Abstract
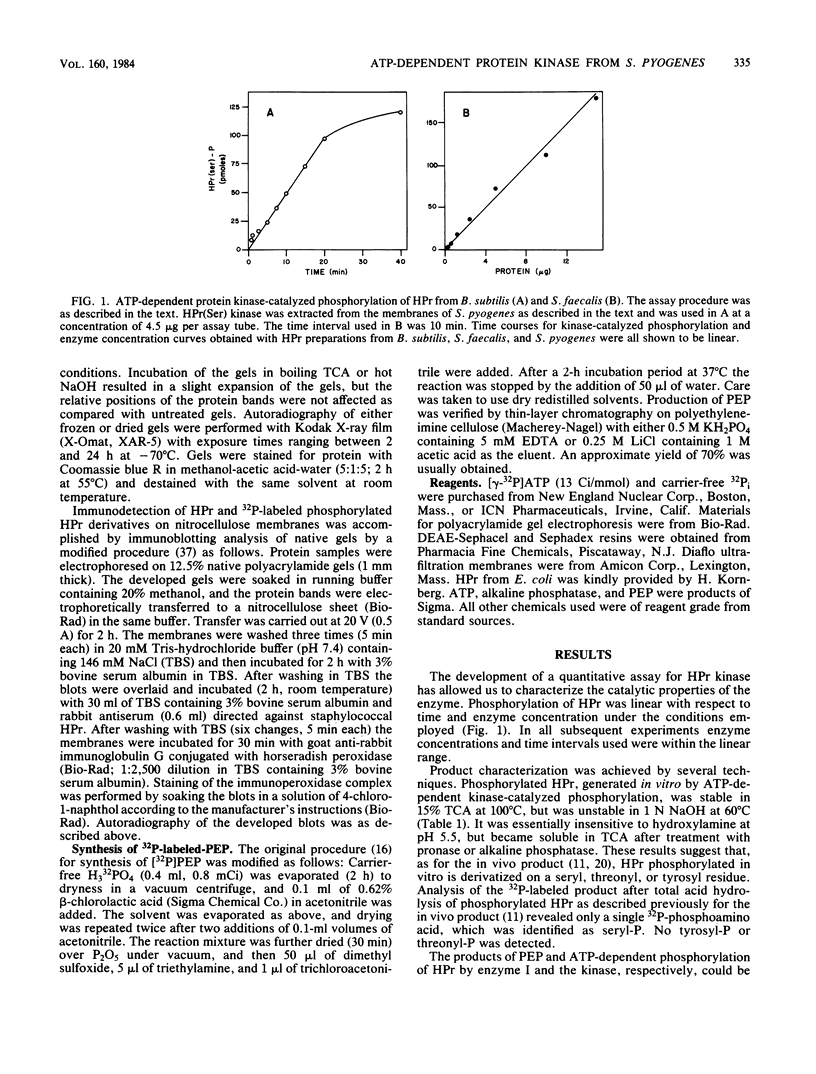
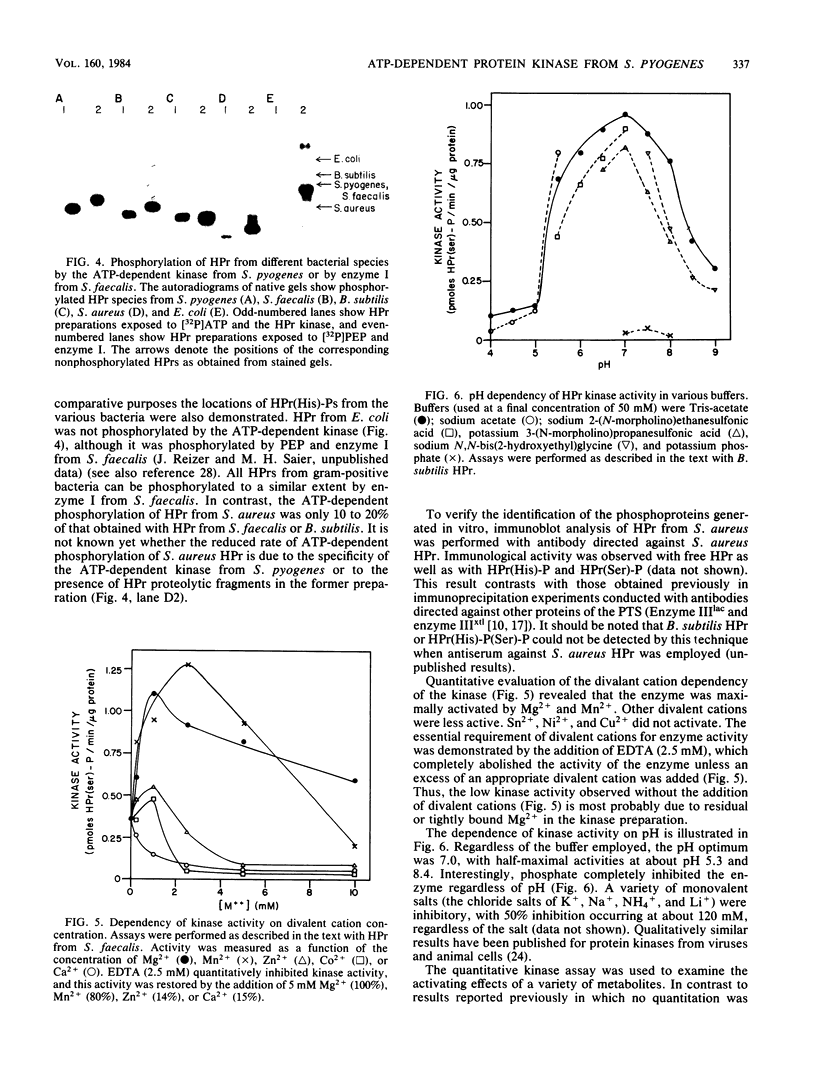
Transport of sugars across the cytoplasmic membranes of gram-positive bacteria appears to be regulated by the action of a metabolite-activated, ATP-dependent protein kinase that phosphorylates a seryl residue in the phosphocarrier protein of the phosphotransferase system, HPr. We have developed a quantitative assay for measuring the activity of this enzyme from Streptococcus pyogenes. The product of the in vitro protein kinase-catalyzed reaction was shown to be phosphoseryl-HPr by several independent criteria (rates of hydrolysis in the presence of various agents, detection of serine-phosphate in acid hydrolysates, immunological assay, and electrophoretic migration rates). HPrs isolated from four different gram-positive bacteria (S. pyogenes, Streptococcus faecalis, Staphylococcus aureus, and Bacillus subtilis) were shown to be phosphorylated by the kinase from S. pyogenes. In contrast, Escherichia coli HPr was not a substrate of this enzyme. The soluble kinase released from the particulate fraction of the cells with high salt in the presence of a protease inhibitor was shown to have an approximate molecular weight of 60,000 as estimated by gel filtration. Its activity was dependent on divalent cations, with Mg2+ and Mn2+ being most active. EDTA, Pi, and high concentrations of salt were strongly inhibitory. The enzyme was optimally active at pH 7.0, exhibited high affinity for its substrates, and was dependent on the presence of one of several metabolites. Of these compounds, fructose 1-6-diphosphate was most active, with gluconate 6-phosphate, 2-phosphoglycerate, 2,3-diphosphoglycerate, phosphoenolpyruvate, and pyruvate exhibiting moderate to low stimulatory activities. Other compounds tested, including a variety of sugar phosphates, pyridine nucleotides, and other metabolites were without effect. The ATP-dependent phosphorylation of HPr on the seryl residue was strongly inhibited by phosphoenolpyruvate-dependent phosphorylation of the active histidyl residue of this protein. Treatment of the kinase with diethyl pyrocarbonate strongly inhibited the ATP-dependent phosphorylation activity, although the sulfhydryl reagents N-ethylmaleimide, p-chloromercuribenzoate, and iodoacetate were without effect. These results serve to characterize the HPr (serine) kinase, which apparently regulates the rates of carbohydrate transport in streptococcal cells via the phosphotransferase system. A primary role of this kinase in the control of cellular inducer levels and carbohydrate metabolic rates is proposed.
Full text
PDF




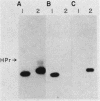
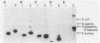
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbe K., Takahashi S., Yamada T. Purification and properties of pyruvate kinase from Streptococcus sanguis and activator specificity of pyruvate kinase from oral streptococci. Infect Immun. 1983 Mar;39(3):1007–1014. doi: 10.1128/iai.39.3.1007-1014.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D-galactose metabolism in group N streptococci: presence of enzymes for both the D-galactose 1-phosphate and D-tagatose 6-phosphate pathways. J Bacteriol. 1974 Jan;117(1):318–320. doi: 10.1128/jb.117.1.318-320.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Mechanism for regulating the distribution of glucose carbon between the Embden-Meyerhof and hexose-monophosphate pathways in Streptococcus faecalis. J Bacteriol. 1971 May;106(2):456–467. doi: 10.1128/jb.106.2.456-467.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Thompson J. Regulation of lactose-phosphoenolpyruvate-dependent phosphotransferase system and beta-D-phosphogalactoside galactohydrolase activities in Lactobacillus casei. J Bacteriol. 1983 Jun;154(3):1195–1203. doi: 10.1128/jb.154.3.1195-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cords B. R., McKay L. L. Characterization of lactose-fermenting revertants from lactose-negative Streptococcus lactis C2 mutants. J Bacteriol. 1974 Sep;119(3):830–839. doi: 10.1128/jb.119.3.830-839.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow V. L., Davey G. P., Pearce L. E., Thomas T. D. Plasmid linkage of the D-tagatose 6-phosphate pathway in Streptococcus lactis: effect on lactose and galactose metabolism. J Bacteriol. 1983 Jan;153(1):76–83. doi: 10.1128/jb.153.1.76-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow V. L., Pritchard G. G. Pyruvate kinase from Streptococcus lactis. Methods Enzymol. 1982;90(Pt E):165–170. doi: 10.1016/s0076-6879(82)90122-7. [DOI] [PubMed] [Google Scholar]
- Crow V. L., Thomas T. D. Arginine metabolism in lactic streptococci. J Bacteriol. 1982 Jun;150(3):1024–1032. doi: 10.1128/jb.150.3.1024-1032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J., Beyreuther K., Sobek H. M., Stüber K., Hengstenberg W. Phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus: factor IIIlac, a trimeric phospho-carrier protein that also acts as a phase transfer catalyst. Biochemistry. 1982 Sep 28;21(20):4867–4873. doi: 10.1021/bi00263a006. [DOI] [PubMed] [Google Scholar]
- Deutscher J., Saier M. H., Jr ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6790–6794. doi: 10.1073/pnas.80.22.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordyce A. M., Moore C. H., Pritchard G. G. Phosphofructokinase from Streptococcus lactis. Methods Enzymol. 1982;90(Pt E):77–82. doi: 10.1016/s0076-6879(82)90109-4. [DOI] [PubMed] [Google Scholar]
- Hengstenberg W., Penberthy W. K., Hill K. L., Morse M. L. Phosphotransferase system of Staphylococcus aureus: its requirement for the accumulation and metabolism of galactosides. J Bacteriol. 1969 Aug;99(2):383–388. doi: 10.1128/jb.99.2.383-388.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbitzer H. R., Hengstenberg W., Rösch P., Muss P., Bernsmann P., Engelmann R., Dörschug M., Deutscher J. HPr proteins of different microorganisms studied by hydrogen-1 high-resolution nuclear magnetic resonance: similarities of structures and mechanisms. Biochemistry. 1982 Jun 8;21(12):2879–2885. doi: 10.1021/bi00541a012. [DOI] [PubMed] [Google Scholar]
- Lauppe H. -F., Rau G., Hengstenberg W. Synthese von [32P]phosphoenolpyruvat. FEBS Lett. 1972 Sep 15;25(2):357–357. doi: 10.1016/0014-5793(72)80524-6. [DOI] [PubMed] [Google Scholar]
- London J., Hausman S. Z. Purification and characterization of the IIIXtl phospho-carrier protein of the phosphoenolpyruvate-dependent xylitol:phosphotransferase found in Lactobacillus casei C183. J Bacteriol. 1983 Nov;156(2):611–619. doi: 10.1128/jb.156.2.611-619.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. W., Carbone D. P., Cushman R. A., Waggoner A. S. The importance of inorganic phosphate in regulation of energy metabolism of Streptococcus lactis. J Biol Chem. 1981 Feb 25;256(4):1861–1866. [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Panos C., Saier M. H., Jr Mechanism of inducer expulsion in Streptococcus pyogenes: a two-step process activated by ATP. J Bacteriol. 1983 Oct;156(1):354–361. doi: 10.1128/jb.156.1.354-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Stuiver I., Saier M. H., Jr Regulation of glycerol uptake by the phosphoenolpyruvate-sugar phosphotransferase system in Bacillus subtilis. J Bacteriol. 1984 Jul;159(1):243–250. doi: 10.1128/jb.159.1.243-250.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Panos C. Regulation of beta-galactoside phosphate accumulation in Streptococcus pyogenes by an expulsion mechanism. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5497–5501. doi: 10.1073/pnas.77.9.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Panos C. Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form. J Bacteriol. 1982 Jan;149(1):211–220. doi: 10.1128/jb.149.1.211-220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Saier M. H., Jr Involvement of lactose enzyme II of the phosphotransferase system in rapid expulsion of free galactosides from Streptococcus pyogenes. J Bacteriol. 1983 Oct;156(1):236–242. doi: 10.1128/jb.156.1.236-242.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert N. D., Blithe D. L., Pastan I. Properties of the src kinase purified from Rous sarcoma virus-induced rat tumors. J Biol Chem. 1982 Jun 25;257(12):7143–7150. [PubMed] [Google Scholar]
- SOKATCH J. T., GUNSALUS I. C. Aldonic acid metabolism. I. Pathway of carbon in an inducible gluconate fermentation by Streptococcus faecalis. J Bacteriol. 1957 Apr;73(4):452–460. doi: 10.1128/jb.73.4.452-460.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. P., Wargnies B., Stalon V. Control of enzyme synthesis in the arginine deiminase pathway of Streptococcus faecalis. J Bacteriol. 1982 Jun;150(3):1085–1090. doi: 10.1128/jb.150.3.1085-1090.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Nakazawa T., Hays J. B., Roseman S. Sugar transport. IV. Isolation and characterization of the lactose phosphotransferase system in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):932–940. [PubMed] [Google Scholar]
- Smorawinska M., Hsu J. C., Hansen J. B., Jagusztyn-Krynicka E. K., Abiko Y., Curtiss R., 3rd Clustered genes for galactose metabolism from Streptococcus mutans cloned in Escherichia coli. J Bacteriol. 1983 Feb;153(2):1095–1097. doi: 10.1128/jb.153.2.1095-1097.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D. Activator specificity of pyruvate kinase from lactic streptococci. J Bacteriol. 1976 Mar;125(3):1240–1242. doi: 10.1128/jb.125.3.1240-1242.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D. Regulation of lactose fermentation in group N streptococci. Appl Environ Microbiol. 1976 Oct;32(4):474–478. doi: 10.1128/aem.32.4.474-478.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Intracellular hexose-6-phosphate:phosphohydrolase from Streptococcus lactis: purification, properties, and function. J Bacteriol. 1983 Oct;156(1):70–80. doi: 10.1128/jb.156.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Novel phosphoenolpyruvate-dependent futile cycle in Streptococcus lactis: 2-deoxy-D-glucose uncouples energy production from growth. J Bacteriol. 1982 Sep;151(3):1454–1465. doi: 10.1128/jb.151.3.1454-1465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Saier M. H., Jr Regulation of methyl-beta-d-thiogalactopyranoside-6-phosphate accumulation in Streptococcus lactis by exclusion and expulsion mechanisms. J Bacteriol. 1981 Jun;146(3):885–894. doi: 10.1128/jb.146.3.885-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Torchia D. A. Use of 31P nuclear magnetic resonance spectroscopy and 14C fluorography in studies of glycolysis and regulation of pyruvate kinase in Streptococcus lactis. J Bacteriol. 1984 Jun;158(3):791–800. doi: 10.1128/jb.158.3.791-800.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberger C. L., Angelo N. Purificationa and properties of a fructose-1,6-diphosphate-activated lactate dehydrogenase from Streptococcus faecalis. J Bacteriol. 1970 Mar;101(3):717–724. doi: 10.1128/jb.101.3.717-724.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]