Abstract
Inherited mutations in the ATM gene lead to a complex clinical phenotype characterized by neuronal degeneration, oculocutaneous telangiectasias, immune dysfunction, and cancer predisposition. Using the yeast two-hybrid system, we demonstrate that ataxia telangiectasia mutated (ATM) binds to β-adaptin, one of the components of the AP-2 adaptor complex, which is involved in clathrin-mediated endocytosis of receptors. The interaction between ATM and β-adaptin was confirmed in vitro, and coimmunoprecipitation and colocalization studies show that the proteins also associate in vivo. ATM also interacts in vitro with β-NAP, a neuronal-specific β-adaptin homolog that was identified as an autoantigen in a patient with cerebellar degeneration. Our data describing the association of ATM with β-adaptin in vesicles indicate that ATM may play a role in intracellular vesicle and/or protein transport mechanisms.
Ataxia telangiectasia (AT) is a rare human autosomal recessive disease with a pleiotropic phenotype characterized by neuronal degeneration, oculocutaneous telangiectasias, immune dysfunction, cancer predisposition, and premature aging (1). AT cells exhibit hypersensitivity to ionizing radiation and multiple defects in cell cycle checkpoints (2). Induction of p53 protein and the p53-regulated gene products GADD45, p21, and MDM2 after ionizing irradiation are defective/delayed in AT cells (3–5), suggesting that the AT mutated (ATM) protein is a participant in this signal transduction pathway regulating cell cycle progression after DNA damage. In addition, AT cells exhibit a variety of cellular abnormalities in culture, including cytoskeletal defects (6), higher trophic factor requirements for cell growth (7, 8), abnormalities in the plasma membrane (9–11), and defects in intracellular signaling (12).
The gene mutated in AT, ATM (13, 14), encodes a 370-kd protein that is predominantly nuclear (15–18). Recently, immunoelectron microscopy and cellular fractionation were used to demonstrate that a fraction of the ATM protein also localizes to cytoplasmic vesicles (17). The ATM protein belongs to a family of protein and lipid kinases (19). One family member, phosphatidylinositol 3 kinase (PI3 kinase), is involved in mitogenic survival signaling as well as the regulation of vesicle transport (20, 21). Other members of this kinase family include the yeast proteins Mec1p, Rad3p, Tel1p, Tor1, Tor2, and the mammalian proteins FKBP12-rapamycin associated protein (FRAP), ataxia-telangiectasia and rad3-related protein (ATR), and DNA-dependent protein kinase. All of these proteins appear to be involved in cell cycle control or DNA repair. The homology between ATM and this kinase family (19) as well as data from a variety of experimental systems (12, 22, 23) suggest that ATM contributes to cellular responses to DNA damage and also may regulate general cellular signaling pathways.
Although the role of ATM remains unknown, the pleiotropic features of AT, the large size of the ATM protein, and its multiple subcellular localizations suggest that ATM may have multiple functions. To explore ATM function, we attempted to identify the proteins that associate with ATM by initially using a yeast two-hybrid screen. Here, we report that an amino terminal domain of ATM interacts with β-adaptin and β-NAP protein fragments. Moreover, both immunoprecipitation and immunofluorescence demonstrate that ATM associates with β-adaptin in cytoplasmic vesicles. These unexpected interactions may provide new insights in understanding AT.
MATERIALS AND METHODS
Plasmid Construction for Yeast Two-Hybrid.
To construct the mouse DNA binding domain (DBD)–mouse ATM protein (designated Atm), FB2F fragment bait, DNA encoding FB2F (amino acids 811–1,283) was amplified by PCR with a Pfu DNA polymerase by using the following primers: 5′ sense 5′-GGTGGTCATATGGCCATGGAGGCCATGAATGA- CATTGCAGATATT-3′, 3′ antisense 5′-CAGTCTAGA- GTCGACTTTCCAGCACTTTTGAAT-3′. The amplified PCR product was digested with SfiI and SalI and was cloned into pASI and pACTII (CLONTECH) for DBD-FB2F and TA-FB2F, respectively. For the deletions of DBD-FB2F, FB2F was released from DBD-FB2F with NcoI/PstI, NcoI/StyI, NdeI, NdeI/SalI, NdeI/BglII, and BglII/SalI and was cloned into pASI to create DBD-FB2F D1, D2, D3, D4, D5, and D6, respectively. DBD-PI3K, DNA encoding Atm PI3K (amino acid 2,712–3,065), was amplified by PCR with primers (5′ sense 5′-GTGGTCATATGGCCATGGAGGCCATAGATTGTGTGGGTTCTGAT-3′, 3′ antisense 5′-CAGTCTTAGAGTCGACTCACACCCAAGCTTTCCA-3′) and cloned into pASI. DBD-ATR, DNA encoding ATR (amino acid 270–2,282), was digested with NheI and was subcloned into pASI.
Yeast Two-Hybrid Screen.
A modified yeast two-hybrid screen was used to identify Atm fragment-interacting proteins as described (24). HF7c yeasts containing DBD-FB2F were transformed with 100 μg of DNA from a mouse embryonic library. Positive clones were tested for specificity with nonspecific baits. For deletion analysis, double transformants were grown in selective media, and β-galactosidase activity was determined by using an o-nitrophenyl β-d-galactoside assay.
Glutathione S-Transferase (GST) Fusion Proteins and in Vitro Binding Assays.
For GST fusion bacterial expression vectors, a β-adaptin DNA fragment (amino acids 159–436) and a β-NAP DNA fragment (amino acids 4–614) were cloned into pGEX 3 (Pharmacia). Production of fusion proteins in BL21(DE3) bacteria and purification were performed by standard techniques.
In vitro transcription/translation was performed by using the T7 in vitro transcription/translation kit (Promega) according to the manufacturer’s directions. Proteins (4 mg) from whole cell lysates (GM00536A lymphoblasts) or 40 μl of in vitro translation reaction were placed in GST binding buffer (50 mM Hepes, pH 7.5/150 mM NaCl/1.5 mM MgCl2/3 mM EGTA/5% glycerol/0.3% Triton X-100) and were cleared with glutathione-Sepharose 4B beads for 2 hr and then were precipitated with immobilized GST-β-adaptin and GST-β-NAP (4 μg) for 5 hr at 4°C. Precipitants were washed with washing buffer (50 mM Hepes, pH7.5/100 mM NaCl/1.5 mM MgCl2/3 mM EGTA/5% glycerol/0.3% Triton X-100) and were analyzed by Western blotting.
Mammalian Expression Plasmids.
Fragments of DNA encoding human FB2F and mouse FB2F were amplified with Pfu polymerase and were epitope tagged by subcloning the fragments into pcDNA3 (Invitrogen) that had a myc- or an hemagglutinin (HA)-epitope tag inserted. A β-adaptin DNA fragment encoding amino acids 159–436 was cloned from the yeast two-hybrid screen. This β-adaptin DNA was released with BglII and was cloned into a modified Flag-tagged pSG5 (25) vector to create a Flag-tagged β-adaptin expression vector. A FB2FD3 DNA also was subcloned into Flag-tagged pSG5 vector. To construct a Flag-tagged ATM expression vector, a full length ATM cDNA containing the Flag epitope was released from pFB-YZ.5 (26) with SalI/XhoI and was subcloned into pcDNA3.
Immunoprecipitation and Confocal Fluorescence Microscopy.
Vero cells or 293T cells were transfected transiently with a mammalian expression vector by using standard calcium phosphate conditions. After 48 hr, immunoprecipitation and immunofluorescence experiments were done. Cells (normal lymphoblasts GM00536A or AT lymphoblasts GM01526) were harvested in buffer A (50 mM Hepes, pH7.5/1 mM EGTA/1.5 mM MgCl2/1 × protease inhibitor mixture from Boehringer Mannheim) and were lysed by freeze thaw in liquid nitrogen. After removing nuclei and unlysed cells by centrifugation, proteins were extracted from supernatants by increasing the concentration of Triton X-100 to 0.3% and NaCl to 150 mM. After centrifugation, supernatants were diluted into IP buffer (buffer A plus 150 mM NaCl and 0.1% Triton X-100). After preclearing with protein A/G agarose and mouse IgG, supernatants were immunoprecipitated following standard protocols. Antibodies used for immunoblotting and immunoprecipitation were anti-HA and Myc (Boehringer), anti-ATM Ab-3 antibody (Oncogen), anti-β β′adaptin (Transduction or Sigma), and anti-Flag M2 (Kodak).
For immunofluorescence experiments, transiently transfected cells were fixed in 1% paraformaldehyde and were permeabilized in 0.2% Triton X-100. The cells were incubated with mouse anti-HA antibody (Boehringer), rabbit anti-Flag antibody (Santa Cruz), or mouse anti-β β′adaptin antibody (Sigma) followed by incubation with a fluorescein isothiocyanate-conjugated goat anti-mouse and a rhodamine-coupled goat anti-rabbit antibody. For confocal microscopy, cells were scanned with a Noran OZ CLSM confocal microscope system with interversion software (Noran Instruments, Middleton, Wisconsin). Series of confocal images were recorded at 0.8-μm optical sections in the Z plane.
RESULTS
β-Adaptin Binds to Atm Fragment in Yeast Two-Hybrid Assays.
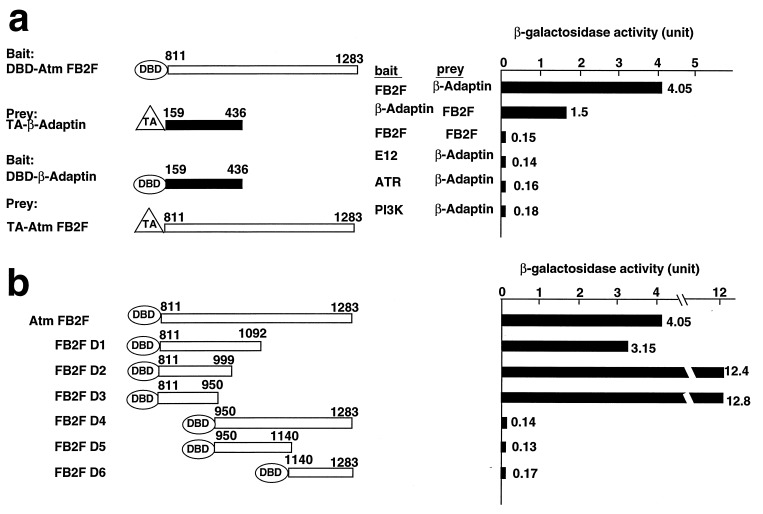
To investigate the mechanism of action of ATM, a yeast two-hybrid assay (24) was used to search for ATM-interacting proteins. ATM protein contains a leucine zipper motif that might be involved in protein–protein interactions. Therefore, using this fragment of Atm (amino acids 811–1,283, designated FB2F) as a bait, we screened a mouse embryonic library and isolated an amino terminal domain of β-adaptin (amino acids 159–436, designated NTBA). The interaction between the Atm fragment and the N-terminal β-adaptin occurred regardless of which fragment was used for bait and prey (Fig. 1a). Specificity was demonstrated because the NTBA did not interact with control baits, including E12, the Atm kinase domain (amino acids 2,712–3,065), or an ATR protein fragment containing a leucine zipper domain (amino acids 270–2,282) (Fig. 1a).
Figure 1.
Yeast two-hybrid binding assays (a) Atm and β-adaptin interact in yeast. The Gal4 DBD was fused to bait (Atm FB2F or β-adaptin), and the Gal4 transactivation domain (TA) was fused to prey (Atm FB2F or β-adaptin). Pairs of plasmids were introduced into HF7c cells, and β-galactosidase activity (Right) was quantitated by using an o-nitrophenyl β-d-galactoside assay. E12, ATR, and Atm PI3K domains were used for negative control baits. (b) Domain analysis of Atm binding site with β-adaptin. Various Atm FB2F deletion baits and TA-β-adaptin were used for baits and prey, respectively. Interaction was assessed as in a.
Deletion analysis of the FB2F fragment was performed to determine the domain that is required for association with β-adaptin (Fig. 1b). Deletion baits of the Atm fragment were individually cotransformed into HF7c yeast cells with NTBA β-adaptin prey, and β-gal activity was determined by quantitative o-nitrophenyl β-d-galactoside assay. Atm deletion fragments did not interact with the Gal4 transactivation domain alone in a filter binding assay (data not shown). An N-terminal region of Atm (amino acids 811–950), but not the leucine zipper domain (amino acids 950–1,283), was sufficient to interact with β-adaptin. Of interest, the putative Atm leucine zipper domain was not capable of self-interaction in this assay.
β-Adaptin Binds to the Atm Fragment in Vitro and in Vivo.
β-adaptin (also called β2 adaptin) is a component of the AP-2 adaptor complex, which is involved in clathrin-mediated endocytosis of receptors (27). Sequences homologous to the amino terminal domain (NTBA) of β-adaptin, which binds to Atm, also are found in β′-adaptin (β1), β-NAP(β3b), β3a adaptin, and β-COP, which are all vesicle-associated proteins that function in vesicle and/or protein transport (28–31). Of interest, β-NAP is a neuronal-specific protein that was identified as an autoimmune antigen in a patient with cerebellar degeneration, a phenotypic hallmark of AT (29). This linkage of ATM to a protein family previously implicated in neuronal processes justified further characterization of this interaction.
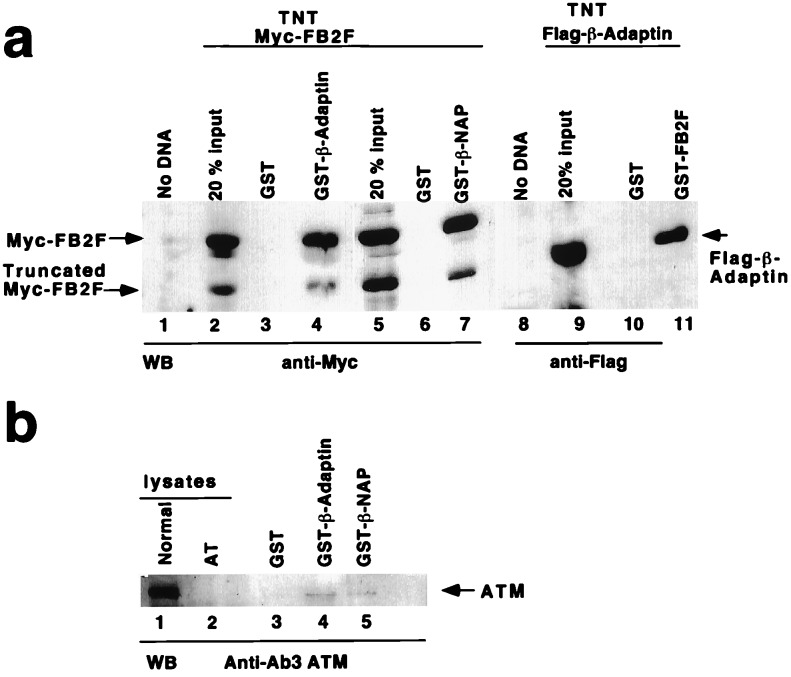
Interaction between the FB2F and NTBA fragments was confirmed in vitro by using GST fusion recombinant proteins. An in vitro-translated Myc-tagged FB2F fragment specifically bound to GST-β-adaptin (Fig. 2a) but not to GST alone. Conversely, an in vitro-translated Flag-tagged β-adaptin bound to GST-FB2F but not to GST alone (Fig. 2a). To determine whether the NTBA can associate with endogenous ATM protein, GST-NTBA was incubated with cell lysates, and precipitants were analyzed by immunoblotting with an anti-ATM antibody. The GST-NTBA was able to bind to the full length ATM protein from cell lysates (Fig. 2b). Moreover, a GST fusion protein containing the N-terminal region of the neuronal specific family member β-NAP (GST-β-NAP, amino acids 4–614) also bound to the in vitro-translated Myc-tagged FB2F and to the full length endogenous ATM from cell lysates (Fig. 2 a and b). Finally, GST-FB2F recombinant protein precipitated the full length β-adaptin from cell lysates (data not shown).
Figure 2.
In vitro binding of Atm FB2F and β-adaptin NTBA. (a) GST and GST fusion proteins (GST-β-adaptin amino acids 159–436, GST-β-NAP amino acids 4–614, and GST-FB2F amino acids 811–1,283) were expressed in Escherichia coli. (Left) In vitro transcribed and translated Myc-tagged FB2F was incubated with immobilized GST (lanes 3 and 6), GST-β-adaptin (lane 4), or GST-β-NAP (lane 7). The bound precipitants were analyzed by Western blotting for Myc-FB2F. (Right) In vitro transcribed/translated Flag-tagged β-adaptin (amino acids 159–436) was incubated with GST (lane 10) and GST-FB2F (lane 11) and was analyzed by Western blotting for Flag-β-adaptin. Mock TNT reactions (No DNA; lanes 1 and 8) or 20% of the input TNT Myc-FB2F (lane 2) or Flag-β-adaptin (lane 9) were included for comparison. A C-terminal truncation of Myc-FB2F was also present in the translation reactions and was bound to the GST fusion product. (b) Extracts made from normal lymphoblasts were mixed with GST (lane 3), GST-β-adaptin (lane 4), and GST-β-NAP (lane 5), and complexes were analyzed by Western blotting with the anti-ATM antibody. Lanes: 1, normal lymphoblast (GM00536A) lysate; 2, AT lymphoblast (GM01526) lysate that lacks ATM protein.
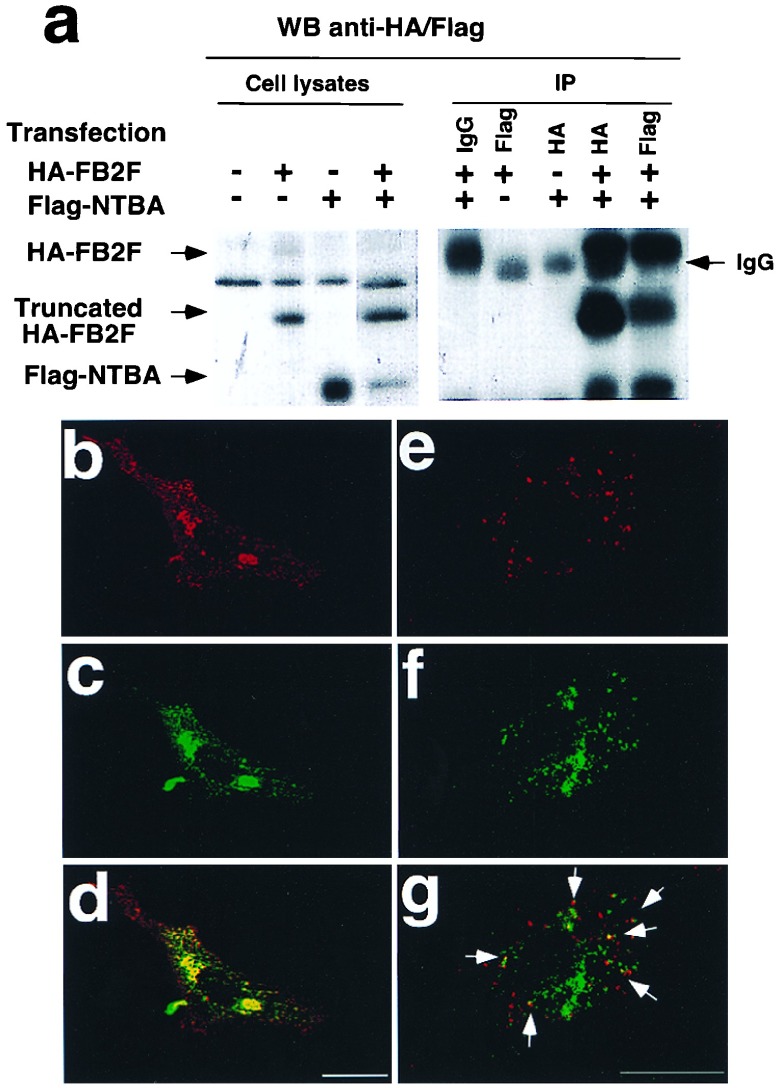
To assess in vivo binding between FB2F and NTBA, we transiently cotransfected the HA-tagged FB2F and Flag-tagged NTBA and performed coimmunoprecipitation and colocalization studies. When the HA-tagged FB2F and Flag-tagged NTBA were cotransfected in 293T cells, immunoprecipitation of NTBA with an anti-Flag antibody brought down the HA-tagged FB2F fragment (Fig. 3a). Conversely, the Flag-tagged NTBA fragment was coprecipitated with an anti-HA antibody (Fig. 3a). Immunofluorescence also was performed to examine the subcellular localization of the two fragments transiently expressed in Vero cells. The immunostaining pattern of Flag-tagged NTBA was similar to that of endogenous β-adaptin, with plasma membrane and punctate vesicular staining (Fig. 3b). A significant fraction of the HA-tagged FB2F fragment was localized to the cytoplasm with a punctate immunostaining whereas a small portion stained nuclei (Fig. 3c). Confocal microscopic analysis demonstrated that these two proteins colocalized at the plasma membrane and at intracellular vesicles (illustrated by the yellow in Fig. 3d). In addition, a Flag-tagged FB2F D3 (amino acids 811–950), which strongly interacted with NTBA in the yeast two-hybrid assay, was transfected into cells, and cells were double labeled with the Flag antibody (Fig. 3e) and β-adaptin antibody (Fig. 3f). We observed partial colocalization of Flag-tagged FB2F D3 with endogenous β-adaptin (shown by yellow in Fig. 3g). This immunofluorescence result is consistent with both the in vitro binding and yeast two-hybrid results. Taken together, these results show that the FB2F fragment of Atm can associate with the amino terminal domains of β-adaptin and β-NAP as well as with endogenous β-adaptin in vivo.
Figure 3.
In vivo binding of Atm FB2F and β-adaptin NTBA. Cells were transfected with plasmids expressing HA-tagged FB2F and Flag-tagged β-adaptin. (a Left) Expression of transfected plasmids was confirmed by immunoblotting with anti-HA and anti-Flag antibodies. (Right) Coimmunoprecipitation of both proteins with either Anti-HA or Anti-Flag. Vero cells cotransfected with both plasmids were immunostained with anti-HA and Anti-Flag and were analyzed by using confocal microscopy. (b) Detection of Flag-β-adaptin with rabbit anti-Flag (red fluorescence). (c) Detection of HA-FB2F Atm immunostaining with mouse Anti-HA (green fluorescence). (d) Colocalization between Flag-β-adaptin and HA-FB2F Atm. Cells also were transfected with Flag-tagged FB2F D3 and were immunostained with Flag antibody and β-adaptin antibody. (e) Detection of Flag-tagged FB2F. (f) Detection of endogenous β-adaptin. (g) Merged image for Flag-FB2F and β-adaptin. Confocal images from each fluorescence were recorded and superimposed to demonstrate colocalization (yellow merge fluorescence). Arrows indicate the colocalization of Flag-FB2F and β-adaptin. (Bar = 25 μm.)
ATM Associates with β-Adaptin in Cytoplasmic Vesicles.
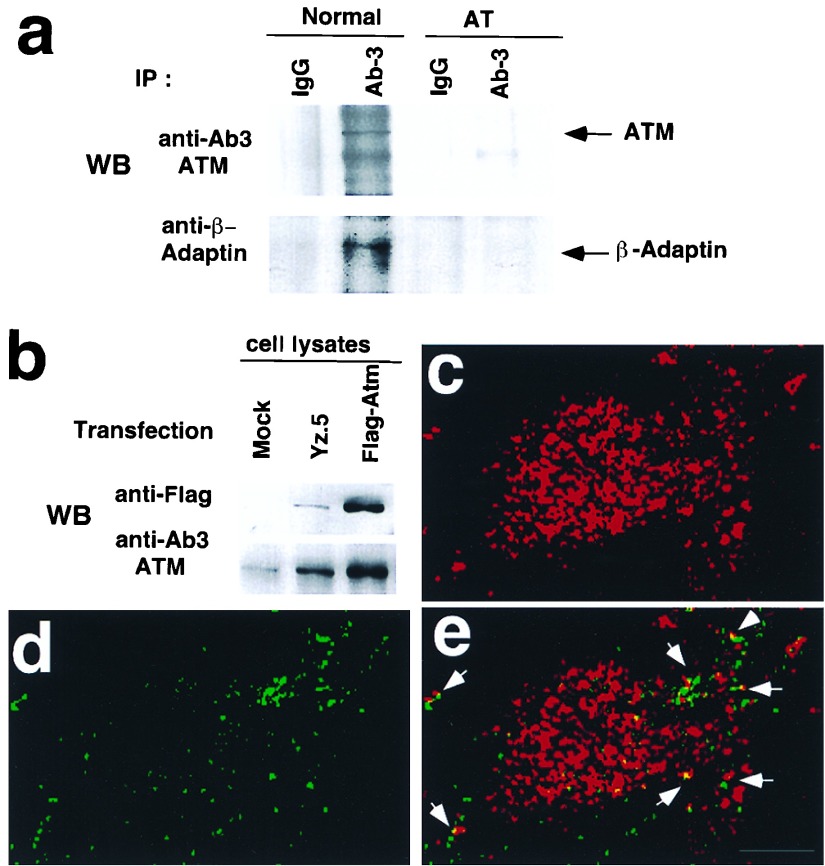
Because fragments of Atm and β-adaptin associate in vitro and in vivo and because both endogenous ATM and β-adaptin proteins have been found in cytoplasmic vesicles (17, 27), we investigated whether endogenous full length proteins colocalize to the same vesicles. Therefore, we examined whether β-adaptin could be coimmunoprecipitated with ATM from normal lymphoblasts. Cells were subfractionated into cytoplasmic and nuclear fractions and cytosolic extracts were used for immunoprecipitation with normal rabbit IgG or an antibody to ATM. As shown in Fig. 4a, a fraction of the endogenous β-adaptin was coimmunoprecipitated with an ATM antibody but not with the normal rabbit IgG. AT lymphoblasts also were used as an negative control for coimmunoprecipitation. ATM protein in AT cells was not detected by Western blots. Immunoprecipitation from AT cytosolic extracts with the anti-ATM antibody or normal rabbit IgG failed to bring down any detectable β-adaptin (Fig. 4a). Thus, the coimmunoprecipitation of β-adaptin with ATM from normal lymphoblasts was not an artifact of antibody cross-reactivity and does not represent nonspecific binding of β-adaptin to the beads but requires the presence of the ATM protein.
Figure 4.
Coimmunoprecipitation and colocalization of β-adaptin with ATM. (a) Coimmunoprecipitation of β-adaptin with ATM. ATM was immunoprecipitated from cytosolic extracts (normal lymphocytes, GM00536A; AT lymphocytes, GM01526) with anti-ATM antibody, and immunoprecipitates were blotted with anti-ATM and anti-β-adaptin antibodies. (b) Transient expression of Flag-tagged ATM. 293T cells were transfected with plasmids [pFB-Yz.5 (26) and Flag-ATM] expressing Flag-tagged ATM, and expression of exogenous Flag-tagged ATM was verified with anti-Flag and anti-ATM antibodies by Western blotting. (c) Labeling of Flag-tagged ATM with a rabbit anti-Flag antibody (red fluorescence). (d) Endogenous β-adaptin immunostaining with mouse anti-β β′-adaptin antibody (green fluorescence). (e) Colocalization between Flag-ATM and β-adaptin. Confocal images from c and d were superimposed to demonstrate colocalization (yellow merge fluorescence). Arrows indicate the colocalization of ATM and β-adaptin. (Bar = 10 μm.)
We also investigated whether full length ATM and β-adaptin colocalize to the same vesicles in cells by using indirect immunostaining. However, because of low levels of endogenous ATM and suboptimal ATM antibodies for immunostaining, we could not perform adequate colocalization experiments of endogenous proteins in cells. Therefore, a Flag-tagged full length ATM expression vector was constructed and was transfected transiently into 293T cells. The expression of Flag-tagged ATM was confirmed by Western blotting (Fig. 4b). Furthermore, the Flag-tagged ATM was coimmunoprecipitated by using anti-β-adaptin antibody after overexpressing the Flag-tagged ATM in cells (data not shown). Immunolocalization of Flag-tagged ATM was similar to that of endogenous ATM, as reported (17, 26). Most of the immunostaining of Flag-tagged ATM was nuclear, although a small portion localized to the cytoplasm with a punctate staining pattern (Fig. 4c). Because the majority of full length ATM localizes to the nucleus in proliferating cells, the amount of cytoplasmic staining is less dramatic than that seen with the FB2F fragment (Fig. 3). Nevertheless, we still observed partial colocalization of the endogenous β-adaptin and Flag-tagged ATM in vesicles as shown by the yellow in Fig. 4e. Of interest, Flag-tagged ATM localized to vesicles that did not costain for β-adaptin, suggesting that ATM also may interact with other vesicle coat proteins.
DISCUSSION
Experiments in vitro and in vivo demonstrate that ATM associates with β-adaptin in cells. The physiological significance of the association between ATM and β-adaptin in vesicles remains unclear, but this interaction may help explain how mutations in the ATM gene cause the pleiotropic nature of the A-T phenotype. The large size of the ATM protein and its multiple subcellular localizations suggest that ATM may have more than one function. Functional alterations in an adaptin pathway caused by lack of ATM protein may contribute to the pleiotropic nature of the A-T phenotype. In the nucleus, ATM may play a role in DNA damage surveillance by signaling to p53 and other proteins that regulate the cell cycle or DNA repair (2, 19). In the cytoplasm, ATM localizes to vesicles and interacts with β-adaptin, one of the components of the AP-2 adaptor complex, which is involved in clathrin-mediated endocytosis of receptors (27). The AP-2 complex also binds to several other proteins including Shc, Grb2, amphiphysin, and eps15(32–35), which have been implicated in endocytosis and cell signaling. Therefore, ATM may have roles in vesicle and/or protein transport similar to other PI3K family proteins, including Vps34 and p85-p110 PI-3 kinase (20). The ATM protein could regulate vesicle transport through phosphorylation of proteins or lipids. Phosphatidylinositol metabolites play a critical role in vesicle trafficking and mitogenic signal transduction (20). Of interest, inositol hexakisphosphate binds to the α-subunit of the AP-2 complex (36), and inositol phosphates and phosphatidyl inositol phosphate inhibit self-assembly of AP-2 and clathrin coat formation (37). Whether ATM in vesicles has a lipid kinase activity like other PI3 kinase isoforms remains to be determined.
A role for ATM in intracellular signaling has been suggested by several of the phenotypic changes observed in AT cells: the high level of growth factors required for cell growth (7, 8), insulin resistance in AT patients (38), altered actin cytoskeleton in AT cells (6), humoral defects, including IgA and IgE deficiency (39, 40), and altered regulation of K+ channels in AT cells (N. Rhodes, personal communication). In addition, signaling through the B-cell receptor is defective in AT cells, including a defect in intracellular mobilization of Ca2+, PLCγ1 activation, and PI-3 kinase activation (12). Of interest, it also has been reported that multivesicular bodies induced by phytohemagglutinin failed to undergo exocytosis in AT lymphocytes (41). In addition, uncharacterized accumulated cytoplasmic lipid cytosomes and an increased number of lysosomes in AT patient samples have been observed in electron microscopic studies (42–44), suggesting altered lipid metabolism or altered lysosomal enzyme activity in AT cells. These reports suggest that ATM may be involved in general signal transduction and in vesicle and/or protein transport in cytoplasmic vesicles. The wide range of defects in AT cells could be explained by altered vesicle and/or protein transport caused by a dysfunction of β-adaptin or other related vesicle-associated proteins. It is likely, however, that the functional ramifications of ATM interacting with adaptin family members will exhibit significant cell-type specificity.
Sequences homologous to the ATM-binding amino terminal domain of β-adaptin also are found in β′-adaptin, β-NAP, β3a, and β-COP, all vesicle-associated proteins that function in vesicle and/or protein transport. Data was presented demonstrating interaction of the Atm fragment with both β-adaptin and β-NAP, and ATM may associate with one or more of these β-adaptin family proteins. β′-adaptin is a component of the AP-1 complex, which mediates vesicle transport from the trans-Golgi network to endosomes and lysosomes. The IgA and IgE deficiency, altered plasma membrane, and failure of multivesicular bodies exocytosis induced by phytohemagglutinin in AT could result from defects in protein or vesicle transport from the trans-Golgi to the plasma membrane. Of interest, it has been proposed that AP-2 and β-NAP play an essential role in synaptic vesicle transport in neuronal cells. Moreover, β-NAP has been shown recently to be required for synaptic vesicle formation (45). Dysfunction of these pathways has been reported to lead to ataxia in humans and fruit flies (29, 46). This interaction between ATM and the vesicle associated proteins may play an important role in regulating vesicle and/or protein transport in neurons. Dysfunction in these pathways may contribute to the progressive cerebellar degeneration of AT patients. Our data describing the association of ATM with β-adaptin in vesicles may provide new insights into ATM function and may illustrate how a complex disease phenotype may result from altered intracellular vesicle and/or protein transport mechanisms.
Acknowledgments
We thank M. Delannoy for help with confocal microscopic analysis; E. Olson for the library utilized in the yeast two-hybrid screen; and K. Cimprich and S. Schreiber for providing the ATR plasmid. This research was supported by National Institutes of Health Grants CA71387 and GM07309. M.B.K is The Steven Birnbaum Scholar of The Leukemia Society of America.
ABBREVIATIONS
- AT
Ataxia telangiectasia
- ATM
Ataxia telangiectasia mutated
- ATR
ataxia-telangietasia and rad3-related protein
- DBD
DNA binding domain
- GST
glutathione S-transferase
- HA
hemagglutinin
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Lavin M F, Shiloh Y. Annu Rev Immunol. 1997;15:177–202. doi: 10.1146/annurev.immunol.15.1.177. [DOI] [PubMed] [Google Scholar]
- 2.Morgan S E, Kastan M B. Adv Cancer Res. 1997;71:1–25. doi: 10.1016/s0065-230x(08)60095-0. [DOI] [PubMed] [Google Scholar]
- 3.Kastan M B, Zhan Q, El-Deiry W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J., Jr Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 4.Khanna K K, Lavin M F. Oncogene. 1993;8:3307–3312. [PubMed] [Google Scholar]
- 5.Canman C E, Wolff A C, Chen C, Fornace A J, Kastan M B. Cancer Res. 1994;54:5054–5058. [PubMed] [Google Scholar]
- 6.McKinnon P J, Burgoyne L A. Eur J Cell Biol. 1985;39:161–166. [PubMed] [Google Scholar]
- 7.Elmore E, Swift M. J Cell Physiol. 1976;89:429–432. doi: 10.1002/jcp.1040890308. [DOI] [PubMed] [Google Scholar]
- 8.Shiloh Y, Tabor E, Becker Y. Exp Cell Res. 1983;140:191–199. doi: 10.1016/0014-4827(82)90169-0. [DOI] [PubMed] [Google Scholar]
- 9.Ozer N K, Ciliv G, Berkel A I, Sanal O, Yegin O, Erosy F. Clin Exp Immunol. 1985;61:118–124. [PMC free article] [PubMed] [Google Scholar]
- 10.Cokugras A N, Karan A, Ozer N K, Berkel A T. Biochem Med. 1986;36:377–381. doi: 10.1016/0885-4505(86)90150-7. [DOI] [PubMed] [Google Scholar]
- 11.Ozer N K, Bashford C L, Carter N D, Pasternak C A. Clin Biochem. 1989;22:469–473. doi: 10.1016/s0009-9120(89)80100-6. [DOI] [PubMed] [Google Scholar]
- 12.Khanna K K, Yan J, Watters D, Hobson K, Beamish H, Spring K, Shiloh Y, Gatti R A, Lavin M F. J Biol Chem. 1997;272:9489–9495. doi: 10.1074/jbc.272.14.9489. [DOI] [PubMed] [Google Scholar]
- 13.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle D A, Smith S, Uziel T, Sfez S, et al. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 14.Savitsky K, Sfez S, Tagle D A, Sartiel A, Collins F S, Shiloh Y, Rotman G. Hum Mol Genet. 1995;4:2025–2032. doi: 10.1093/hmg/4.11.2025. [DOI] [PubMed] [Google Scholar]
- 15.Lakin N, Weber P, Stankovic T, Rottinghaus S, Taylor A, Jackson S. Oncogene. 1996;13:2707–2716. [PubMed] [Google Scholar]
- 16.Chen G, Lee E. J Biol Chem. 1996;271:33693–33697. doi: 10.1074/jbc.271.52.33693. [DOI] [PubMed] [Google Scholar]
- 17.Watters D, Khanna K K, Beamish H, Birrell G, Spring K, Kedar P, Gatei M, Stenzel D, Hobson K, Kozlov S, et al. Oncogene. 1997;14:1911–1921. doi: 10.1038/sj.onc.1201037. [DOI] [PubMed] [Google Scholar]
- 18.Brown K D, Ziv Y, Sadanandan S N, Chessa L, Collins F S, Shiloh T, Tagle D A. Proc Natl Acad Sci USA. 1997;94:1840–1845. doi: 10.1073/pnas.94.5.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter T. Cell. 1995;83:1–4. doi: 10.1016/0092-8674(95)90225-2. [DOI] [PubMed] [Google Scholar]
- 20.De Camilli P, Emr S D, McPherson P S, Novick P. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- 21.Seaman M N J, Burd C G, Emr S D. Curr Opin Cell Biol. 1996;8:549–556. doi: 10.1016/s0955-0674(96)80034-2. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez Y, Desany B A, Jones W J, Liu Q, Wang B, Elledge S J. Science. 1996;271:357–360. doi: 10.1126/science.271.5247.357. [DOI] [PubMed] [Google Scholar]
- 23.Cliby W A, Roberts C J, Cimprich K A, Stringer C M, Lamb J R, Schreiber S L, Friend S H. EMBO J. 1998;17:159–169. doi: 10.1093/emboj/17.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durfee T, Becherer K, Chen P-L, Yeh S-H, Yang Y, Kilburn A E, Lee W-H, Elledge S J. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- 25.Green S, Issemann I, Sheer E. Nucleic Acids Res. 1988;16:369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziv Y, Bar-Shira A, Pecker I, Russell P, Jorgensen T J, Tsarfati I, Shiloh Y. Oncogene. 1997;15:159–167. doi: 10.1038/sj.onc.1201319. [DOI] [PubMed] [Google Scholar]
- 27.Robinson M S. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 28.Serafini T, Stenbeck G, Brecht A, Lottspeich F, Orci L, Rothman J E, Wieland F T. Nature (London) 1991;349:215–220. doi: 10.1038/349215a0. [DOI] [PubMed] [Google Scholar]
- 29.Newman L S, McKeever M O, Okano H J, Darnell R B. Cell. 1995;82:773–783. doi: 10.1016/0092-8674(95)90474-3. [DOI] [PubMed] [Google Scholar]
- 30.Simpson F, Peden A A, Christopoulou L, Robinson M S. J Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duden R, Griffiths G, Frank R, Argos P, Kreis T E. Cell. 1991;64:649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- 32.Okabayashi Y, Sugimoto Y, Totty N F, Hsuan J, Kido Y, Sagaguchi K, Gout I, Waterfield M D, Kasuga M. J Biol Chem. 1996;271:5265–5269. doi: 10.1074/jbc.271.9.5265. [DOI] [PubMed] [Google Scholar]
- 33.David C, McPherson P S, Mundigl O, de Camilli P A. Proc Natl Acad Sci USA. 1996;93:331–335. doi: 10.1073/pnas.93.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L-H, Sudhop T C, Anderson R G W. J Biol Chem. 1995;270:10079–10083. doi: 10.1074/jbc.270.17.10079. [DOI] [PubMed] [Google Scholar]
- 35.Benmerah A, Begue B, Dautry-Varsat A, Cerf-Bensussan N. J Biol Chem. 1996;271:12111–12116. doi: 10.1074/jbc.271.20.12111. [DOI] [PubMed] [Google Scholar]
- 36.Voglmaier S M, Keen J H, Murphy J-E, Feriis C D, Prestwich G D, Snyder S H, Theibert A B. Biochem Biophys Res Commun. 1992;187:158–163. doi: 10.1016/s0006-291x(05)81473-1. [DOI] [PubMed] [Google Scholar]
- 37.Beck K A, Keen J H. J Biol Chem. 1991;266:4442–4447. [PubMed] [Google Scholar]
- 38.Bar R S, Lewis W R, Rechler M M, Harrison L C, Siebert C, Podskalny J, Roth J, Muggeo M. N Engl J Med. 1978;298:1164–1171. doi: 10.1056/NEJM197805252982103. [DOI] [PubMed] [Google Scholar]
- 39.Epstein W, Fudenberg H, Reed W. Int Arch Allergy Appl immunol. 1966;30:15–29. [PubMed] [Google Scholar]
- 40.Ammann A, Cain W, Ischizaka K, Hong R, Good R. N Engl J Med. 1969;281:469–504. doi: 10.1056/NEJM196908282810904. [DOI] [PubMed] [Google Scholar]
- 41.O’Connor R D, Linthicum D S. Clin Immunol Immunopathol. 1980;15:66–75. doi: 10.1016/0090-1229(80)90021-5. [DOI] [PubMed] [Google Scholar]
- 42.Gardner M, Goodman W N. Bull Los Angeles Neurol Soc. 1969;34:23–38. [PubMed] [Google Scholar]
- 43.Jerusalem F, Bischoff A. Z Neurol. 1972;202:128–138. [PubMed] [Google Scholar]
- 44.Schoonderwaldt H, Joosten E, Gabreels L V, Gabreels-Festen A, Korten J J. Psychiatr Neurol Neurochir. 1973;76:459–472. [PubMed] [Google Scholar]
- 45.Faundez V, Horng J-T, Kelly R B. Cell. 1998;93:423–432. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Gaitan M, Jackle H. Cell. 1997;88:767–776. doi: 10.1016/s0092-8674(00)81923-6. [DOI] [PubMed] [Google Scholar]