Abstract
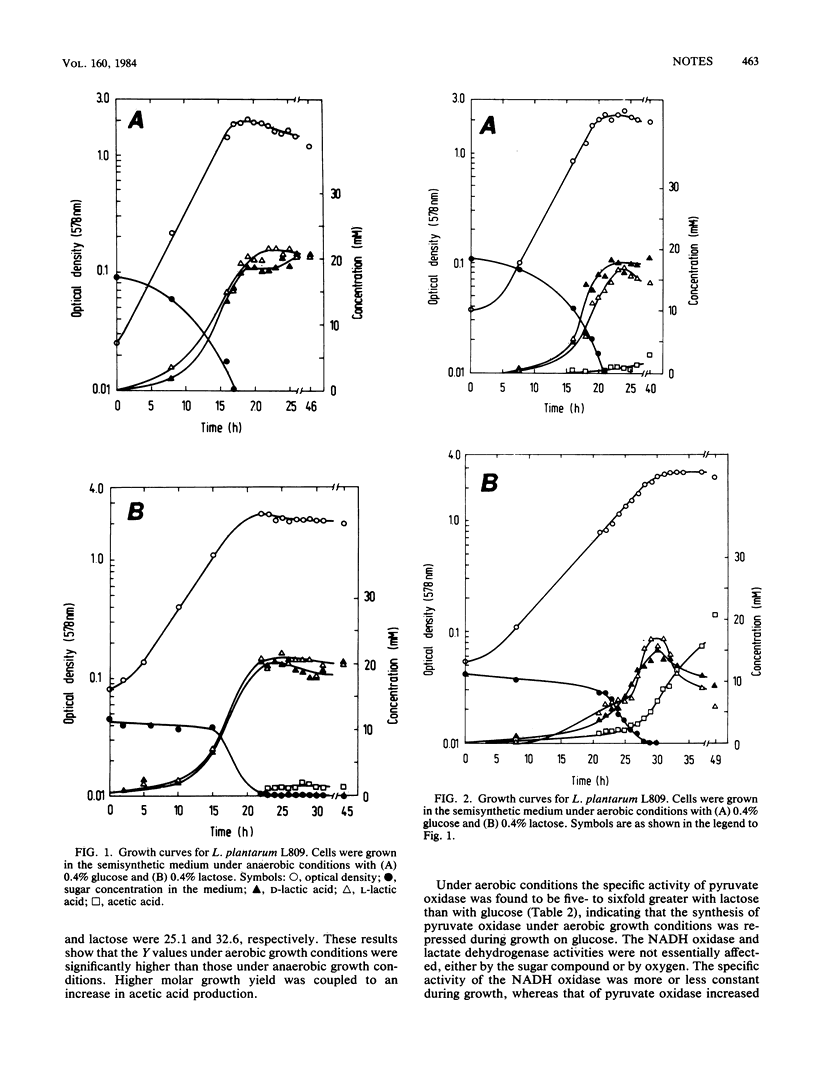
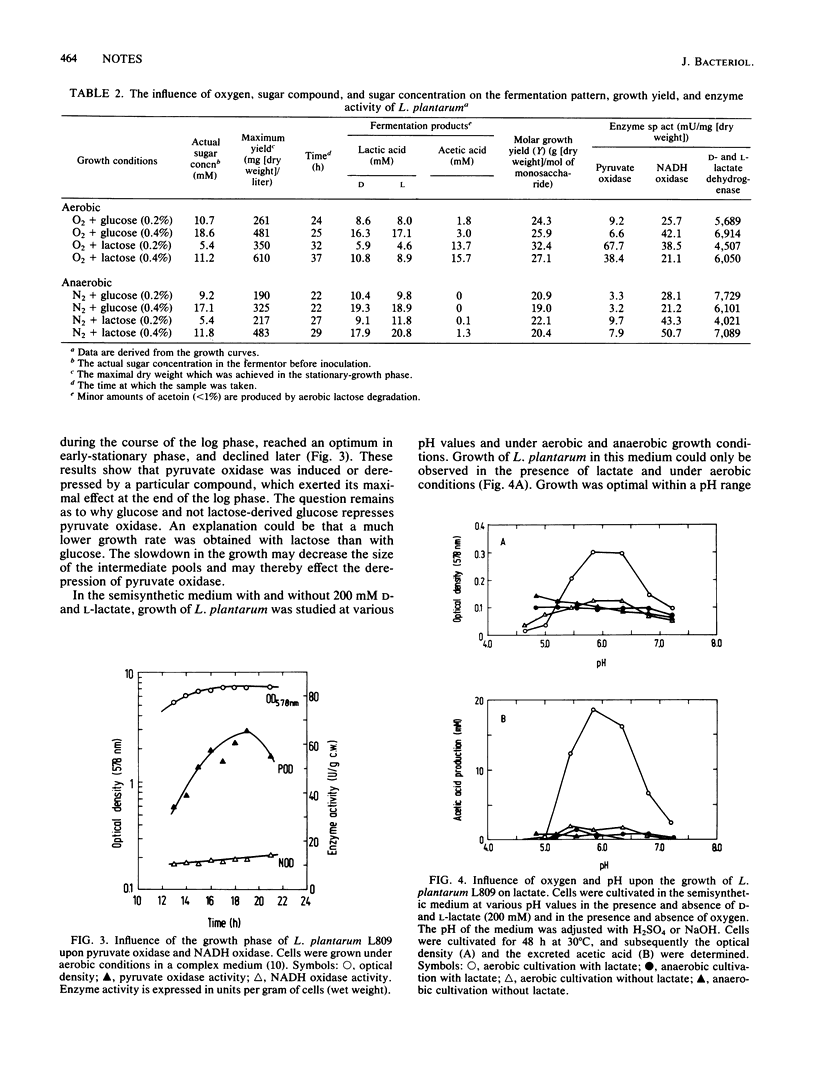
Under aerobic growth conditions Lactobacillus plantarum produced acetic acid in addition to lactic acid. It was found that lactic acid was predominantly produced at first, and then when the carbohydrate was nearly exhausted, lactic acid was metabolized further to acetic acid. The most likely enzyme involved in the aerobic metabolism of L. plantarum is pyruvate oxidase. Its activity is enhanced in the presence of oxygen and is reduced in the presence of glucose. The specific activity of pyruvate oxidase is highest at the beginning of the stationary-growth phase, where a strong increase in acetic acid production was also observed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. P., VanDemark P. J. Respiration of Lactobacillus casei. Can J Microbiol. 1968 Aug;14(8):829–835. doi: 10.1139/m68-141. [DOI] [PubMed] [Google Scholar]
- Dirar H., Collins E. B. Aerobic utilization of low concentrations of galactose by Lactobacillus plantarum. J Gen Microbiol. 1973 Oct;78(2):211–215. doi: 10.1099/00221287-78-2-211. [DOI] [PubMed] [Google Scholar]
- Dirar H., Collins E. B. End-products, fermentation balances and molar growth yields of homofermentative lactobacilli. J Gen Microbiol. 1972 Nov;73(2):233–238. doi: 10.1099/00221287-73-2-233. [DOI] [PubMed] [Google Scholar]
- Götz F., Schleifer K. H. Biochemical properties and the physiological role of the fructose-1,6-bisphosphate activated L-lactate dehydrogenase from Staphylococcus epidermidis. Eur J Biochem. 1978 Oct 16;90(3):555–561. doi: 10.1111/j.1432-1033.1978.tb12635.x. [DOI] [PubMed] [Google Scholar]
- Götz F., Sedewitz B., Elstner E. F. Oxygen utilization by Lactobacillus plantarum. I. Oxygen consuming reactions. Arch Microbiol. 1980 Apr;125(3):209–214. doi: 10.1007/BF00446878. [DOI] [PubMed] [Google Scholar]
- OXENBURGH M. S., SNOSWELL A. M. USE OF MOLAR GROWTH YIELDS FOR THE EVALUATION OF ENERGY-PRODUCING PATHWAYS IN LACTOBACILLUS PLANTARUM. J Bacteriol. 1965 Mar;89:913–914. doi: 10.1128/jb.89.3.913-914.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- STRITTMATTTER C. F. Electron transport to oxygen in lactobacilli. J Biol Chem. 1959 Oct;234:2789–2793. [PubMed] [Google Scholar]
- Sedewitz B., Schleifer K. H., Götz F. Purification and biochemical characterization of pyruvate oxidase from Lactobacillus plantarum. J Bacteriol. 1984 Oct;160(1):273–278. doi: 10.1128/jb.160.1.273-278.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



