Abstract
The lymphatic vascular system mediates fluid homeostasis, immune defense, and tumor metastasis. Only a handful of genes are known to affect the development of the lymphatic vasculature, and even fewer represent therapeutic targets for lymphatic diseases. Adrenomedullin (AM) is a multifunctional peptide vasodilator that transduces its effects through the calcitonin receptor–like receptor (calcrl) when the receptor is associated with a receptor activity–modifying protein (RAMP2). Here we report on the involvement of these genes in lymphangiogenesis. AM-, calcrl-, or RAMP2-null mice died mid-gestation after development of interstitial lymphedema. This conserved phenotype provided in vivo evidence that these components were required for AM signaling during embryogenesis. A conditional knockout line with loss of calcrl in endothelial cells confirmed an essential role for AM signaling in vascular development. Loss of AM signaling resulted in abnormal jugular lymphatic vessels due to reduction in lymphatic endothelial cell proliferation. Furthermore, AM caused enhanced activation of ERK signaling in human lymphatic versus blood endothelial cells, likely due to induction of CALCRL gene expression by the lymphatic transcriptional regulator Prox1. Collectively, our studies identify a class of genes involved in lymphangiogenesis that represent a pharmacologically tractable system for the treatment of lymphedema or inhibition of tumor metastasis.
Introduction
The lymphatic vascular system is an open-ended network of endothelial cell–lined vessels that works to transport extravasated tissue fluid, proteins, metabolites, and cells from the interstitial space back to the circulatory system via the thoracic duct (1). Lymphatic capillaries, which lack a basement membrane, are made of overlapping endothelial cells that adhere to the extracellular matrix through fibrillin-rich, pressure-sensing anchoring filaments. Under conditions of interstitial edema, lymphatic endothelial cells (LECs) stretch apart to collect excess fluid and large proteins. Propulsion of lymph fluid into the larger collecting lymph vessels is largely dependant on the vascular smooth muscle contractions of the upper circulatory trunks. Lymphatic vessels also mediate lipid uptake from the gut and transport white blood cells and antigen-presenting cells to lymphoid organs. Moreover, the lymphatic system plays important roles in pathological conditions such as inflammation, scarring, and tumor metastasis (2–5). Despite its central roles in both normal and disease physiology, our understanding of the development and molecular regulation of the lymphatic vascular system lags far behind that of the parallel blood vascular system (6, 7).
In recent years, advanced genetic engineering techniques have supported the concept that during development, LECs bud from veins to form primary lymph sacs, which then proliferate and sprout into the periphery to form lymphatic capillaries and vessels. One of the earliest molecular markers distinguishing venous endothelial cells with a lymphatic bias is the transcription factor prospero-related homeobox 1 (Prox1) (8). Prox1 is expressed in a polarized fashion in the venous endothelium and serves as a master switch for establishing LEC competence (9). Knockout mice for Prox1 show ablated budding and sprouting of primary lymph sacs and die at E14.5 with severe edema (10). Genetic mouse models with loss of VEGFC signaling also develop severe interstitial edema due to failed lymphatic budding and migration (11). The eventual separation of lymphatic vessels from the venous circulation is largely mediated by the tyrosine kinase Syk and adaptor protein SLP76. Mice with homozygous deletions for the genes encoding these proteins develop arteriovenous shunts and abnormal lymphatic-venous connections (12). Finally, several genes have been implicated in the later stages of lymphatic vessel patterning and postnatal maturation, including podoplanin (13), neuropillin2 (14), FoxC2 (15), and angiopoietin-2 (16). While a few of these gene products, such as VEGFC and angiopoietins, show promise as potential targets for the stimulation, inhibition, or modulation of lymphangiogenesis and lymphatic function in lymphedematous tissues or tumors of adult animals (17), there remains a crucial need to identify additional genetic and pharmacologically tractable targets that regulate the development and function of the lymphatic vascular system (1, 18).
Adrenomedullin (AM) is a multifunctional, 52-aa peptide vasodilator that is pathologically elevated in a variety of tumors (19) and cardiovascular conditions (20). In addition to its function as a potent vasodilator, AM is also recognized as an important vascular factor involved in angiogenesis and endothelial cell proliferation. AM treatment potently induces the proliferation, migration, and capillary tube formation of cultured HUVECs and directly promotes endothelial cell growth and survival through activation of MAPK/ERK downstream signaling pathways (21–26). Similar results have been recapitulated in vivo through the use of a hind-limb ischemia model (27) and a differentiated ES cell culture model (28) in which AM treatment promotes an angiogenic response. Taken together, these data demonstrate that AM signaling in the blood vasculature plays an important role in vessel development, remodeling, and function; however, its role in the lymphatic vascular system has yet to be addressed.
AM binds and signals through a GPCR, calcitonin receptor–like receptor (calcrl, gene; CLR, protein). The ligand-binding affinity of CLR (and other class II GPCRs) can be changed via receptor interaction with a class of single-pass transmembrane proteins called receptor activity–modifying proteins (RAMPs) (29). Since the AM receptor and the 3 mammalian RAMPs are highly expressed in the vasculature, this paradigm of cell signaling is being explored pharmaceutically for the potential treatment of conditions such as migraine (30), pulmonary hypertension (31), and other cardiovascular disorders (32), and for the modulation of cancer progression (33). However, determining the functional significance of AM signaling on the vasculature during embryogenesis and adulthood remains an area of intense investigation that we here address with genetically engineered animals that either lack AM, calcrl, or RAMP2 globally or that lack calcrl specifically in endothelial cells.
Results
AM–/–, calcrl–/–, and RAMP2–/– embryos failed to survive and developed severe interstitial edema without hemorrhage by mid-gestation (Figure 1, A, D, and G). Histology of the AM–/–, calcrl–/–, and RAMP2–/– embryos confirmed the presence of extreme interstitial edema and the notable absence of hemorrhagic plaques (Figure 1, B, C, E, F, H, and I). The onset of edema between the different lines was temporally staggered by approximately 24 hours, after which all null embryos were found dead 1–2 days later. The delay in phenotypic onset in RAMP2–/– embryos suggests that some compensatory signaling for AM, perhaps through a RAMP3-CLR complex, may still exist during embryonic development. However, our previous observations that RAMP3-null mice survive to adulthood with few phenotypic defects (34) demonstrates that RAMP3 is unable to fully replenish RAMP2-mediated AM signaling in vivo. Moreover, we found that RAMP2–/– embryos displayed developmental defects remarkably similar to those we have previously described for AM–/– and calcrl–/– mice (35, 36) (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI33302DS1). Therefore, this conserved phenotypic series from 3 independent knockout mouse lines provides compelling genetic evidence to define CLR and RAMP2 as the receptor components required for AM signaling during embryogenesis.
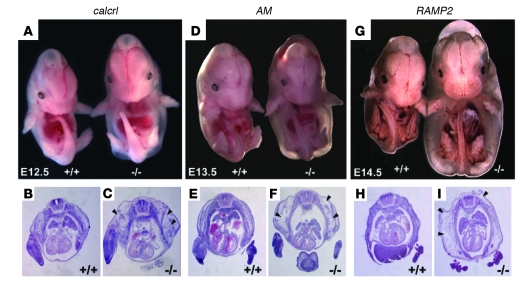
Figure 1. Phenotypic series of AM signaling–null mice.
Genetic deletion of genes responsible for AM signaling results in a phenotypic series of null mice with generalized edema and lethality at mid-gestation. (A) Generalized interstitial edema, without hemorrhage, was observed throughout calcrl–/– embryos at E12.5. Similar edema was present by E13.5 in AM–/–embryos (D) and by E14.5 in RAMP2–/– embryos (G). Edema was not observed 1 day before onset, and no viable embryos were recovered 1–2 days after edema onset. H&E-stained transverse sections of wild-type littermates (B, E, and H) and calcrl–/–, AM–/–, and RAMP2–/– (C, F, and I) embryos at E12.5, E13.5, and E14.5, respectively. Arrowheads indicate interstitial fluid accumulation. Original magnification, ×10 (A, D, and G); ×20 (B, C, E, F, H, and I).
Generalized edema and mid-gestation lethality can be caused by a multitude of developmental defects during embryogenesis. To determine whether the loss of AM signaling in vascular endothelial cells could account for the embryonic edema and/or lethality of global AM–/–, calcrl–/–, and RAMP2–/– embryos, we generated mice with a conditional allele for the calcrl gene. Using the Cre-loxP gene targeting strategy, we generated homozygous floxed mice for the calcrl gene and confirmed correct gene targeting by Southern blot and genomic PCR (Figure 2, A–C). Importantly, our gene targeting approach ensured that the loxP sites flanked the same genomic regions as those deleted in the original calcrl-knockout mouse (36), thereby eliminating the possibility of generating allelic phenotypes. Homozygous floxed calcrl mice (calcrlFlox/Flox) appeared normal, bred well, and had levels of calcrl gene expression that were indistinguishable from those of wild-type animals (Figure 2D).
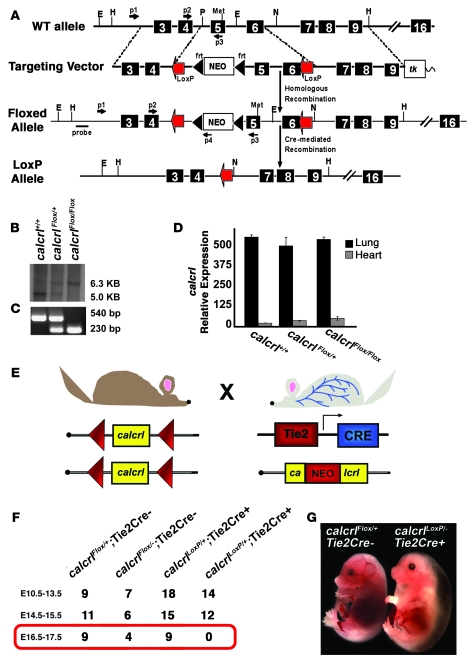
Figure 2. Generation and characterization of conditional calcrl line.
(A) Schematic diagram depicting strategy used for generation of a floxed calcrl allele by gene targeting. The top figure shows the endogenous wild-type calcrl allele. The targeting vector was designed so that loxP sites would flank the same exons that were deleted in the calcrl global knockout (36). The third line depicts the targeted calcrlFloxallele, and the fourth line depicts the calcrlLoxP allele after Cre-mediated excision. Primers used for isolation of correctly targeted ES cells and for routine genotyping are indicated by small arrows. (B) Correctly targeted ES cells were confirmed by Southern blot analysis using the probe depicted in A. (C) PCR genotyping for the calcrlFlox allele. (D) Quantitative RT-PCR was performed on RNA isolated from lungs and hearts of wild-type and homozygous calcrlFlox/Flox mice and revealed no significant differences in the expression of the calcrlFlox allele before Cre-mediated excision. (E) Schematic representation of breeding scheme used to generate mice with calcrl expression deleted specifically in endothelial cells by use of the Tie2Cre transgene. (F) Results of cross demonstrate that no viable calcrlLoxP/–Tie2Cre+mice were found beyond E16.5. (G) Compared with calcrlLoxP/+Tie2Cre+control littermates, the calcrlLoxP/–Tie2Cre+ mice displayed remarkable hydrops without hemorrhage, which phenocopied the global calcrl–knockout phenotype, yet often occurred substantially later, at E16.5. Original magnification, ×10.
As shown in the schematic of Figure 2E, we bred the calcrlFlox/Flox mice to mice that were heterozygous for the calcrl gene knockout and positive for expression of Cre recombinase in endothelial cells via expression of a Tie2-Cre transgene (37). By breeding onto a calcrl+/– genetic background, we ensured robust excision of a single floxed allele and thereby reduced the incidence of mosaic cellular excision. Importantly, calcrl+/– mice are born at the expected Mendelian ratio and survive to adulthood with few phenotypic defects (34). The resulting mice from the Cre-loxP breeding strategy, calcrlLoxP/–Tie2Cre+, were therefore global heterozygotes for calcrl and lacked calcrl expression specifically in endothelial cells as early as E10.5. We noticed a slight reduction in the Mendelian distribution of calcrlFloxP/–Tie2Cre– mice (Figure 2F), which is consistent with the leakiness of the Tie2Cre transgene in the female germline leading to global calcrl excision and embryonic lethality (37). Consistently, the calcrlLoxP/–Tie2Cre+ mice were embryonic lethal and developed extreme interstitial edema without hemorrhage, sometimes as early as E13.5 (Figure 2, F and G). However, in contrast to the global calcrl–/– mice, which die by E13.5, the onset of edema and eventual demise of the calcrlLoxP/–Tie2Cre+mice was often substantially delayed to approximately E16.5, 3–4 days later than the global calcrl–/– mice. Thus, we conclude that endothelium-specific deletion of calcrl prolonged the survival of AM signaling mutants during development but nevertheless resulted in generalized edema without hemorrhage similar to that in global calcrl-knockout mice. These data demonstrate that the cellular basis of the embryonic edema and eventual lethality in AM signaling mutant mice is due to loss of AM signaling in endothelial cells.
Despite the well-established role for AM in maintaining vascular permeability (38, 39), we very rarely observed blood hemorrhage, and only occasionally in embryos that were visibly deteriorated or near death. For example, of 72 wild-type embryos, 7 (9.7%) exhibited some form of visible hemorrhage, and only 4 of 56 RAMP2–/– littermates (7.1%) showed evidence of hemorrhage. Thus, the remarkable lack of embryonic hemorrhage in the AM–/–, calcrl–/–, and RAMP2–/– embryos suggested to us that the developing blood vasculature remained largely intact during embryonic development. Moreover, presence of high-MW proteins in the interstitial fluid confirmed that the null mice suffered from embryonic lymphedema as opposed to osmotic imbalance (Supplemental Figure 2). Thus, based on the absence of blood hemorrhage, the presence of embryonic lymphedema, and the fact that the edematous phenotype of AM signaling–null mice more closely resembled that of other mouse models with defects in lymphangiogenesis (10, 11, 14) rather than defects in blood vascular permeability (40–43), we hypothesized that AM signaling might be principally important for orchestrating normal lymphangiogenesis.
First, we demonstrated that AM, calcrl, and RAMP2 are expressed in adult lymphatic vessels at levels similar to those observed in whole embryonic RNA extracts (Figure 3A). To determine whether AM was also expressed in lymphatic vessels during embryonic development, we made use of an EGFP that was inserted at the initiator methionine of the endogenous AM gene by homologous recombination and serves as a biological marker of AM gene expression in AM+/– and AM–/– mice (35). As shown in Figure 3B, AM was expressed in endothelial cells lining the jugular vein and budding lymph sac at E12.5, which correlates with the earliest stages of lymphatic vascular development. Interestingly, we noticed that the pattern of AM expression in the jugular vein was often polarized toward the developing lymph sac, similar in pattern to that of the master transcriptional regulator of lymphatic specification, Prox1 (9). We therefore conclude that the expression of genes required for AM signaling is robust in adult and developing LECs and is similar in pattern to that of Prox1 during embryogenesis.
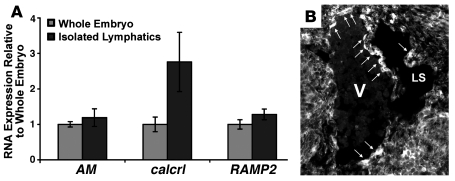
Figure 3. AM signaling components are expressed in adult and developing lymphatic vessels.
(A) Isolated adult lymphatic vessels express AM, calcrl, and RAMP2 at levels similar to whole embryo RNA extracts. Adult mouse lymphatic vessels were identified by the uptake of Evans blue dye injected into the hind paw and were subsequently microdissected for RNA isolation. Expression of AM, calcrl, and RAMP2 mRNAs in these vessels was comparable to mRNA levels in E10–E12.5 embryos by quantitative RT-PCR. n > 4 samples, analyzed twice in triplicate. (B) To determine whether LECs express AM during development, we used an EGFP that serves as a biological marker of AM gene expression in AM gene–targeted mice (35). Unstained transverse sections through the jugular region of an E12.5 AM+/– embryo show that AM is expressed in endothelial cells of both the jugular vein (V) and jugular lymph sac (LS) (arrows). Note the polarized expression of AM in the progenitor cells, which will eventually migrate to form the lymph sac. Original magnification, ×200.
We next performed immunohistochemistry using LEC markers to determine whether AM signaling was required for the initial formation of jugular lymph sacs. Distinct lymph sacs were present in wild-type, RAMP2–/–, and AM–/–embryos by E13.5 (Figure 4, A–C) and stained positively for PECAM and the lymphatic-specific makers VEGFR3 and Prox1, indicating normal lymphatic differentiation from venous vasculature. Similar results were observed in calcrl–/– embryos (data not shown). We also identified distinct VEGFR3-positive vessels in the skin of RAMP2–/– embryos (Figure 4, D and E), indicating that the development of the dermal lymphatic vasculature remains intact in AM signaling–mutant mice. From these findings, we conclude that AM signaling is not required for the early stages of lymphangiogenesis, including differentiation and migration of LECs and the formation of primary lymph sacs and dermal lymphatic vessels.
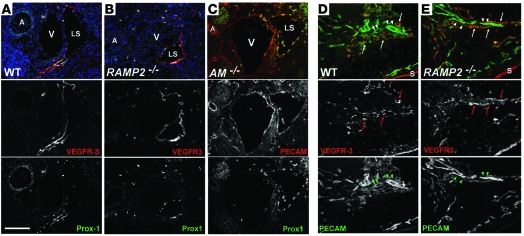
Figure 4. Differentiated lymph sacs and dermal lymphatics are present in AM signaling–null embryos.
Immunofluorescent staining of transverse sections through the jugular region (A–C) and sagittal sections through the skin (D and E) of E13.5 embryos. (A) Endothelial cells of lymph sacs from wild-type mice at E13.5 expressed VEGFR3 (red) and Prox1 (green). (B) Similar staining was observed in the lymph sacs of RAMP2–/– littermates. Nuclei stained with Hoechst are indicated in blue (A and B). (C) Prox1 (green) was also correctly coexpressed with PECAM (red) in lymph sacs of AM–/– embryos. (D and E) Dermal lymphatics (arrows) were present in wild-type (D) and RAMP2–/– (E) littermates at E14.5 as shown by VEGFR3 (red) and PECAM (green) staining. Dermal blood endothelium (arrowheads) expresses PECAM but not VEGFR3. n > 6 embryos per genotype. A, carotid artery, A; S, skin. Original magnification, ×200. Scale bar: 100 μM.
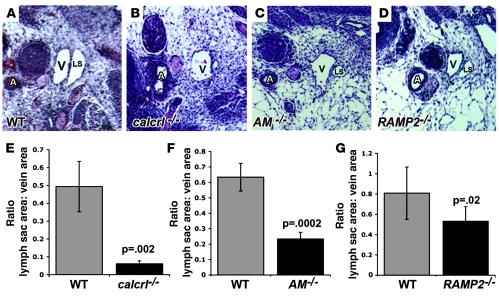
Although immunohistochemistry revealed the presence of lymph sacs in the AM signaling–null mouse models, we noticed from histological sections that the jugular lymph sacs appeared strikingly smaller than those of litter-matched wild-type embryos (Figure 5, A–D). To confirm the apparent hypoplasia of the lymph sacs, we used computerized morphometry to calculate lymph sac area and found that all null mice showed significantly reduced lymph sac area compared with wild-type embryos at E13.5 (Figure 5, E–G). Thus, despite excessive interstitial lymphedema (which would normally cause lymphatic vessel distension), the lymph sacs of the AM signaling–null embryos remained significantly smaller than wild-type lymph sacs, suggesting either a functional failure of the early lymphatics to take up extravasated fluid or an abnormality in LEC growth and proliferation.
Figure 5. Loss of AM signaling results in hypoplastic lymph sacs.
Lymph sacs of calcrl–/–, AM–/–, and RAMP2–/– embryos were significantly smaller than those of their WT littermates at E13.5. (A–D) H&E-stained sections through transverse sections of the jugular lymph sacs of wild-type (A), calcrl–/– (B), AM–/– (C), and RAMP2–/– (D) embryos at E13.5. Note the remarkable decrease in size of lymph sacs relative to jugular vein and carotid artery in the AM signaling–mutant embryos, despite massive interstitial edema. (E–G) Quantitation of lymph sac area, normalized to jugular vein area in calcrl–/– (E), AM–/– (F), and RAMP2–/– (G) embryos (black bars) and their wild-type littermates (gray bars) at E13.5. E, F, and G have different scales. n > 6 embryos per genotype. Error bars indicate SEM. P values were determined using Student’s s test. Original magnification, ×200.
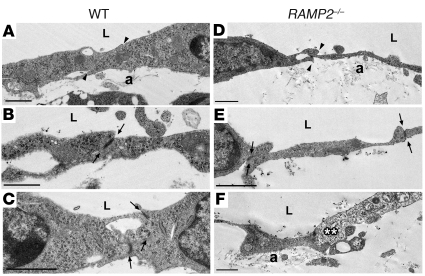
To determine whether an ultrastructural defect in LECs could lead to a functional failure of lymphatic vessels during development, we performed transmission electron microscopy of RAMP2–/– and wild-type mice at E14.5. LECs of the jugular lymphatic vessels in wild-type mice displayed typical features, including overlapping cell-cell contacts (Figure 6A, arrowheads), electron-dense tight junctions (Figure 6, B and C, arrows), and anchoring filaments (Figure 6A). Wild-type lymphatic vessels at this stage of development also lacked a basement membrane and lymphatic smooth muscle cells (see also Supplemental Figure 3), thus precluding the possibility that failure of smooth muscle recruitment could account for the lymphatic phenotypes observed in the AM signaling–null mice. In RAMP2–/– mice the overlapping cell-cell contacts, tight junctions, and anchoring filaments remained intact (Figure 6, D and E). Occasionally, necrotic LECs were observed in RAMP2–/– mice (Figure 6F), but the tight junctions nevertheless remained intact. Consistently, we found that RAMP2–/– LECs appeared markedly thinner than wild-type LECs. Based on these data, we found no substantial evidence to suggest that the lymphatic vasculature of the RAMP2–/– mice would fail to function due to ultrastructural abnormalities.
Figure 6. LECs of RAMP2–/– mice are thin with otherwise normal ultrastructural features.
Transmission electron microscopy of wild-type (A–C) and RAMP2–/– (D–F) lymph sacs at E14.5. In general, the LECs of RAMP2–/– mice appeared significantly thinner than those of wild-type littermates. Although obvious edema was present in the interstitial space surrounding both the veins and lymph sacs in RAMP2–/– embryos, endothelial cells could be followed continuously around both vessel types, without obvious breaks or gaps. Other typical ultrastructural features of lymphatic vessels were identified in both wild-type and RAMP2–/– mice including: overlapping contacts (arrowheads), tight junctions (arrows), and anchoring filaments (A). Occasionally necrotic endothelial cells (**) were observed in RAMP2–/– mice, but the associated tight junctions remained structurally intact. n = 2 wild-type and 3 RAMP2–/–. L; lumen. Scale bar: 1 μm.
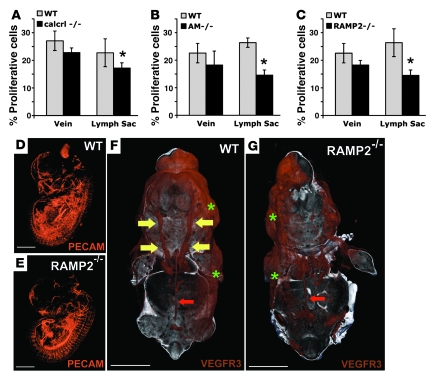
To determine whether the smaller lymph sacs of AM signaling–mutant mice could be due to abnormal endothelial cell proliferation, we used a BrdU incorporation assay to count proliferating cells in both lymph sacs and adjoining jugular veins. In AM–/–, RAMP2–/–, and calcrl–/– embryos, there were significantly fewer proliferative endothelial cells in the lymph sacs; the proliferation of the venous endothelial cells was somewhat reduced in the null mice, but the difference compared with wild-type mice was not statistically significant (Figure 7, A–C). Taken together these results demonstrate that lack of AM signaling during embryonic development leads to a specific reduction in lymphatic, but not venous, endothelial cell proliferation.
Figure 7. AM signaling is required for the proliferation and growth of jugular lymphatic vessels during embryonic development.
(A–C) The percentage of proliferating cells relative to total cells was determined in the jugular vein and neighboring jugular lymph sac of wild-type (gray bars) and calcrl–/–, AM–/–, and RAMP2–/– (black bars, A–C, respectively) littermate embryos. Percent proliferative cells was defined as the number of BrdU-positive endothelial cells divided by the total number of endothelial cells in each vessel. Error bars indicate SEM. *P = 0.01 for calcrl–/– (A), P = 0.004 for AM–/– (B), and P = 0.01 for RAMP2–/– (C); Student’s t test. n > 6 animals per genotype. (D and E) Whole mount immunofluorescence of developing vasculature identified by PECAM staining and visualized by 3D OPT of wild-type (D) and RAMP2–/– (E) littermate embryos at E13.5. (F and G) Whole mount immunofluorescence of developing lymphatic vasculature identified by VEGFR3 staining and visualized by 3D OPT of wild-type (F) and RAMP2–/– (G) littermate embryos at E14.5. Compare the presence of well-formed jugular lymph sacs in the wild-type embryo (yellow arrows in F) with the relative lack of jugular lymphatic vessels in the RAMP2–/– littermate (G). Note that the retroperitoneal lymph vessel (red arrows) and dermal lymphatic vessels (green asterisks) of RAMP2–/– mice appear normal compared with those of wild-type embryos. Three embryos from 2 different litters were stained for PECAM and VEGFR3. The online supplemental data contains movies for enhanced, high-resolution, 3D viewing (Supplemental Figures 4 and 5). Scale bar: 2,000 μM.
To confirm that loss of AM signaling preferentially affected the development of the lymphatic versus blood vascular system, we used whole mount immunohistochemistry for VEGFR3 or PECAM followed by 3D optical projection tomography (OPT) (44) in RAMP2–/– mice. While we found no obvious differences in the morphological development of the blood vascular system between wild-type and knockout mice (Figure 7, D and E), we did observe extraordinary differences in the jugular lymphatic trunks (Figure 7, F and G, large yellow arrows). While wild-type mice had large, well-formed jugular lymphatic trunks, the same vessels were essentially absent in the RAMP2–/– littermates (Figure 7 and Supplemental Movies 4 and 5). Notably, the retroperitoneal lymph vessel (which develops into the thoracic duct) and the dermal lymphatic vessels of RAMP2–/– embryos appeared indistinguishable from those of wild-type littermates (Figure 7, F and G, small red arrows, and Figure 4, D and E). Therefore, we conclude that AM signaling is preferentially required for the normal proliferation and morphological integrity of the jugular lymphatic vessels during the later stages of lymphangiogenesis.
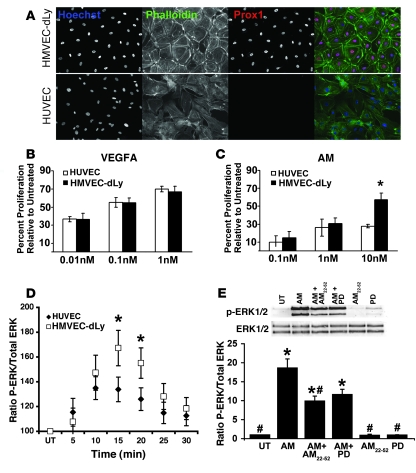
To determine the downstream signaling cascades that are associated with the enhanced activity of AM signaling in LEC proliferation, we made use of 2 distinct endothelial cell lines: HUVECs and human dermal lymphatic microvascular endothelial cells (herein referred to as HMVEC-dLy). Histology and immunohistochemistry revealed that HMVEC-dLys and HUVECs differed in cellular morphology and their expression of Prox1, a finding that is consistent with the characterization of the former as lymphatic endothelial cells (Figure 8A). Stimulation of HUVECs and HMVEC-dLys with VEGFA resulted in an expected and dose-dependant increase in cellular proliferation that did not differ significantly between the 2 cell lines (Figure 8B). In contrast, while AM elicited a modest yet significant increase in HUVEC proliferation, the proliferative effects of AM on HMVEC-dLys were substantially more robust and significantly different from the effects of AM on HUVEC proliferation at 10 nM (Figure 8C). Direct cell count determinations verified that HMVEC-dLys treated with 10 nM AM for 48 hours showed 2-fold more proliferation than similarly treated HUVECs (81% vs. 37% more than control, P < 0.01; data not shown).
Figure 8. AM signaling preferentially mediates enhanced ERK activation in HMVEC-dLys compared with HUVECs.
(A) Cultured HMVEC-dLys and HUVECs are morphologically and genetically distinct cell lines based on histology and expression pattern of Prox1. Original magnification, ×400. (B) Stimulation of HUVECs and HMVEC-dLys with the potent growth factor VEFGA resulted in a dose-dependant increase in cell proliferation that was not significantly different between the 2 cell lines. Data represent averages of 4 independent experiments, each performed in duplicate. (C) Stimulation of HUVECs and HMVEC-dLys with AM peptide resulted in a dose-dependant increase in cell proliferation that was significantly greater in HMVEC-dLys compared with HUVECs. *P < 0.05. Data represent averages of 4 independent experiments, each performed in duplicate. (D) Stimulation of HUVECs and HMVEC-dLys with 10 nM AM peptide resulted in a significantly greater induction of ERK phosphorylation in HMVEC-dLys compared with HUVECs over a 30-minute time course. *P < 0.03 at 15- and 20-minute time points. Data represent averages of 3 independent experiments, each performed in duplicate. (E) Induction of ERK activation by AM stimulation in HMVEC-dLys was significantly reduced by the RAMP2-specific peptide inhibitor AM22-52 and completely blocked by the MAPK inhibitor PD98057 (PD). *P < 0.05 compared with untreated; #P < 0.05 compared with AM-treated. Data represent averages of 3 independent experiments, each performed in duplicate.
To determine the downstream signaling pathways associated with the effects of AM signaling on HMVEC-dLy proliferation, we quantitatively assessed the activation of ERK in HUVECs and HMVEC-dLys that were treated with 10 nM AM. Using a Gaussian fit curve analysis, we found that AM stimulation of HMVEC-dLys resulted in approximately twice the overall levels of phosphorylated ERK over a 30-minute time course compared with HUVECs (Figure 8D). Inhibition of AM-mediated ERK phosphorylation by a RAMP2-specific AM inhibitor, AM22–52 (45), and by the MAPK inhibitor PD98057 confirmed that ERK activation by AM signaling in HMVEC-dLys is mediated through a RAMP2-CLR receptor complex (Figure 8E). Taken together, these comparative studies in cultured endothelial cells demonstrate that AM signaling, through a RAMP2-CLR receptor complex, has a preferential and enhanced effect on ERK-mediated proliferation of HMVEC-dLys compared with HUVECs.
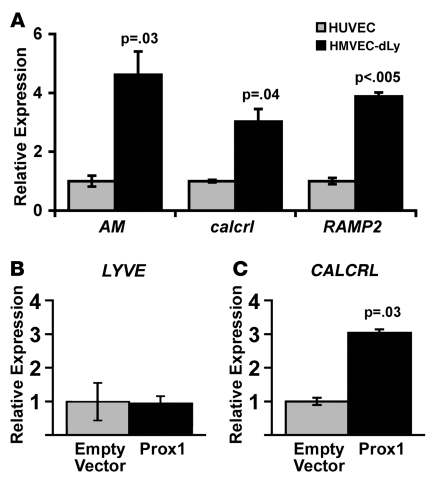
Several comparative transcriptional profiling studies have shown enhanced expression of AM signaling genes in lymphatic versus blood endothelial cells (46, 47) and lymphedematous versus normal vasculature (48). Therefore, we hypothesized that the enhanced sensitivity of LECs to AM signaling could be due to preferential upregulation of the receptor components required for AM signaling. Quantitative RT-PCR for AM, calcrl, and RAMP2 confirmed our assumptions and showed approximately 4-fold higher levels of gene expression in cultured HMVEC-dLys compared with HUVECs (Figure 9A). Because we had previously observed that the expression of AM in vivo closely matched the pattern of Prox1 expression in the developing lymphatic sacs of E13.5 embryos (Figure 3B), we next wanted to determine whether the AM receptor, calcrl, could be a downstream target gene of Prox1. Therefore, we transfected a hProx1 expression plasmid into cultured HMVEC-dLys and measured mRNA production of endogenous LYVE and calcrl. Consistent with previous studies showing that Prox1 is not required for the lymphatic-specific expression of LYVE (10), overexpression of Prox1 in HMVEC-dLys did not induce the expression of LYVE (Figure 9B). In contrast, overexpression of Prox1 resulted in a 3-fold increase in endogenous calcrl expression levels compared with empty vector control transfections (Figure 9C). We therefore conclude that the expression of genes required for AM signaling is preferentially robust in HMVEC-dLys compared with HUVECs and furthermore inducible by Prox1.
Figure 9. AM signaling genes are preferentially upregulated in LECs via Prox1 induction.
(A) Comparative gene expression analysis for hAM, hcalcrl, and hRAMP2 in cultured HUVECs versus LECs, demonstrating significantly higher levels of gene expression in LECs compared with HUVECs. Data represent averages of 3 independent experiments, each performed in duplicate. (B) As expected, the endogenous expression of the lymphatic-specific marker LYVE was not induced by transient overexpression of hProx1 in LECs. (C) In contrast, expression of endogenous calcrl was potently induced by transient overexpression of hProx1 in LECs. Data presented in B and C are from 1 experiment and representative of 3 independent transfections each performed in triplicate.
Discussion
We have performed a comparative phenotypic characterization of 3 independent gene-knockout models for genes that encode proteins purported to be involved in signaling for AM peptide. The remarkable conservation in phenotypes between knockout mice with targeted loss of either AM peptide, the GPCR CLR or its modifying protein, RAMP2, provides compelling genetic evidence to define the receptor components required for AM signaling during embryogenesis. The paradigm of RAMP/GPCR signaling offers the exciting opportunity to generate pharmacological compounds that specifically interact at the RAMP-GPCR interface for modulating the activity of several peptide agonists. As an example, the small-molecule compound BIBN4096BS (49) specifically binds to RAMP1-CLR complexes to antagonize CGRP activity and is currently in clinical trials for treatment of migraine (50). Our conserved phenotypic series of null mouse models suggest that compounds specific to the RAMP2-CLR interface would provide the best pharmacological target for modulation of AM activity in vivo.
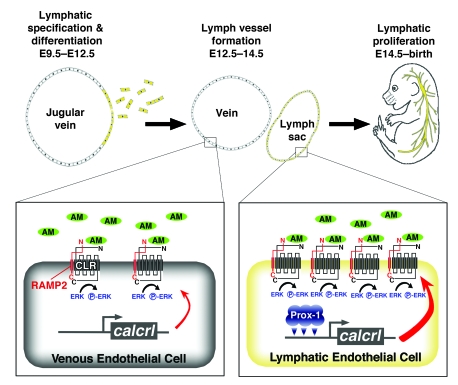
AM is a secreted peptide vasodilator that is highly expressed in blood endothelial cells and induced by hypoxia to promote angiogenesis and inhibit vascular permeability (31, 51). Here we show that AM and its receptors are more highly expressed in HMVEC-dLys under the control of the lymphatic-specific transcriptional regulator Prox1. Therefore, the enhanced level of CLR receptor expression disposes LECs to a more robust AM response than blood endothelial cells (Figure 10). As a consequence, global genetic loss of AM, calcrl, or RAMP2 causes marked and preferential reduction in the proliferation of LECs of the jugular lymphatic vessels. Our current explanation for the cause of edema in these mouse models is that lack of lymphatic proliferative signals during lymphangiogenesis results in smaller, lower-capacity jugular lymphatic vessels that are unable to accommodate the normal uptake of extravasated fluid and thus exacerbates massive interstitial edema. It is also worth emphasizing that the precise cause of embryonic lethality has yet to be determined.
Figure 10. Model for role of AM signaling in lymphangiogenesis.
Preferential upregulation of AM signaling genes in LECs leads to robust AM signaling and enhanced activation of ERK phosphorylation in lymphatic versus blood endothelial cells, which is essential for maintaining normal lymphatic vessel proliferation from E14.5 to birth.
A multitude of developmental defects during embryogenesis can lead to edema and mid-gestation lethality. For example, we cannot at this time exclude the possibility that global loss of AM signaling might also cause excessive permeability of the blood vasculature, contributing to the overall hydrops phenotype. In this regard, our data suggest that global loss of AM, calcrl, or RAMP2 results in thin vascular smooth muscle coverage of large blood vessels (35, 36) (Supplemental Figure 1, E and F). However, based on the absence of hemorrhage and the remarkable similarity of our phenotype to that of other genetically engineered mouse models with defects in lymphangiogenesis (10, 11, 14) (rather than mouse models with leaky blood vasculature or thin vascular smooth muscle walls; refs. 40–43), we contend that the major contribution to the overall hydrops phenotype in AM signaling–null mice comes from the loss of gene function in the lymphatic vasculature. We have also shown that global loss of AM, calcrl, or RAMP2 results in smaller hearts with thin compact zones and ventricular trabeculae (35, 36) (Supplemental Figure 1, A–D), and heart failure can sometimes contribute to embryonic edema. However, since the AM signaling–null mice do not suffer from hepatic congestion or bradycardia and since the edema is characteristically generalized interstitial edema (as opposed to pericardial effusion, which most often accompanies embryonic heart failure), we believe that it is unlikely that heart failure is the principal cause of embryonic edema.
Our generation and characterization of mice with conditional loss of calcrl in vascular endothelial cells further supports our interpretation for a principal and essential role for AM signaling in endothelial cells during embryonic development. Since expression of the Tie2Cre transgene precedes the onset of lymphangiogenesis in embryonic veins and since the Tie2 promoter is active in developing LECs (52), it is likely that excision of the calcrl gene also occurs robustly in the developing LECs of the calcrlLoxP/–Tie2Cre+ mice. However, the Tie2Cre transgene is also expressed in cells of the hematopoietic lineage, which have modest expression of AM signaling components (53, 54) and have been previously demonstrated to contribute to lymphatic vascular development (55). Therefore, we cannot exclude the possibility that loss of AM signaling in the hematopoietic lineage also contributes to the overall phenotype of the calcrlLoxP/–Tie2Cre+ mice. Similarly, we cannot exclude the possibility that loss of AM signaling in cardiac structures derived from Tie2Cre-expressing progenitors (for example, mesenchymal cells of the atrioventricular canal and the proximal outflow tract) may contribute to the phenotype. Future studies with conditional deletion of the calcrl gene exclusively in blood endothelial cells, LECs, or hematopoietic cells will help address these caveats, but suitable Cre-transgenic mouse lines with exclusive expression of Cre in these cell types must first be generated.
Hypoplastic growth of the lymphatic vasculature during development has also been described in mice that lack neuropillin2 (14), a coreceptor for the lymphangiogenic growth factor VEGFC. One assumption that can be made from these apparently similar phenotypes is that VEGFC and AM signaling may interact simultaneously to control the proliferation of the developing lymphatic vessels during embryogenesis. However, in contrast to the AM signaling–null phenotype, loss of neuropillin2 selectively and transiently compromised the proliferation of smaller lymphatic vessels and capillaries, but not jugular lymphatic vessels, during development. Together, these results demonstrate that the development of small and large lymphatic vessel populations is under the control of different lymphangiogenic factors and that these growth factors may work autonomously in lymphatic vessels that arise from different progenitor populations.
Our current study and those of others (21–26) show that AM signaling directly promotes endothelial cell growth and survival through activation of MAPK/ERK downstream signaling pathways. In contrast, the Sprouty/Spred family of proteins mediates an important negative regulation of growth factor– and cytokine-induced MAPK/ERK activation. Recently, Taniguchi et al. showed that mice with double genetic deletion of Spred-1 and -2 develop marked edema and dilated, blood-filled lymphatic vessels (56). While the number of blood vessels and blood endothelial cells in Spred-1/2–deficient embryos did not differ from that in wild-type embryos, the authors found a significant increase in the number of LECs and lymphatic vessels that was due to abnormal loss of VEGFC/VEGFR-3 inhibition and subsequent higher ERK activation. The phenotype of hyperplastic lymphatic vascular growth by enhanced ERK activation in Spred-1/2–knockout mice stands in notable contrast to our AM signaling–null phenotype of hypoplastic lymphatic vascular growth caused by decreased ERK activation in HMVEC-dLys. Taken together, the comparative findings from the two studies are entirely consistent with the notion that the tight control of ERK activation by vascular growth factors is essential for the controlled proliferation of LECs during development.
Failure or insufficient function of the lymphatic vascular system results in disfiguring, disabling, and sometimes life-threatening swelling of the limbs, called lymphedema (57), which most often occurs as a result of a parasitic filarial infection or secondary to surgical removal of lymph nodes, radiation therapy, or infection (2). Recent studies have also brought the lymphatic system to the forefront as an important route of tumor metastasis (3, 6), the major cause for treatment failure and decreased survival in cancer patients. Thus, a concerted effort is being made to identify new genetic and/or pharmacological targets for the management and treatment of lymphedema and for inhibiting the metastatic spread of cancer cells. Ideally, these treatments would improve lymphatic permeability, induce the controlled proliferation of the existing lymphatic vasculature, or inhibit tumor lymphangiogenesis. The elucidation of a previously unrecognized role for AM signaling in orchestrating embryonic lymphangiogenesis considerably expands our understanding of the relatively small repertoire of molecular regulators controlling this process. Moreover, the conserved phenotypic series of mice is the first genetic, in vivo data to our knowledge confirming that RAMP2 interaction with calcrl is required for AM signaling during embryonic development. Therefore, further characterization of AM signaling and its pharmacological modulation via RAMP2-CLR effectors might lead to novel therapeutic strategies for the treatment of lymphedema or inhibition of tumor metastasis.
Methods
An expanded Methods section can be found in Supplemental Methods.
Animals.
The generation and characterization of mice with targeted deletions of the AM, calcrl, and RAMP2 genes has been described previously (34–36). Gene targeting for the AM, calcrl, and RAMP2 genes was performed with targeting vectors and ES cells of the SvEv129/6-TC1 background. Chimeric male mice were then bred to SvEv129/6-TC1 female mice in order to establish completely isogenic colonies. In the case of RAMP2, we have previously reported that on the SvEv129/6-TC1 isogenic background, there are profound fertility defects (34). These fertility defects are rescued on an F1 (SvEv129/6-TC1:C57Bl6) background, and so animals derived from F1 intercrosses were used for analysis in the current study.
To generate calcrlFlox/Flox mice, a calcrl floxed targeting vector was generated using a 129S6/SvEv phage clone (described in ref. 36) containing exons 3–9 of the calcrl gene. loxP sites were inserted outside of exons 5 and 6. The long arm of homology included exons 7, 8, and 9, while the short arm included exons 3 and 4. Standard gene targeting methods were utilized to generate ES cells and mice with the floxed calcrl allele. Briefly, E14 ES cells were electroporated with the linearized targeting vector shown in Figure 2A. After positive (G418) and negative (ganciclovir) selection, 7 positive ES cell clones were identified from 96 selected clones. Primers p1 and p4 were used for PCR-based screening of ES cells and primers p2, p3, and p4 were used for genotyping of mice (Figure 2A and Supplemental Figure 6). Male chimeric mice that transmitted the targeted allele were bred to C57BL/6J females, and homozygous calcrlFlox/Flox mice were generated. Targeting was confirmed by Southern blotting using a probe indicated in Figure 2A.
B6.Xcg-Tg (tek-cre)12Flv/J (Tie2Cre) mice were obtained from the Jackson Laboratory. Endothelium-specific calcrl-null embryos were generated by crossing calcrl+/– female mice to Tie2Cre+ male mice. The resulting calcrl+/–Tie2Cre+ animals were then bred to calcrlFlox/Flox mice, resulting in experimental animals for this study.
For all studies, littermate controls were used. The day of the vaginal plug was E0.5. All experiments were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Antibodies and growth factors.
The following primary antibodies were used in this study: rat anti-mouse CD31 (PECAM) monoclonal (BD Biosciences), rabbit anti-Prox1 (Chemicon), monoclonal anti–α–smooth muscle actin clone 1A4 (Sigma-Aldrich), goat anti-mouse VEGFR3 (R&D Systems), mouse anti–phospho-ERK1/2 (p44/42) antibody (Thr202/Tyr204), and rabbit anti-ERK antibody (Cell Signaling Technology). F-actin was stained with FITC-phalloidin (Sigma-Aldrich), and nuclei were stained with Hoechst 33258 (Sigma-Aldrich). Human AM and human AM22–52 were purchased from American Peptide, PD98057 from Sigma-Aldrich, and recombinant human VEGFA from Pierce Biotechnology.
In vivo proliferation assay.
Pregnant females were injected intraperitoneally with 100 μg/g body weight BrdU (Sigma-Aldrich). After 1 hour, mice were euthanized and embryos recovered for immunohistochemistry as described in Supplemental Methods. BrdU-positive cells were detected with the BrdU staining kit (Zymed) followed by staining in Mayer hematoxylin. Percent proliferative cells was determined by blinded quantitation of BrdU-positive cells in the lymphatic or vascular endothelium divided by total hematoxylin-stained nuclei. Data were analyzed using the 2-tailed Student’s t test assuming unequal variance. A P value < 0.05 was considered significant.
High-resolution, 3D OPT.
Whole mount immunofluorescence OPT was performed by BIOPTONICS following the manufacturer’s protocol. Briefly, E13.5 (PECAM) and E14.5 (VEGFR3) embryos were fixed in DENTS fixative overnight, permeabilized in acetone, and washed with PBST. Embryos were incubated in primary antibodies and 5% goat serum, 1% BSA, and 1% DMSO in PBST for 8–10 days. Specimens were washed in multiple changes of PBST for up to 4 days, and secondary antibodies in PBST were added. After 8 days, embryos were washed in PBST followed by PBS and then imaged by BIOPTONICS OPT scanner 3001 (MRC Technology).
Cell culture.
HUVECs were obtained from Lonza and maintained in endothelial cell medium provided by the supplier and used at passages 3–7. HMVEC-dLys (product no. CC-2810) were purchased from Lonza and maintained in endothelial cell medium provided by the supplier and used at passages 3–7. Culture media was changed every 2–3 days, and cells were passed when they achieved 90% confluency.
In vitro proliferation assay.
Cell proliferation was measured using a modified MTS assay as previously described (58). Briefly, HMVEC-dLys and HUVECs were plated at 8 × 104 cells/ml on a 96-well plate. After 24 hours in normal growth conditions, media was changed to basic medium containing 0.5% FBS for 24 hours. Cells were then treated with appropriate concentrations of peptide (AM or VEGFA in replicates of 5) under low-serum conditions for 48 hours, at which point 20 μl of MTS tetrazolium compound (CellTiter 96Aquesous; Promega) was added to each well and incubation was continued for 3 hours. Absorbance at 490 nm was recorded, and background was corrected by subtracting the average absorbance observed in wells containing no cells. Percent proliferation defined as the corrected absorbance in treated samples divided by corrected absorbance of untreated samples.
For total cell count determinations, HMVEC-dLys and HUVECs were plated at 2 × 105 cells/ml in each well of 6-well plates. After 24 hours in normal growth conditions, media was changed to basic medium containing 0.5% FBS for 24 hours. Cells were then treated with 10 nm of AM peptide under low-serum conditions for 48 hours. At 48 hours, the cells were lifted off the plate by application of 0.25% trypsin (Gibco; Invitrogen) and counted visually with a hemocytometer. Percent proliferation was a measure of treated samples divided by untreated samples. Experiments were performed in duplicate, and data are representative of 3 separate experiments.
Prox1 transfections.
For electroporation of HUVECs and HMVEC-dLys, the plasmid pFlag, either empty or containing full-length hProx1 (59), a kind gift from Eckard Truter, Karolinska Institute, Sweden, were electroporated in triplicate. HUVECs or HMVEC-dLys (1 × 106) were electroporated using the HUVEC Nucleofector kit (Amaxa). Twenty-four hours after transfection, RNA was generated for quantitative RT-PCR as described above.
Supplementary Material
Acknowledgments
We thank Eckardt Treuter for generously providing us with the hProx1 expression plasmid. We also thank Guillermo Oliver, Suk-Won Jin, Vicki Bautch, James Faber, Mark Majesky, and members of the Caron laboratory for helpful advice and discussions. We thank Armin Just, Kirk McNaughton, Vicki Madden, and members of Cam Patterson’s laboratory for expert technical assistance. This work was supported in part by The Burroughs Wellcome Fund, an NIH grant (HD046970), and an American Heart Association grant (0555424U) to K.M. Caron.
Footnotes
Nonstandard abbreviations used: AM, adrenomedullin; calcrl, calcitonin receptor–like receptor; HMVEC-dLy, human dermal lymphatic microvascular endothelial cells; LEC, lymphatic endothelial cell; OPT, optical projection tomography; Prox1, prospero-related homeobox 1; RAMP, receptor activity–modifying protein.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:40–50 (2008). doi:10.1172/JCI33302
Kimberly L. Fritz-Six and William P. Dunworth contributed equally to this work.
See the related Commentary beginning on page 29.
References
- 1.Alitalo K., Tammela T., Petrova T.V. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 2.Karkkainen M.J., Alitalo K. Lymphatic endothelial regulation, lymphoedema, and lymph node metastasis. Semin. Cell Dev. Biol. 2002;13:9–18. doi: 10.1006/scdb.2001.0286. [DOI] [PubMed] [Google Scholar]
- 3.Karkkainen M.J., Makinen T., Alitalo K. Lymphatic endothelium: a new frontier of metastasis research. Nat. Cell. Biol. 2002;4:E2–E5. doi: 10.1038/ncb0102-e2. [DOI] [PubMed] [Google Scholar]
- 4.Scavelli C., Vacca A., Di Pietro G., Dammacco F., Ribatti D. Crosstalk between angiogenesis and lymphangiogenesis in tumor progression. Leukemia. 2004;18:1054–1058. doi: 10.1038/sj.leu.2403355. [DOI] [PubMed] [Google Scholar]
- 5.Skobe M., et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y. Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat. Rev. Cancer. 2005;5:735–743. doi: 10.1038/nrc1693. [DOI] [PubMed] [Google Scholar]
- 7.Oliver G., Alitalo K. The lymphatic vasculature: recent progress and paradigms. Annu. Rev. Cell Dev. Biol. 2005;21:457–483. doi: 10.1146/annurev.cellbio.21.012704.132338. [DOI] [PubMed] [Google Scholar]
- 8.Hong Y.K., Detmar M. Prox1, master regulator of the lymphatic vasculature phenotype. Cell Tissue Res. 2003;314:85–92. doi: 10.1007/s00441-003-0747-8. [DOI] [PubMed] [Google Scholar]
- 9.Wigle J.T., et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wigle J.T., Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 11.Karkkainen M.J., et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 12.Abtahian F., et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schacht V., et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan L., et al. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 15.Petrova T.V., et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat. Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 16.Shimoda H., et al. Abnormal recruitment of periendothelial cells to lymphatic capillaries in digestive organs of angiopoietin-2-deficient mice. Cell Tissue Res. 2007;328:329–337. doi: 10.1007/s00441-006-0360-8. [DOI] [PubMed] [Google Scholar]
- 17.Yla-Herttuala S., Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nat. Med. 2003;9:694–701. doi: 10.1038/nm0603-694. [DOI] [PubMed] [Google Scholar]
- 18.Tammela T., Petrova T.V., Alitalo K. Molecular lymphangiogenesis: new players. Trends Cell Biol. 2005;15:434–441. doi: 10.1016/j.tcb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Zudaire E., Martinez A., Cuttitta F. Adrenomedullin and cancer. Regul. Pept. 2003;112:175–183. doi: 10.1016/s0167-0115(03)00037-5. [DOI] [PubMed] [Google Scholar]
- 20.Gibbons C., Dackor R., Dunworth W., Fritz-Six K., Caron K.M. Receptor activity-modifying proteins: RAMPing up adrenomedullin signaling. Mol. Endocrinol. 2007;21:783–796. doi: 10.1210/me.2006-0156. [DOI] [PubMed] [Google Scholar]
- 21.Zhou M., Simms H.H., Wang P. Adrenomedullin and adrenomedullin binding protein-1 attenuate vascular endothelial cell apoptosis in sepsis. Ann. Surg. 2004;240:321–330. doi: 10.1097/01.sla.0000133253.45591.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Sauze S., et al. Effects of adrenomedullin on endothelial cells in the multistep process of angiogenesis: involvement of CRLR/RAMP2 and CRLR/RAMP3 receptors. Int. J. Cancer. 2004;108:797–804. doi: 10.1002/ijc.11663. [DOI] [PubMed] [Google Scholar]
- 23.Kato H., Shichiri M., Marumo F., Hirata Y. Adrenomedullin as an autocrine/paracrine apoptosis survival factor for rat endothelial cells. Endocrinology. 1997;138:2615–2620. doi: 10.1210/endo.138.6.5197. [DOI] [PubMed] [Google Scholar]
- 24.Miyashita K., et al. Adrenomedullin promotes proliferation and migration of cultured endothelial cells. Hypertens. Res. 2003;26(Suppl.):S93–S98. doi: 10.1291/hypres.26.s93. [DOI] [PubMed] [Google Scholar]
- 25.Kim W., et al. Angiogenic role of adrenomedullin through activation of Akt, mitogen-activated protein kinase, and focal adhesion kinase in endothelial cells. FASEB J. 2003;17:1937–1939. doi: 10.1096/fj.02-1209fje. [DOI] [PubMed] [Google Scholar]
- 26.Miyashita K., et al. Adrenomedullin provokes endothelial Akt activation and promotes vascular regeneration both in vitro and in vivo. FEBS Lett. 2003;544:86–92. doi: 10.1016/s0014-5793(03)00484-8. [DOI] [PubMed] [Google Scholar]
- 27.Iimuro S., et al. Angiogenic effects of adrenomedullin in ischemia and tumor growth. Circ. Res. 2004;95:415–423. doi: 10.1161/01.RES.0000138018.61065.d1. [DOI] [PubMed] [Google Scholar]
- 28.Yurugi-Kobayashi T., et al. Adrenomedullin/cyclic AMP pathway induces Notch activation and differentiation of arterial endothelial cells from vascular progenitors. Arterioscler. Thromb. Vasc. Biol. 2006;26:1977–1984. doi: 10.1161/01.ATV.0000234978.10658.41. [DOI] [PubMed] [Google Scholar]
- 29.McLatchie L.M., et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 30.Arulmani U., Maassenvandenbrink A., Villalon C.M., Saxena P.R. Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur. J. Pharmacol. 2004;500:315–330. doi: 10.1016/j.ejphar.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 31.Nagaya N., Mori H., Murakami S., Kangawa K., Kitamura S. Adrenomedullin: angiogenesis and gene therapy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R1432–R1437. doi: 10.1152/ajpregu.00662.2004. [DOI] [PubMed] [Google Scholar]
- 32.Ishimitsu T., Ono H., Minami J., Matsuoka H. Pathophysiologic and therapeutic implications of adrenomedullin in cardiovascular disorders. Pharmacol. Ther. 2006;111:909–927. doi: 10.1016/j.pharmthera.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Julian M., et al. Adrenomedullin: a new target for the design of small molecule modulators with promising pharmacological activities. Eur. J. Med. Chem. 2005;40:737–750. doi: 10.1016/j.ejmech.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Dackor R., Fritz-Six K., Smithies O., Caron K. Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J. Biol. Chem. 2007;282:18094–18099. doi: 10.1074/jbc.M703544200. [DOI] [PubMed] [Google Scholar]
- 35.Caron K.M., Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc. Natl. Acad. Sci. U. S. A. 2001;98:615–619. doi: 10.1073/pnas.021548898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dackor R.T., et al. Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene. Mol. Cell. Biol. 2006;26:2511–2518. doi: 10.1128/MCB.26.7.2511-2518.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koni P.A., et al. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J. Exp. Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hippenstiel S., et al. Adrenomedullin reduces endothelial hyperpermeability. Circ. Res. 2002;91:618–625. doi: 10.1161/01.res.0000036603.61868.f9. [DOI] [PubMed] [Google Scholar]
- 39.Kis B., et al. Chronic adrenomedullin treatment improves blood-brain barrier function but has no effects on expression of tight junction proteins. Acta. Neurochir. Suppl. 2003;86:565–568. doi: 10.1007/978-3-7091-0651-8_115. [DOI] [PubMed] [Google Scholar]
- 40.Feng Y., et al. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19836–19841. doi: 10.1073/pnas.0609628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braren R., et al. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J. Cell Biol. 2006;172:151–162. doi: 10.1083/jcb.200506184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zwerts F., et al. Lack of endothelial cell survivin causes embryonic defects in angiogenesis, cardiogenesis, and neural tube closure. Blood. 2007;109:4742–4752. doi: 10.1182/blood-2006-06-028068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim K., et al. Polycystin 1 is required for the structural integrity of blood vessels. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1731–1736. doi: 10.1073/pnas.040550097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharpe J., et al. Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science. 2002;296:541–545. doi: 10.1126/science.1068206. [DOI] [PubMed] [Google Scholar]
- 45.Hay D.L., et al. CL/RAMP2 and CL/RAMP3 produce pharmacologically distinct adrenomedullin receptors: a comparison of effects of adrenomedullin22-52, CGRP8-37 and BIBN4096BS. Br. J. Pharmacol. 2003;140:477–486. doi: 10.1038/sj.bjp.0705472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrova T.V., et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirakawa S., et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am. J. Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabibiazar R., et al. Inflammatory Manifestations of Experimental Lymphatic Insufficiency. PLoS Med. 2006;3:e254. doi: 10.1371/journal.pmed.0030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doods H., et al. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br. J. Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doods H. Development of CGRP antagonists for the treatment of migraine. Curr. Opin. Investig. Drugs. 2001;2:1261–1268. [PubMed] [Google Scholar]
- 51.Ribatti D., Nico B., Spinazzi R., Vacca A., Nussdorfer G.G. The role of adrenomedullin in angiogenesis. Peptides. 2005;26:1670–1675. doi: 10.1016/j.peptides.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 52.Morisada T., et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- 53.Harzenetter M.D., et al. Regulation and function of the CGRP receptor complex in human granulopoiesis. Exp. Hematol. 2002;30:306–312. doi: 10.1016/s0301-472x(02)00772-5. [DOI] [PubMed] [Google Scholar]
- 54.Del Pup L., et al. Adrenomedullin is expressed in cord blood hematopoietic cells and stimulates their clonal growth. Int J Mol Med. 2003;11:157–160. [PubMed] [Google Scholar]
- 55.Sebzda E., et al. Syk and Slp-76 mutant mice reveal a cell-autonomous hematopoietic cell contribution to vascular development. Dev. Cell. 2006;11:349–361. doi: 10.1016/j.devcel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Taniguchi K., et al. Spreds are essential for embryonic lymphangiogenesis by regulating vascular endothelial growth factor receptor-3 signaling. Mol. Cell. Biol. 2007;27:4541–4550. doi: 10.1128/MCB.01600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Witte M.H., Bernas M.J., Martin C.P., Witte C.L. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc. Res. Tech. 2001;55:122–145. doi: 10.1002/jemt.1163. [DOI] [PubMed] [Google Scholar]
- 58.Nakagami H., et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler. Thromb. Vasc. Biol. 2005;25:2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 59.Steffensen K.R., et al. Functional conservation of interactions between a homeodomain cofactor and a mammalian FTZ-F1 homologue. EMBO Rep. 2004;5:613–619. doi: 10.1038/sj.embor.7400147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.