorption and distribution, and allowing the exclusion of stereoisomers.
Figure 1.
Properties of the antioxidant BO-653. (a) Structure of BO-653, 2,3-dihydro-5-hydroxy-2,2-dipentyl-4,6-di-tert-butylbenzofuran, and the structures of the antioxidants probucol and α-tocopherol. (b) Inhibition of BO-653 on chemiluminescence induced by linoleic acid peroxyl radicals. Linoleic acid peroxyl radicals were generated by autoxidation and detected by chemiluminescence using MCLA. (c) Inhibition of BO-653 on rabbit LDL oxidation. LDL and the test compound were incubated at 37°C for 24 hr with CuSO4 or soybean lipoxygenase (LO) and the lipid peroxides were determined as TBARS. (d) Inhibition by BO-653 of 125I-LDL degradation induced by oxidation. 125I-LDL was incubated at 37°C for 24 hr with CuSO4 and the degradation was carried out by incubation with mouse peritoneal macrophages. All results are shown as mean ± SD of triplicate (b and c) or duplicate (d) samples. ∗, P < 0.05; ∗∗, P < 0.01.
As theoretically expected (17), this benzofuran structure showed the most potent activity as a hydrogen donor. It was demonstrated by testing BO-653 against the autoxidation of linoleic acid, and Fig. 1b shows the effect on MCLA-dependent chemiluminescence during such autoxidation. The chemiluminescence is generated by the peroxyl radicals produced by lipid peroxidation, and was suppressed by BO-653 in a dose-dependent manner similar to that of α-tocopherol. Details of the kinetic results and theoretical analysis have been presented by Noguchi et al. (27).
Activity in the lipid core was demonstrated by inhibition of lipid peroxide generation in rabbit LDL oxidized by Cu2+ or lipoxygenase, in which assay BO-653 was found to be more potent than either probucol or α-tocopherol, especially against lipoxygenase oxidation (Fig. 1c). BO-653 also inhibited degradation of LDL by macrophages 3-fold more potently than did probucol (Fig. 1d). Taken together, these results indicate that BO-653 potently inhibits LDL oxidation.
Antiatherogenic Effect.
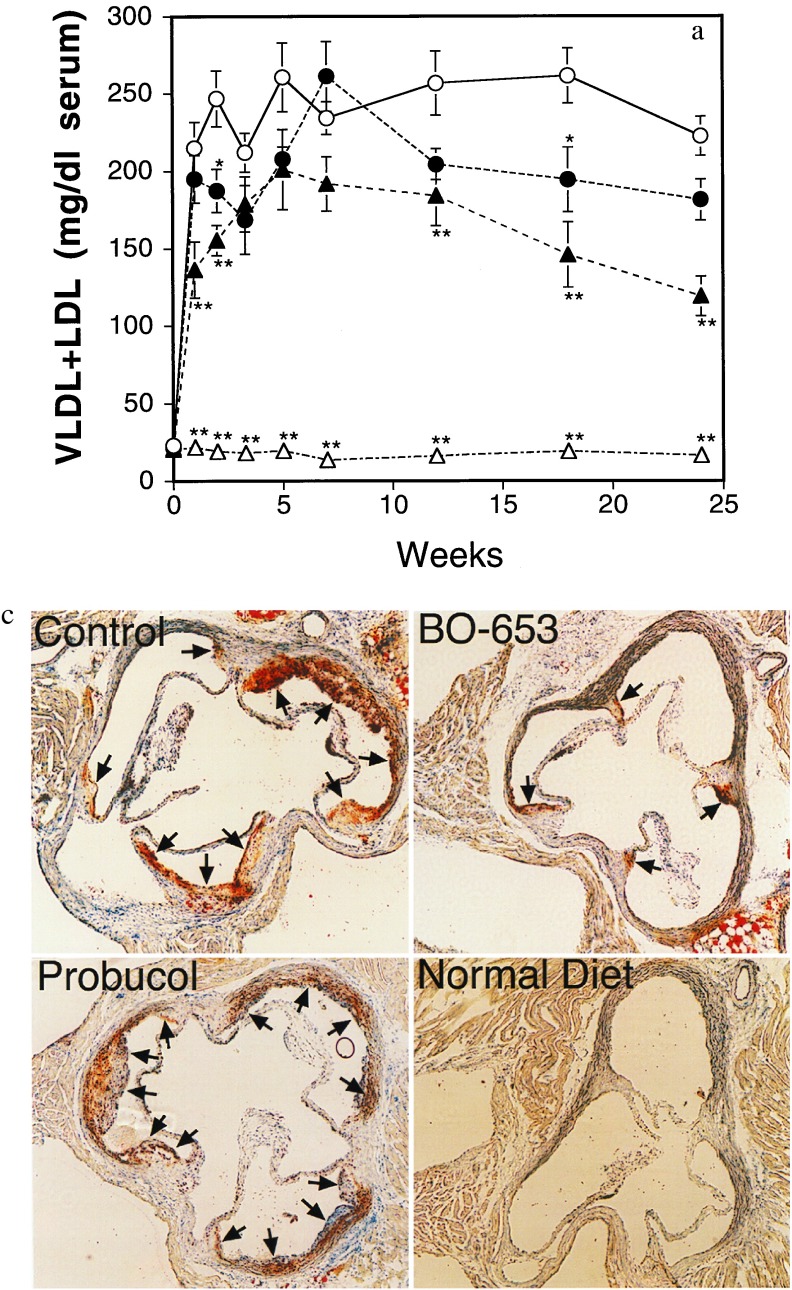
Antiatherogenic potency was examined in three different animal models. First, we tested in a WHHL rabbit model of atherosclerosis (28) that is reportedly suppressed by probucol (29). Following the established protocol, we maintained the rabbits on a diet that contained probucol (1%) or BO-653 (0.2% or 0.5%). As shown in Table 1, WHHL rabbits showed high plasma very low density lipoprotein (VLDL) and LDL level but a reduced HDL level compared with wild-type JW rabbits. Plasma cholesterol level declined slightly throughout the experiment, and that in the probucol group tended to be lower in the first half of the experimental period than that in the other groups. After 6 months, no decrease of HDL cholesterol level was observed in the BO-653-treated group whereas it decreased in the probucol-treated group. LDL was prepared individually at the end of the experiment, and LDL oxidizability was examined by incubating LDL with Cu2+ or lipoxygenase. The administration of either antioxidant compound suppressed TBARS generation in either system (Table 1). An evaluation of atherosclerotic lesions on the inner surface of the aorta revealed the control rabbit aorta to be covered with opaque, white lipid plaques (Fig. 2a), with the most advanced lesions appearing in the aortic arch. Both compounds reduced these lesions by approximately 30% (Fig. 2b). In terms of the percentage of lesions over the total area, BO-653 exhibited the same inhibitory effect as probucol at half the dose (Fig. 2c). Plasma and organ concentrations of the compounds were consistent with the high affinity of BO-653 for LDL (Fig. 2d). The BO-653 levels were twice those of probucol and were characteristically distributed in exactly these aortic vessels.
Table 1.
Plasma cholesterol, VLDL + LDL, and HDL levels and LDL oxidizability in WHHL rabbits
| Measurement | WHHL rabbits
|
||||
|---|---|---|---|---|---|
| Control | Probucol 1% | BO-653
|
Normal rabbits | ||
| 0.5% | 0.2% | ||||
| Plasma cholesterol, mg/dl | |||||
| Total | |||||
| Start | 951 ± 59 | 984 ± 91 | 972 ± 94 | 970 ± 92 | — |
| Six months | 751 ± 61 | 640 ± 88 | 679 ± 84 | 793 ± 52 | 49 ± 5* |
| VLDL + LDL cholesterol | 723 ± 67 | 630 ± 78 | 608 ± 70 | 747 ± 47 | 11 ± 0* |
| HDL cholesterol | 8.6 ± 0.6 | 5.6 ± 0.3 | 8.5 ± 1.2 | 10.4 ± 0.4 | 38 ± 6* |
| TBARS content in LDL, nmol/mg protein | |||||
| Unincubated | 1.5 ± 0.3 | 1.2 ± 0.2 | 1.0 ± 0.3 | 1.4 ± 0.2 | — |
| Incubated with Cu2+ | 83.3 ± 6.2 | 3.5 ± 0.3* | 2.4 ± 0.4* | 3.4 ± 0.1* | — |
| Incubated with lipoxygenase | 37.9 ± 2.3 | 5.5 ± 0.5* | 2.0 ± 0.4* | 10.5 ± 5.3* | — |
The lipoprotein concentrations were evaluated as cholesterol concentrations of respective fractions by gel-permeation chromatography. LDL samples were prepared individually from the plasma samples and the oxidizability was evaluated as the TBARS content generated by incubating for 24 hr with Cu2+ or lipoxygenase. All results are shown as mean ± SE, N = 4 or 5.
P < 0.01 compared with control.
Figure 2.
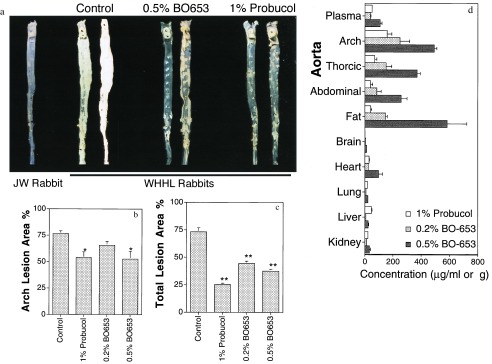
Inhibitory effect of BO-653 on the atherosclerosis of WHHL rabbits. Male rabbits were maintained for 6 months on a diet containing the indicated concentration of the compound. (a) Representative photographs of aortic inner surfaces of WHHL rabbits. The aorta of a wild-type JW rabbit is presented as normal aortic inner surface. (b and c) Effect of BO-653 on intimal surface area with atherosclerotic lesions of aortic arch (b) and whole aorta (c). (d) Tissue concentrations of the compounds in WHHL rabbits treated for 6 months. All results are shown as mean ± SE. n = 4 or 5. ∗, P < 0.05; ∗∗, P < 0.01.
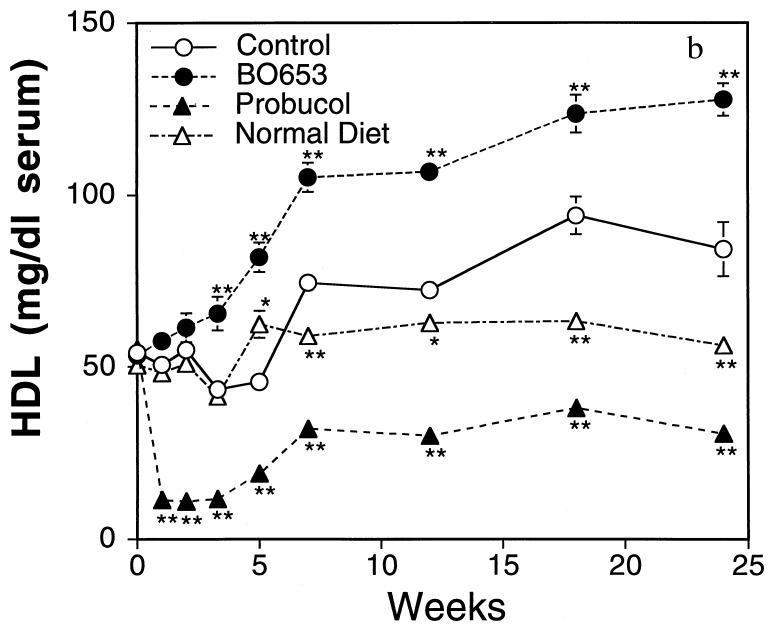
C57BL/6J mice are well established as developing atherosclerotic lesions when maintained on a high-fat diet (30). The effects of BO-653 and probucol were compared in this model. Both decreased VLDL/LDL levels (Fig. 3a). In the BO-653 group there was a gradual increase of HDL to a final level of +40% over a period of 2 months, whereas in the probucol group HDL there was a decrease of 60% that started in the first week (Fig. 3b). At 7 months both the control and probucol groups had developed distinct atherosclerotic lesions on their aortic valves (Fig. 3c for representative cross sections), whereas the lesions in the BO-653 group were comparable to those in the normal diet group. Fig. 3d highlights the contrasting effects of probucol and BO-653.
Figure 3.
Inhibitory effect of BO-653 on the atherosclerosis of high-fat-fed mice. Female mice were maintained on a high-fat diet containing 0.5% of the compound for 28 weeks. (a and b) Serial changes of VLDL + LDL concentration (a) and HDL concentration (b). The lipoprotein concentrations were evaluated as cholesterol concentrations of respective fractions by gel-permeation chromatography. (c) Representative cross sections of aortic valvesin hearts of high-fat-fed mice. Atherosclerotic lesions are shown by arrows. (×100.) (d) Effect of BO-653 on atherosclerotic lesions in high-fat-fed mice. All results are shown as mean ± SE, n = 5 (normal diet group) or 10 or 11. ∗, P < 0.05; ∗∗, P < 0.01.
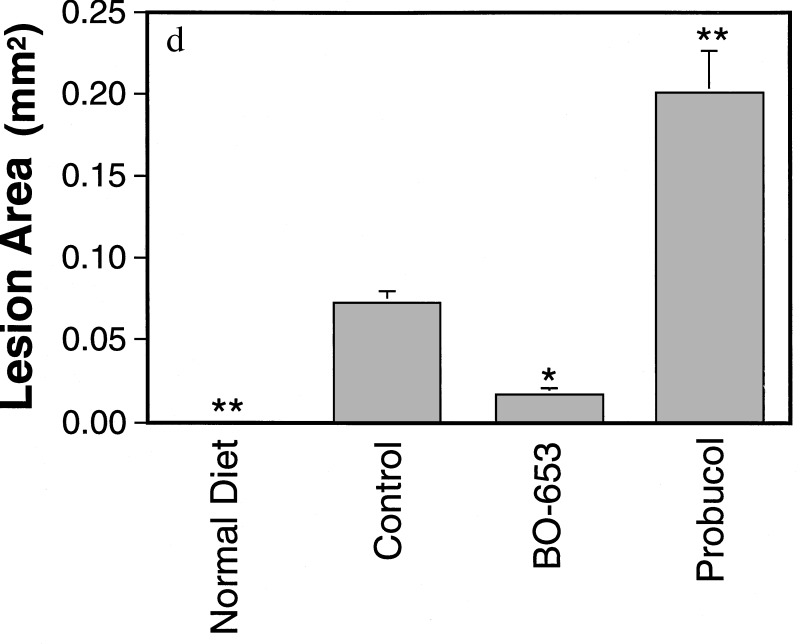
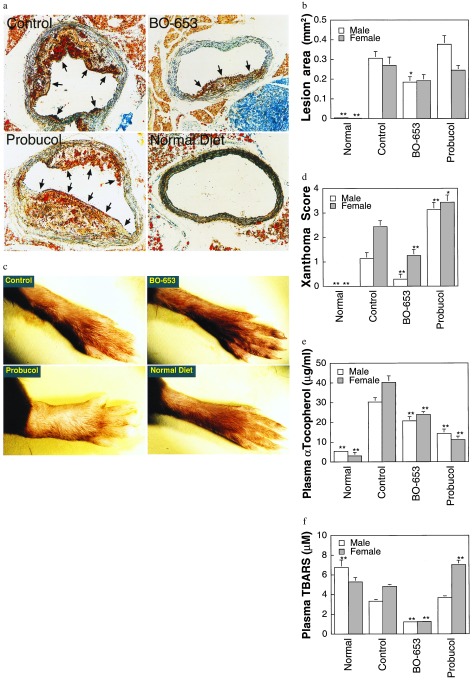
A further examination of atherosclerosis in an LDL-receptor knockout mouse model fed a high-fat diet was carried out (31). The serial lipoprotein changes were similar to those described in the C57BL/6J model and reached quantitative significance in this model (data not shown). Again, pronounced lesions were visible only in the control and probucol groups, whereas in the BO-653 group they were noticeably less severe and in the normal diet group there were none (Fig. 4a; total lesion area Fig. 4b). Xanthomas were found throughout the body in the control group and especially in the probucol group, most evident in the leg joints. The probucol group exhibited grossly swollen hind legs (Fig. 4c). BO-653 lowered the xanthoma score, but the converse was true for probucol (Fig. 4d).
Figure 4.
Inhibitory effect of BO-653 on the atherosclerosis of LDL-receptor knockout mice. Male and female mice, 6 weeks old, were maintained on a high-fat diet containing 0.5% of the compound for 13 weeks (a, b, d–f) or 21 weeks (c). (a) Representative cross sections of aortic arches of male LDL-receptor knockout mice fed a high-fat diet. Atherosclerotic lesions are indicated by arrows. (×132.) (b) Effect of BO-653 on atherosclerotic lesions in LDL-receptor knockout mice. (c) Hind leg xanthoma of female LDL-receptor knockout mice. (d) Effect of BO-653 on xanthoma. Xanthoma score was evaluated by scoring appearances observed mainly in forelimbs. (e and f) Effect of BO-653 on serum α-tocopherol (e) and TBARS (f) concentrations in LDL-receptor knockout mice. All results are shown as mean ± SE, n = 5 (female normal diet group) or 7–10. ∗, P < 0.05; ∗∗, P < 0.01.
DISCUSSION
The theoretical design of a novel antioxidant was aimed at achieving a combination structure that might overcome the respective drawbacks of probucol and of α-tocopherol. The optimal compound chosen from a newly synthesized library based on these characteristics, BO-653, further exhibited good absorption and distribution into the target aortic vessels, the latter characteristic being highly desirable in antiatherosclerotic agents. Furthermore, this lipophilic antioxidant had no apparent toxic effects as measured by serum biochemical values and autopsy studies in three different animal models (data not shown), whereas most potent antioxidants have shown some toxicity or mutagenicity. This safety may be a property of highly lipophilic antioxidants such as α-tocopherol.
Whereas probucol inhibited lesion formation in WHHL rabbits, it actually increased lesion severity in the mouse models, in agreement with the findings of Zhang et al. (32) in the apolipoprotein-E-knockout mice. The reason may well be that mice are less sensitive to exogenous antioxidants than are rabbits. In both WHHL rabbits and LDL-receptor knockout mice, the plasma concentrations of BO-653 were higher than those of probucol, and they were 5-fold or more higher than those of endogenous α-tocopherol. Although the kinetic constant for reaction of probucol with peroxyl radicals is much lower than that of either α-tocopherol (10) or BO-653 (27), it had less of a lowering effect on endogenous α-tocopherol levels than did BO-653 in mice (Fig. 4e). Thus, probucol administration paradoxically leads to increased levels of lipid peroxides in serum (Fig. 4f). BO-653, however, brings about a decrease in serum lipid peroxides in line with its antioxidative potency. Although probucol has been linked to its observable diminution of HDL and α-tocopherol levels (8, 33), the in vivo animal model results clearly indicate that BO-653 reduces such diminution effect and is an agent with potential clinical utility.
In summary, we have shown that a powerful lipophilic antioxidant that suppresses LDL oxidation without affecting HDL levels in the case of WHHL rabbits has potential as an antiatherogenic agent. In the mouse models there was actually an increase in HDL and an attenuation of VLDL/LDL, which may have contributed to the observed antiatherogenic effects. An interesting question to be explored is whether the effects of BO-653 on lipoprotein levels are linked in any way to its antioxidant activity. Further studies will be needed to determine whether BO-653 has therapeutic utility.
Acknowledgments
We are grateful to Drs. H. Nabata, Y. Naito, M. Yoshida, M. Tanigawa, and H. Suzuki for encouragement. We are grateful to Prof. T. Kita at Kyoto Univ., Prof. H. Yamaguchi at Juntendo Univ., and Prof. N. Maeda at North Carolina Univ. for valuable advice and to Dr. K. Boru for help in preparing the manuscript.
ABBREVIATIONS
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- VLDL
very low density lipoprotein
- MCLA
Cypridina luciferin analog 2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo[1,2-a]pyrazin-3-one
- WHHL
Watanabe heritable hyperlipidemic
- TBARS
thiobarbituric acid-reactive substances
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Brown M S, Goldstein J L. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg D, Parthasarathy S, Carew T E, Khoo J C, Witztum J L. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 3.Witztum J L, Steinberg D. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi T, Higashi T, Suzuki T, et al. Nature (London) 1997;386:292–297. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 5.Walldius G, Erikson U, Olsson A, Bergstrand L, Hådell K, Johansson J, Kaijser L, Lassvik C, Mölgaard J, Nilsson, et al. Am J Cardiol. 1994;84:875–883. doi: 10.1016/0002-9149(94)90579-7. [DOI] [PubMed] [Google Scholar]
- 6.Duell P B. J Nutr. 1996;126:1067S–1071S. doi: 10.1093/jn/126.suppl_4.1067S. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg D. Lancet. 1995;346:36–38. doi: 10.1016/s0140-6736(95)92657-7. [DOI] [PubMed] [Google Scholar]
- 8.Johansson J, Olsson A, Bergstrand L, Elinder L S, Nilsson S, Erikson U, Mölgaard J, Holme I, Walldius G W. Arterioscler Thromb Vasc Biol. 1995;15:1049–1056. doi: 10.1161/01.atv.15.8.1049. [DOI] [PubMed] [Google Scholar]
- 9.Miller N E. Am Heart J. 1987;113:589–597. doi: 10.1016/0002-8703(87)90638-7. [DOI] [PubMed] [Google Scholar]
- 10.Gotoh N, Shimizu K, Komuro E, Tsuchiya J, Noguchi N, Niki E. Biochim Biophys Acta. 1992;1128:147–154. doi: 10.1016/0005-2760(92)90300-k. [DOI] [PubMed] [Google Scholar]
- 11.Williams R J, Motteram J M, Sharp C H, Gallagher P J. Atherosclerosis. 1992;94:153–159. doi: 10.1016/0021-9150(92)90240-h. [DOI] [PubMed] [Google Scholar]
- 12.Janero D R. Free Radical Biol Med. 1991;11:129–144. doi: 10.1016/0891-5849(91)90193-7. [DOI] [PubMed] [Google Scholar]
- 13.Godfried S L, Combs G F, Saroka J M, Dillingham L A. Br J Nutr. 1989;61:507–617. doi: 10.1079/bjn19890148. [DOI] [PubMed] [Google Scholar]
- 14.Rimm E B, Stampfer M J, Ascherio A, Giovannucci E, Colditz G A, Willett W. N Engl J Med. 1993;328:1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 15.Blot W J, Li J, Taylor P R, Guo W, Dawsey S, Wang G, Yang C S, Zheng S, Gail M, Li G, et al. J Natl Cancer Inst. 1993;85:1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 16.ATBC Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. [Google Scholar]
- 17.Ingold K U, Burton G W, Zuker M, Hughes L, Lacelle S, Lusztyk E, Slaby M. FEBS Lett. 1986;205:117–120. doi: 10.1016/0014-5793(86)80877-8. [DOI] [PubMed] [Google Scholar]
- 18.McLean L R, Hagaman K A. Biochim Biophys Acta. 1990;1029:161–166. doi: 10.1016/0005-2736(90)90449-x. [DOI] [PubMed] [Google Scholar]
- 19.Perly B, Smith I C P, Hughes L, Burton G W, Ingold K U. Biochim Biophys Acta. 1985;819:131–135. doi: 10.1016/0005-2736(85)90203-2. [DOI] [PubMed] [Google Scholar]
- 20.Mellies M J, Gartside P S, Glatfelter L, Vink P, Guy G, Schonfeld G, Glueck C J. Metabolism. 1980;29:956–964. doi: 10.1016/0026-0495(80)90039-6. [DOI] [PubMed] [Google Scholar]
- 21.Adams D O. Methods Enzymol. 1979;58:494–506. doi: 10.1016/s0076-6879(79)58164-6. [DOI] [PubMed] [Google Scholar]
- 22.Nakano M. Methods Enzymol. 1990;186:585–591. doi: 10.1016/0076-6879(90)86154-n. [DOI] [PubMed] [Google Scholar]
- 23.Cynshi O, Takashima Y, Suzuki T, Kawabe Y, Ohba Y, Kodama T. J Atheroscler Thromb. 1994;1:87–97. doi: 10.5551/jat1994.1.87. [DOI] [PubMed] [Google Scholar]
- 24.Bilheimer D W, Eisenberg S, Levy R I. Biochim Biophys Acta. 1972;260:212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- 25.Nishina P M, Verstuyft J, Paigen B. J Lipid Res. 1990;31:859–869. [PubMed] [Google Scholar]
- 26.Zhang S H, Reddick R L, Burkey B, Maeda N. J Clin Invest. 1994;94:937–945. doi: 10.1172/JCI117460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noguchi N, Iwaki Y, Takahashi E, Kato Y, Tamura K, Cynshi O, Kodama T, Niki E. Arch Biochem Biophys. 1997;342:236–243. doi: 10.1006/abbi.1997.9994. [DOI] [PubMed] [Google Scholar]
- 28.Bilheimer D W, Watanabe Y, Kita T. Proc Natl Acad Sci USA. 1982;79:3305–3309. doi: 10.1073/pnas.79.10.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kita T, Nagano Y, Yokode M, Ishii K, Kume N, Ooshima A, Yoshida H, Kawai C. Proc Natl Acad Sci USA. 1987;84:5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishina P M, Wang J, Toyofuku W, Kuypers F A, Ishida B Y, Paigen B. Lipids. 1993;28:599–605. doi: 10.1007/BF02536053. [DOI] [PubMed] [Google Scholar]
- 31.Ishibashi S, Brown M S, Goldstein J L, Gerard R D, Hammer R E, Herz J. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S H, Reddick R L, Avdievich E, Surles L K, Jones R G, Reynolds J B, Quarfordt S H, Maeda N. J Clin Invest. 1997;99:2858–2866. doi: 10.1172/JCI119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elinder L S, Hådell K, Johansson J, Mölgaard J, Holme I, Olsson A, Walldius G. Arterioscler Thromb Vasc Biol. 1995;15:1057–1063. doi: 10.1161/01.atv.15.8.1057. [DOI] [PubMed] [Google Scholar]