Abstract
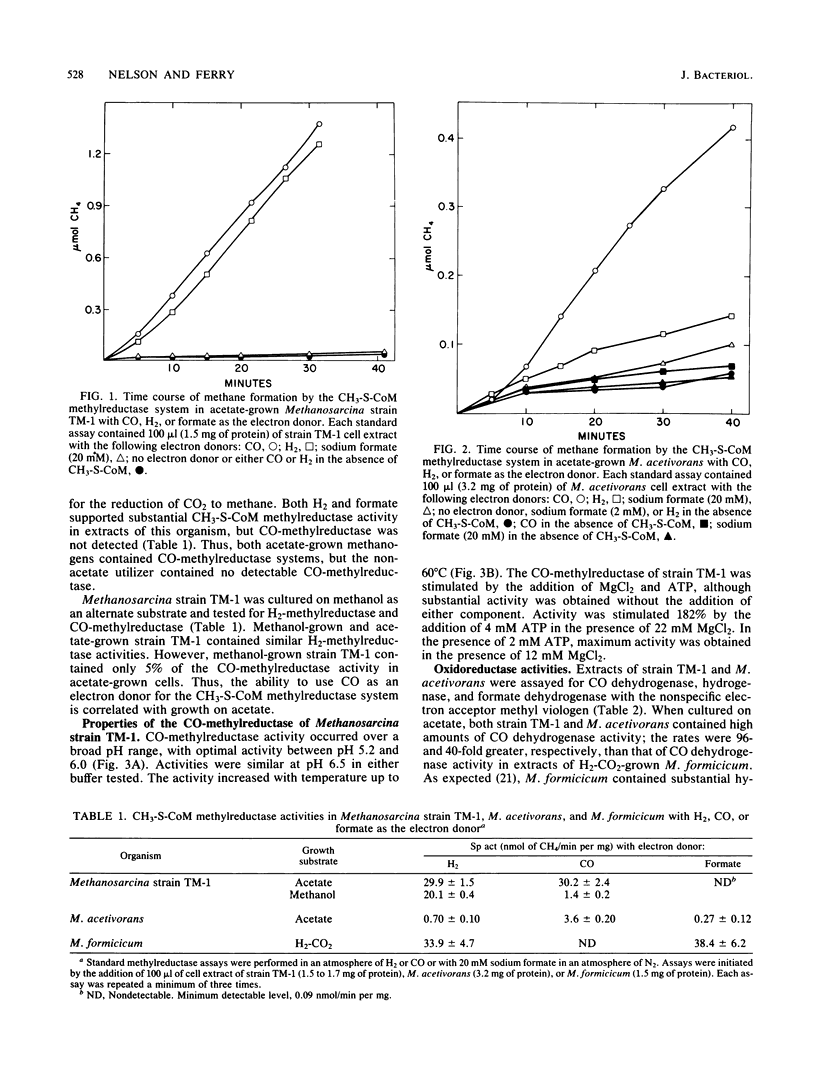
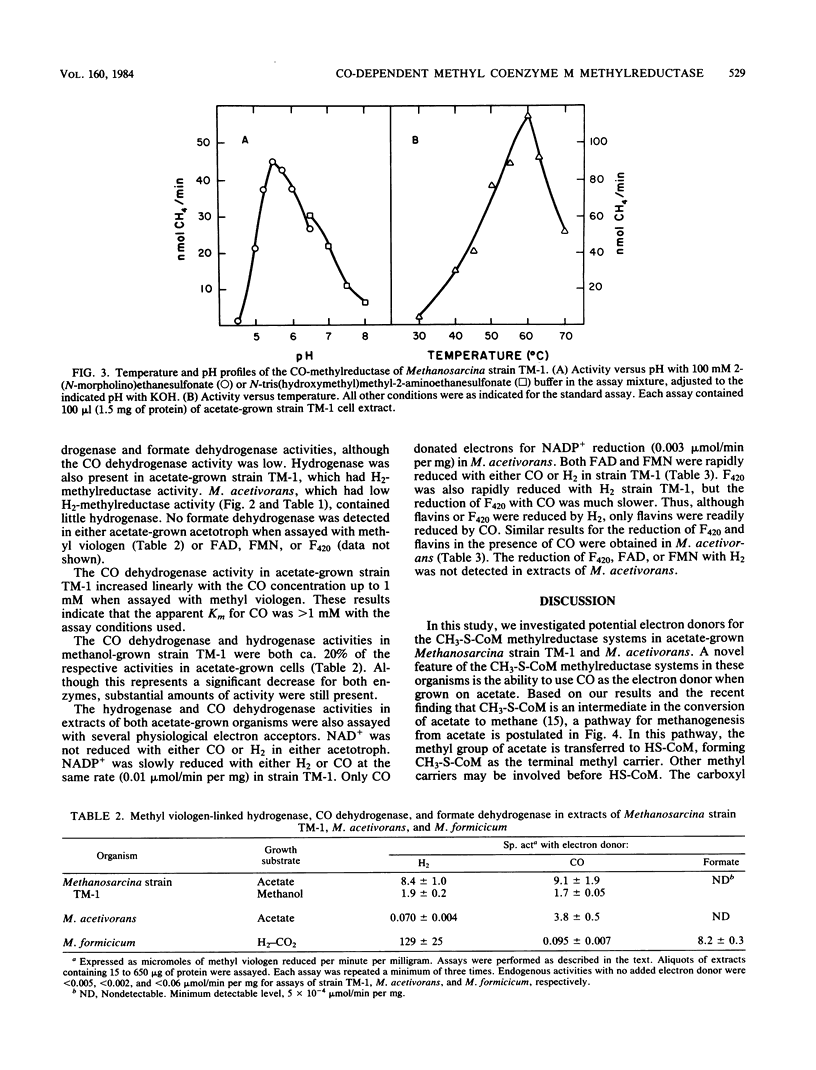
Cell extracts of acetate-grown Methanosarcina strain TM-1 and Methanosarcina acetivorans both contained CH3-S-CoM methylreductase activity. The methylreductase activity was supported by CO and H2 but not by formate as electron donors. The CO-dependent activity was equivalent to the H2-dependent activity in strain TM-1 and was fivefold higher than the H2-dependent activity of M. acetivorans. When strain TM-1 was cultured on methanol, the CO-dependent activity was reduced to 5% of the activity in acetate-grown cells. Methanobacterium formicicum grown on H2-CO2 contained no CO-dependent methylreductase activity. The CO-dependent methylreductase of strain TM-1 had a pH optimum of 5.5 and a temperature optimum of 60 degrees C. The activity was stimulated by the addition of MgCl2 and ATP. Both acetate-grown strain TM-1 and acetate-grown M. acetivorans contained CO dehydrogenase activities of 9.1 and 3.8 U/mg, respectively, when assayed with methyl viologen. The CO dehydrogenase of acetate-grown cells rapidly reduced FMN and FAD, but coenzyme F420 and NADP+ were poor electron acceptors. No formate dehydrogenase was detected in either organism when grown on acetate. The results suggest that a CO-dependent CH3-S-CoM methylreductase system is involved in the pathway of the conversion of acetate to methane and that free formate is not an intermediate in the pathway.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baresi L., Wolfe R. S. Levels of coenzyme F420, coenzyme M, hydrogenase, and methylcoenzyme M methylreductase in acetate-grown Methanosarcina. Appl Environ Microbiol. 1981 Feb;41(2):388–391. doi: 10.1128/aem.41.2.388-391.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Daniels L., Fuchs G., Thauer R. K., Zeikus J. G. Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol. 1977 Oct;132(1):118–126. doi: 10.1128/jb.132.1.118-126.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Component C of the methylreductase system of Methanobacterium. J Biol Chem. 1981 May 10;256(9):4259–4262. [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Role of component C in the methylreductase system of Methanobacterium. J Biol Chem. 1980 Sep 25;255(18):8388–8389. [PubMed] [Google Scholar]
- Ferguson T. J., Mah R. A. Effect of H(2)-CO(2) on Methanogenesis from Acetate or Methanol in Methanosarcina spp. Appl Environ Microbiol. 1983 Aug;46(2):348–355. doi: 10.1128/aem.46.2.348-355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. ATP activation and properties of the methyl coenzyme M reductase system in Methanobacterium thermoautotrophicum. J Bacteriol. 1978 Sep;135(3):851–857. doi: 10.1128/jb.135.3.851-857.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Methyl coenzyme M reductase from Methanobacterium thermoautotrophicum. Resolution and properties of the components. J Biol Chem. 1980 Mar 10;255(5):1891–1895. [PubMed] [Google Scholar]
- Hammel K. E., Cornwell K. L., Diekert G. B., Thauer R. K. Evidence for a nickel-containing carbon monoxide dehydrogenase in Methanobrevibacter arboriphilicus. J Bacteriol. 1984 Mar;157(3):975–978. doi: 10.1128/jb.157.3.975-978.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. I., Drake H. L., Wood H. G. Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and coenzyme A by enzymes from Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):440–448. doi: 10.1128/jb.149.2.440-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzycki J. A., Wolkin R. H., Zeikus J. G. Comparison of unitrophic and mixotrophic substrate metabolism by acetate-adapted strain of Methanosarcina barkeri. J Bacteriol. 1982 Jan;149(1):247–254. doi: 10.1128/jb.149.1.247-254.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzycki J. A., Zeikus J. G. Characterization and purification of carbon monoxide dehydrogenase from Methanosarcina barkeri. J Bacteriol. 1984 Apr;158(1):231–237. doi: 10.1128/jb.158.1.231-237.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., White R. H., Ferry J. G. Identification of methyl coenzyme M as an intermediate in methanogenesis from acetate in Methanosarcina spp. J Bacteriol. 1984 Nov;160(2):521–525. doi: 10.1128/jb.160.2.521-525.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Wolfe R. S. A new coenzyme of methyl transfer, coenzyme M. Biochemistry. 1971 Jun 8;10(12):2317–2324. doi: 10.1021/bi00788a022. [DOI] [PubMed] [Google Scholar]
- Nagle D. P., Jr, Wolfe R. S. Component A of the methyl coenzyme M methylreductase system of Methanobacterium: resolution into four components. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2151–2155. doi: 10.1073/pnas.80.8.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINE M. J., BARKER H. A. Studies on the methane fermentation. XII. The pathway of hydrogen in the acetate fermentation. J Bacteriol. 1956 Jun;71(6):644–648. doi: 10.1128/jb.71.6.644-648.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezacka E., Wood H. G. The synthesis of acetyl-CoA by Clostridium thermoaceticum from carbon dioxide, hydrogen, coenzyme A and methyltetrahydrofolate. Arch Microbiol. 1984 Jan;137(1):63–69. doi: 10.1007/BF00425809. [DOI] [PubMed] [Google Scholar]
- Romesser J. A., Balch W. E. Coenzyme M: preparation and assay. Methods Enzymol. 1980;67:545–552. doi: 10.1016/s0076-6879(80)67067-0. [DOI] [PubMed] [Google Scholar]
- Royburman P., Royburman S., Visser D. W. Incorporation of 5,6-dihydrouridine triphosphate into ribonucleic acid by DNA-dependent RNA polymerase. Biochem Biophys Res Commun. 1965 Jul 26;20(3):291–297. doi: 10.1016/0006-291x(65)90362-1. [DOI] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Metabolism of formate in Methanobacterium formicicum. J Bacteriol. 1980 Jun;142(3):800–807. doi: 10.1128/jb.142.3.800-807.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Properties of formate dehydrogenase in Methanobacterium formicicum. J Bacteriol. 1982 Apr;150(1):1–7. doi: 10.1128/jb.150.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S., Wolfe R. S. Methyl-coenzyme M, an intermediate in methanogenic dissimilation of C1 compounds by Methanosarcina barkeri. J Bacteriol. 1980 Feb;141(2):728–734. doi: 10.1128/jb.141.2.728-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupperich E., Hammel K. E., Fuchs G., Thauer R. K. Carbon monoxide fixation into the carboxyl group of acetyl coenzyme A during autotrophic growth of Methanobacterium. FEBS Lett. 1983 Feb 7;152(1):21–23. doi: 10.1016/0014-5793(83)80473-6. [DOI] [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. Structure and methylation of coenzyme M(HSCH2CH2SO3). J Biol Chem. 1974 Aug 10;249(15):4879–4885. [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Whitman W. B., Wolfe R. S. Activation of the methylreductase system from Methanobacterium bryantii by ATP. J Bacteriol. 1983 May;154(2):640–649. doi: 10.1128/jb.154.2.640-649.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Mah R. A. Isolation and Characterization of a Thermophilic Strain of Methanosarcina Unable to Use H(2)-CO(2) for Methanogenesis. Appl Environ Microbiol. 1979 Nov;38(5):996–1008. doi: 10.1128/aem.38.5.996-1008.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



