Abstract
Novel biophysical approaches combined with modeling and new biochemical data have helped to recharge the lipid raft field and have contributed to the generation of a refined model of plasma membrane organization. In this review, we summarize new information in the context of previous literature to provide new insights into the spatial organization and dynamics of lipids and proteins in the plasma membrane of live cells. Recent findings of large-scale separation of liquid-ordered and liquid-disordered phases in plasma membrane vesicles demonstrate this capacity within the complex milieu of plasma membrane proteins and lipids. Roles for membrane heterogeneity and reorganization in immune cell activation are discussed in light of this new information.
Keywords: cholesterol, liquid-ordered, lipid probes, IgE receptors, mast cells
The Lipid Raft Hypothesis
Cell membranes define the limiting boundary of eukaryotic cells, actively restricting access to the cellular interior by the external environment, and playing a crucial regulatory role in the exchange of nutrients and metabolites and transduction of external signals. Plasma membranes are approximately half lipids and half proteins by mass (1). The fluid mosaic model (2) represents an early effort to portray the two dimensional organization of proteins and lipids in the plasma membrane, and it depicts the plasma membrane as a multi-component milieu of functionally active proteins interspersed in an essentially homogeneous lipid bilayer. This model does not assign functional significance to physical heterogeneities in the lipid organization that can arise out of thermal fluctuations and non-ideal mixing. However, in recent years a large number of studies have provided a considerably more complex picture of the organization of lipids and proteins in the plasma membrane. In particular, the “lipid raft” hypothesis (3–8) has captured the imagination of researchers interested in the role of membrane organization in signaling and vesicular trafficking. The lipid raft hypothesis is underpinned by the concept that lipids in the plasma membrane have different biophysical propensities to associate with each other, and, in its simplest form, proposes the presence of lateral heterogeneities in the plasma membrane arising out of the tighter packing of cholesterol with saturated and mono-unsaturated phospholipids than with poly-unsaturated phospholipids. This hypothesis associates functional significance with lateral heterogeneities present in the plasma membrane and proposes that membrane domains resulting from these heterogeneities play active roles in various physiological processes including signal transduction (9–11), vesicle trafficking (12, 13), cell adhesion and motility (14), and entry of pathogenic viruses and bacteria (15, 16).
Phase separation in model membranes
The coexistence of a cholesterol-poor, liquid disordered (Ld) phase and a liquid-ordered (Lo) phase enriched in sphingolipids and cholesterol has been demonstrated in ternary mixtures of sphingomyelin (SM):unsaturated phosphatidylcholine (PC):cholesterol with a wide range of compositions and temperatures (17–19). Cholesterol is crucial for the formation of the Lo phase, which is characterized by a high degree of acyl chain ordering, but with translational mobility similar to that of the Ld phase. The acyl chains in the Lo phase are more tightly packed and consequently there is a reduction in cross-sectional area per lipid. Fluorescently labeled glycosylphosphatidylinositol(GPI)-anchored proteins such as Thy-1, glycosphingolipids such as ganglioside GM1, and saturated phospholipid probes such as N-(7-nitro-2-1,3-benzoxadiazol-4-yl)-dipalmitoylphosphatidylethanolamine (NBD-DPPE) were found to partition preferentially into an Lo phase in macroscopically phase-separated model membranes, whereas lipids with short or unsaturated acyl chains, and most transmembrane proteins, are preferentially excluded from the Lo phase (Figure 1; 20–23). Silvius demonstrated the presence of nanoscale domains (~10–40 nm) in lipid bilayers with compositions modeling that of the outer leaflet of the plasma membrane at physiological temperatures (24), suggesting that even in absence of macroscopic phase separation, thermal fluctuations can lead to transient, small-scale Lo domains or condensed complexes with significant lifetimes.
Figure 1.
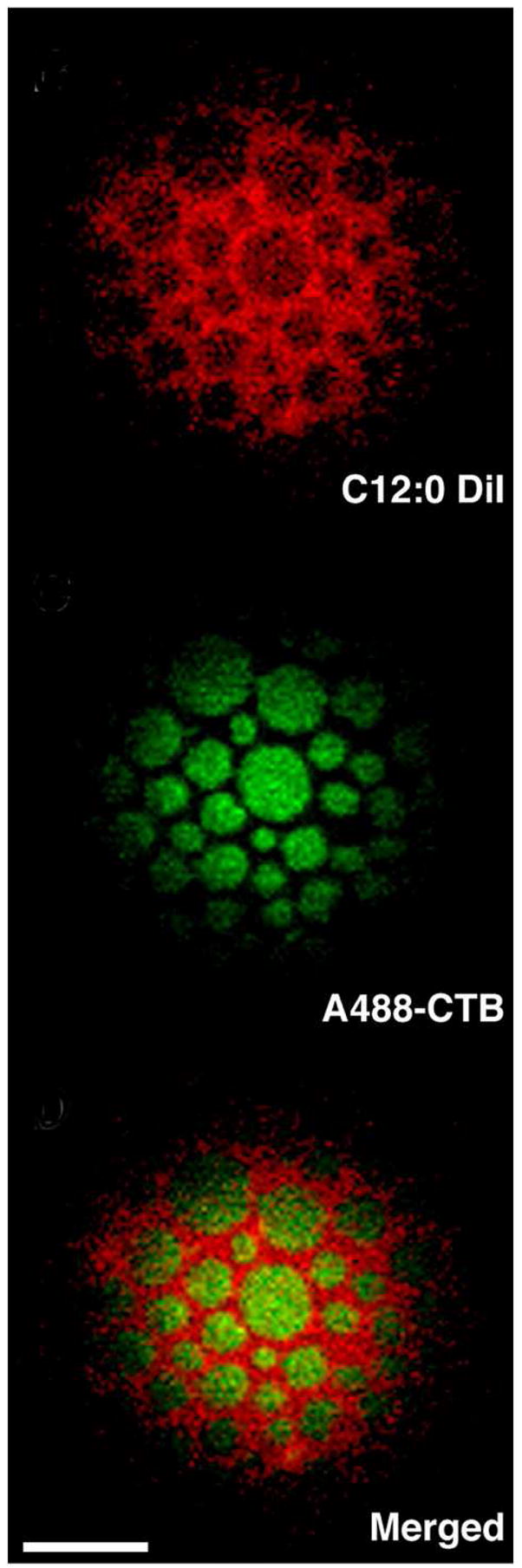
Micron-scale fluid-fluid phase separation in giant unilamellar vesicles (GUVS) composed of cholesterol, SM, DOPC, and ganglioside GM1. Tangential confocal section of GUV imaged at 23 ° C. Alexa488-cholera toxin B (A488-CTB) bound to Lo-preferring GM1 partitions complementarily to the Ld-preferring carbocyanine lipid probe C12:0 DiI in phase separated GUVs (scale bar, 5 μm). Image adapted from ref. 23.
What is the physical basis for the association of cholesterol and sphingolipids? The packing of cholesterol with saturated acyl chains of sphingolipids is entropically more favorable than with unsaturated acyl chains (25). Dipolar interactions between sphingolipids, and possible hydrogen bonding between the hydroxyl group of cholesterol and the amide group of sphingolipids and ceramides can also contribute to the favorable association of cholesterol with sphingolipids (3–6). In addition, many body interactions such as hydrophobic shielding or the “umbrella effect” proposed by Huang and Feigenson (26) can provide energetically favorable interactions. According to the umbrella model, cholesterol segregates into regions of membrane with strongly hydrated large head groups, like those of sphingolipids, where the sterol rings can be more effectively shielded from the aqueous environment. An alternative hypothesis, put forward by McConnell and colleagues, proposes that cholesterol forms reversible, condensed complexes of defined stoichiometry with sphingolipids and saturated phospholipids (27, 28).
Another interesting possibility has been suggested by molecular dynamics simulations of a ternary mixture of cholesterol, dioleoylPC (DOPC) and SM (29). These results predict that cholesterol preferentially localizes at the interface between SM-enriched and DOPC-enriched regions, with entropically favorable packing of the saturated acyl chains of SM against the smooth α-face of cholesterol, and the disordered unsaturated DOPC acyl chains packing more readily with the protruding methyl groups of the rougher cholesterol β-face. In this simulation, small, curvilinear nanoscale domains formed but did not increase in size during the simulation time course, suggesting that nanoscopic domains can form spontaneously with cholesterol, playing a crucial role in stabilizing these by decreasing the line tension between Lo and Ld domains. Interestingly, these nanoscale domains generated by computer simulations are reminiscent of the nanoscopic heterogeneities reported by Silvius in ternary mixtures of cholesterol/palmitoyloleoylPC/SM at 37°C (24). Phase separation and clustering observed in model membranes (17–24, 30) is likely due to a combination of all these intermolecular forces.
Lipid rafts and domains in the plasma membrane
A subset of plasma membrane components was found to be insoluble in nonionic detergents, such as Triton-X 100, at low temperatures (31). Experiments with model membranes also demonstrated that the Lo-phase, unlike the Ld-phase, is resistant to solubilization by cold, non-ionic detergents (32). Detergent-resistant membrane (DRM) fractions prepared from model membranes and cell membranes were found to be enriched in cholesterol and SM and to contain a subset of lipid-anchored proteins, including the GPI-anchored proteins, and certain transmembrane proteins (33–35). This led to the picture that the plasma membrane is organized into microdomains with lipid structure akin to the Lo phase of model membranes that is dispersed in a contiguous sea of disordered lipids in an Ld phase (3). Subsequently, biophysical studies on model membranes, plasma membrane vesicles and reconstituted plasma membrane vesicles demonstrated that the packing of lipid acyl chains in DRMs is similar to Lo phases in model membranes (35–37). Thus, association of various membrane proteins and lipids with DRMs after incubation with cold Triton X-100 has been used to infer their association with lipid rafts in intact membranes. Furthermore, functional perturbation by cholesterol depletion has been used to implicate the involvement of lipid rafts in various cellular processes (38, 39).
A major obstacle to realistic understanding of this area of cell biology is that lipid rafts have been, by most definitions, considered to be discrete physical entities. This is largely because they have been characterized principally in terms of biochemical criteria, i.e., resistance to solubilization by mild detergents and cholesterol depletion. However, detergents can selectively extract membrane components irrespective of their preference for Lo, and they can cause mixing of domains or even membrane segregation in some situations (40, 41). Thus, DRMs do not provide a clear representation of the native structure of Lo plasma membrane domains; rather, as for any biochemical preparation of isolated membranes, they provide only limited information about in situ characteristics of those membranes. The effects of cholesterol depletion are also more complicated and wide-ranging than initially recognized, including perturbation of the actin cytoskeleton (42) and clathrin mediated endocytosis (43), so that such experiments cannot be interpreted solely in terms of disruption of membrane rafts. Thus, it is increasingly recognized that biochemical perturbations provide only limited information about the nanoscale organization of biological membranes.
Recently we found that giant plasma membrane vesicles (GPMV), produced from RBL mast cells by chemically-induced blebbing, spontaneously separate into coexisting fluid phases at temperatures below 20°C (Figure 2; 44). We also observed phase separation in a small fraction of GPMVs at temperatures as high as 37°C. These results demonstrate that the complex mixture of lipids and proteins of biological membranes can support fluid-fluid phase coexistence, and they are consistent with nanoscopic Lo/Ld fluctuations in live cell plasma membranes described below. These GPMVs offer an unique opportunity to study the phase preference of lipids and proteins in complex biological membranes, and they provide clear evidence for the relevance of Lo/Ld fluid/fluid lipid segregation to plasma membrane structure and function.
Figure 2.
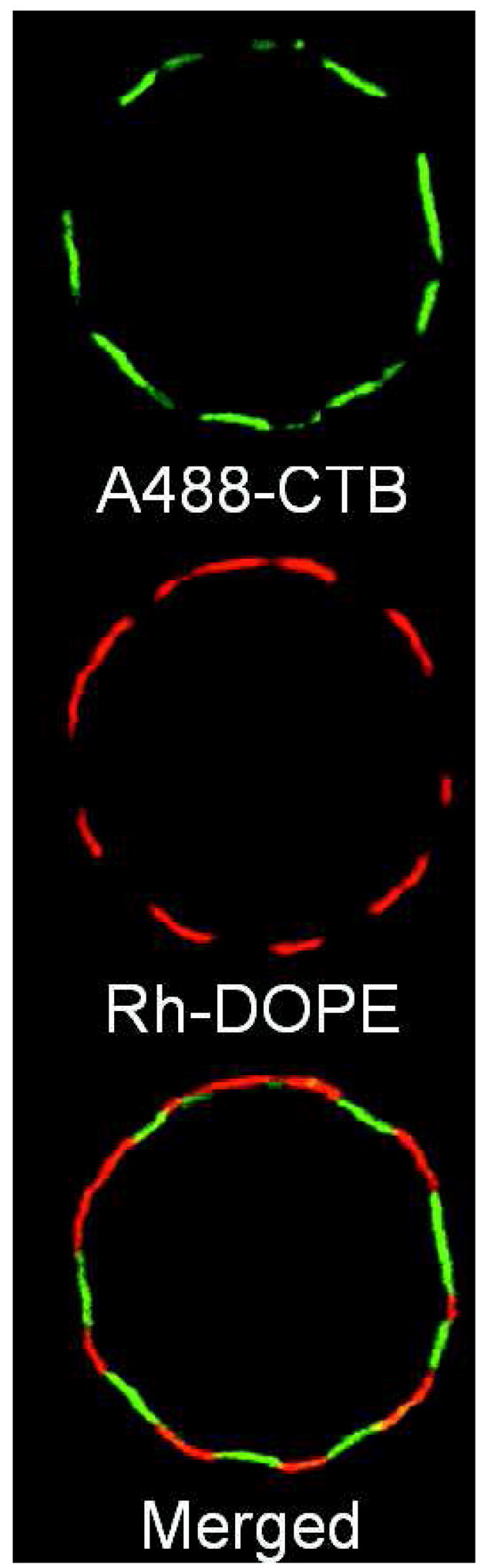
Giant plasma membrane vesicles (GPMVs) isolated from RBL-2H3 mast cells spontaneously phase separate into coexisting fluid phases. GPMVs were generated from cells pre-labeled with Alexa488-cholera toxin B and lissamine rhodamine B sulfonyl-DOPE (Rh-DOPE). Equatorial confocal section of a GPMV (~25 μm diameter) was imaged at 15° C. A488-CTB bound to GM1 shows partitioning complementary to Rh-DOPE and prefers the Lo-phase in GPMVs. Image adapted from ref. 44.
What then is the real nature of membrane heterogeneity in live cells? To address this complex and challenging question satisfactorily, we need to visualize directly membrane domains with sufficiently high spatial and temporal resolution. Fluorescence microscopy of live, unperturbed cells has consistently failed to reveal optically resolvable segregation of membrane domains enriched in major raft-components (as characterized by biochemical criteria), such as GPI-anchored proteins. This indicates that if functionally significant plasma membrane heterogeneities exist in live cells, they are either too close together to be spatially resolved (< 200 nm), or too dynamic to be temporally resolved.
Plasma membranes are non-equilibrium systems, and accumulation of some components in a particular area due to exocytosis or an enzyme-catalyzed reaction can lead to transient formation of domains as a purely kinetic process. For example, the hydrolysis of sphingomyelin by sphingomyelinase or phosphatidylinositol-4,5-bisphosphate by phospholipase C can lead to a local increase in ceramide or diacylglycerol concentration, respectively. However, these newly produced lipid molecules would be expected to diffuse away and dissipate fairly quickly on the micrometer scale (within a few microseconds, Dlipids~5 × 10−9 – 10−8 cm2/sec), and such transient heterogeneities must be stabilized by other thermodynamic interactions if they are to contribute significantly to membrane heterogeneity in the present context.
It is expected that small domains formed due to differential interactions between membrane constituents will coalesce to decrease thermodynamic line tension. However, proteins and cholesterol can act as surface-active agents to decrease the line tension, thereby helping to stabilize smaller domains in the cell membrane. An intriguing possibility is that the cell membrane is in a mixed, quasi-equilibrated phase, poised at the brink of a phase boundary/phase separation, and perturbations like crosslinking of membrane components or depletion of cholesterol can drive the membrane phase behavior across the boundary as manifested by large scale segregation of lipids and proteins. Recently, we found that crosslinking of a minor membrane component, GM1, by pentameric cholera toxin B leads to micrometer-scale fluid-fluid phase separation in a homogeneous mixture of cholesterol/SM/DOPC when the initial composition of the GUVs was chosen to be single phase but close to a two-phase boundary (23). Although this situation might not pertain exactly for cell membranes, it clearly demonstrates that crosslinking of a single membrane species can lead to wide scale changes in the intermolecular interactions and spatial distributions of other membrane species. It is expected that the cell membrane, with such a large repertoire of lipids and proteins, will harness the wide array of intermolecular interactions to respond to small and subtle changes in the concentration or the aggregation state of specific species. Linkages with other cellular components, including internal membranes and the cytoskeleton, further influence these interactions.
Socci and colleagues recently used theoretical calculations to show that for biologically relevant diffusion and membrane recycling rates, and under the non-equilibrium conditions applicable for plasma membranes, there can be spontaneous formation of domains in the range of tens of nanometers, with membrane recycling to prevent the formation of larger assemblies (45). Thus, continuous trafficking to-and-from the plasma membrane can also contribute to the overall steady state membrane organization to minimize large-scale heterogeneities in the plasma membrane, such as the phase separation observed in GPMVs. In addition, it is likely that the membrane-associated cytoskeleton acts to regulate the formation of large-scale membrane domains. For example, inhibition of actin polymerization by cytochalasin D or lactrunculin A dramatically alters the lipid composition of DRMs similarly to cell activation mediated by IgE receptors (Han, X., Smith, N., Sil, D., Holowka, D., McLafferty, F. and Baird, B., submitted for publication).
Current ideas of plasma membrane structure and dynamics in live cells
Interest in the lipid raft hypothesis, in particular, and overall functional membrane organization in general, has led to the development and refinement of new technologies and biophysical approaches to examine the membrane architecture at hitherto inaccessible temporal and spatial resolution. The use of these sophisticated biophysical techniques has heralded a new era in studying membrane architecture and is providing fresh insights regarding spatial and temporal associations of cell surface molecules.
Spatial organization of the plasma membrane
A combination of high resolution imaging and mathematical modeling is providing the view that raft proteins are organized into high density nanoclusters with radii ranging over 5–20 nm (46–50). Using measurements of fluorescence resonance energy transfer between the same probes (homo-FRET), Mayor and colleagues reported that a fraction (20–40%) of GPI-anchored proteins are organized into high density clusters of 4–5 nm radius, each consisting of a few molecules and different GPI-anchored proteins, on the surface of CHO cells (46). In studies utilizing quantitative immunoelectron microscopy, Hancock and colleagues found that different Ras isoforms are organized into distinct microdomains in the inner leaflet of plasma membrane with characteristic radius ranging 6–11 nm with as few as 6–8 proteins present in each cluster (47, 48). These high resolution “snapshots” provide indications of spatial heterogeneity of both inner and outer leaflet proteins, and they reveal nanometer-scale clustering of both raft and non-raft proteins.
An important issue not addressed by these FRET and EM studies is the percentage of plasma membrane that has Lo character. Initially, it was assumed that lipid rafts constitute a small fraction of the plasma membrane surface and facilitate protein-protein interactions by concentrating them into a small area. However, fluorescence anisotropy and recent ESR experiments show that a substantial (60–70%) portion of the plasma membrane lipid can be in a Lo state (36, 50). Consistent with this, imaging studies by Maxfield and colleagues reveal large, inter-connected regions of plasma membrane containing lipid raft markers under conditions of membrane perturbation by nonionic detergent (51) or cholesterol depletion (52).
In a recent study, FRET measured between carbocyanine lipid probes has been used to investigate the lateral organization of lipid components of the plasma membrane of living RBL-2H3 mast cells. A combination of hetero-FRET between donor/acceptor pairs and homo-FRET measured by fluorescence anisotropy indicate that carbocyanine lipid probes segregate in the outer leaflet of the plasma membrane based on their alkyl chain structure. The results are consistent with order-dependent segregation of lipids in the plasma membrane of live cells on a spatial scale of tens of nanometers. These results, together with ESR evidence for Lo/Ld-like domain segregation in live cells (50), provide strong evidence for the relevance of fluid/fluid phase separation, as characterized in model membranes and isolated plasma membrane vesicles, to fundamental cell physiology.
Dynamic properties of membrane domains
Pulse EPR spin-labeling experiments used to determine the half-life of lipids in rafts suggest that either rafts are very short lived and/or raft molecules rapidly diffuse in and out at a time scale of 100 microseconds or less (53). There have been a number of single particle experiments examining the diffusion behavior of raft components, and experiments involving observation at video rate (30.3 milliseconds/frame) from different groups suggest that raft sizes can be anywhere in the range of 26–500 nm (54–56). Kusumi and colleagues measured the diffusion of fluorescent lipids and GPI proteins using extremely high time resolution (25 microseconds/frame). They conclude from these results that the plasma membrane is compartmentalized by membrane skeleton fences (picket and fence model) into 30–230 nanometer domains, depending on cell type, with membrane molecules undergoing confined diffusion at short time scales and hop diffusion over longer times (57, 58). Their results suggest that single antibody conjugates of raft molecules diffuse as extremely small species, i.e. either as monomers or as very small preexisting assemblies, but not as part of large-scale stable rafts.
According to the hydrodynamic theory of Saffman and Delbruck (59), lateral diffusion of individual membrane molecules and small clusters of molecules would not be very different in the two-dimensional plane, as the diffusion coefficient has a weak dependence on the size in this case. Thus, single-molecule diffusion of non-raft molecules and raft molecules trapped in small rafts would be similar. However, in the presence of cytoskeletal fences and pickets, as envisioned in Kusumi’s model, there can be appreciable resistance to larger clusters whereas single molecules/small clusters can escape through dynamic gaps between the picket proteins bound to membrane skeleton fence. The translation diffusion of putative raft and non-raft molecules were reported to be similar by Vrlijic (60, 61) and Kenworthy (62), and structural determinants like transmembrane anchors were found to be the primary determinants of diffusion rates. If raft components are stably confined to Lo domains, then these domains must be small enough to diffuse through the gaps between the cytoskeletal proteins, suggesting a radius on the order of 2–9 nm, the average gap between pickets in different cell types (63). However, as individual raft markers are expected to diffuse in and out of Lo domains with only small differences in their diffusion coefficient in each environment (56), preference for a Lo environment does not, a priori, predict reduced diffusion of individual molecules, even for the case of larger Lo domains.
Membrane domains in immune signaling
A number of recent studies provide considerable evidence that protein compartmentalization is integral to the regulation of immune signaling. Biochemically defined lipid rafts have been shown to play significant roles in modulating protein-protein interactions in resting and activated T-cells, B-cells and mast cells (9–11), and co-aggregation of raft-associated components following receptor ligation is believed to be a general mechanism for promoting immune cell signaling. For example, in RBL-2H3 mast cells crosslinking of cell surface IgE receptors results in decreased detergent solubilization of the crosslinked receptors (64, 65), and ordered microdomains actively promote stable phosphorylation of receptor aggregates by activated Lyn kinase by sequestering them from transmembrane phosphatases (66).
Fluorescence cross-correlation analysis shows that full length Lyn co-diffuses with crosslinked IgE-receptor in RBL-2H3 mast cells, but PM-EGFP, containing GFP attached to the minimal myristoyl-palmitoyl-membrane anchor sequence of Lyn kinase, shows no detectable association with crosslinked receptors (67). In a separate study, Lyn-EGFP was found to colocalize with A488-IgE-FcεRI clusters on micropatterned surfaces after several minutes at 37°C, and receptor phosphorylation mediated by Lyn was detected (by labeled, specific antibodies) at even earlier times. In contrast, PM-EGFP co-localized with the receptor patches only after significantly longer times of stimulation (68). Douglass and Vale, using single molecule tracking, recently detected stable interactions of full length Lck-GFP with TCR-signaling domains containing LAT and CD2. However, similar to the IgE-receptor signaling, the minimal lipid anchor of Lck did not colocalize with the signaling domains (69). These results suggest that the cytoplasmic portions of these proteins contain an essential protein interaction domain that is crucial for their stable association with activated receptors. Thus, stimulated interactions of minimally anchored and full length Src-family kinases are not identical, and protein-protein interactions, in addition to inner leaflet anchoring by saturated acyl chains, play roles in the organization of functional signaling domains.
Implications for functional rafts
Recent results summarized above suggest that lipid rafts are very small (5–20 nm) and/or short lived (millisecond or less), with preferentially partitioning molecules diffusing in and out of the rafts. Considering that a cluster of radius 5 nm would only contain ~30–40 lipids and ~6–10 proteins, one is confronted with the question: can domains this small and transient be of biological utility? In a recent study, Hancock and colleagues used stochastic Monte Carlo modeling of dynamics of proteins in the presence of microdomains, and they found that dynamic partitioning of proteins into small (6–14 nm in diameter) and mobile rafts can maximize functional interactions via inter-protein collisions to act as efficient nanochambers of signal transduction (70). In this model, the association of different lipids is not strong enough to be thermodynamically stable at physiological temperatures, and consequently, these nanodomains will have a tendency to dissipate and reform. Proteins at the boundaries of these domains can potentially act to decrease the line tension and to stabilize the domains. Crosslinking of such boundary proteins could change their spatial localization, leading to coalescence of small domains. In contrast, endocytosis and vesicular trafficking can function to keep the membrane well mixed and prevent any large scale coalescence of raft membranes.
It is increasingly appreciated that proteins can play an active role in the organization of functional membrane microdomains. Using FRET, Zacharias et al. showed PM-EGFP to be organized into nanoclusters in the inner leaflet of the plasma membrane (71). As described above, the full-length protein kinase counterparts of this construct, Lyn and Lck, colocalize with crosslinked receptor aggregates in RBL mast cells and T cells, respectively, whereas the GFP-labeled minimal lipid anchor sequences of Lyn and Lck do not show the same spatio-temporal associations (67–69). It is not yet clear whether the full-length kinases and the minimal anchor sequences have different spatial distributions in resting cells. The minimal anchor of H-Ras partitions into Lo domains, whereas the full length H-Ras is organized into discrete, functional non-raft microdomains representing the hot spots of Ras activation (48). These results show that the cytoplasmic domains of plasma membrane-anchored proteins can play important roles in the spatial organization of these proteins, and in some cases might override the partitioning affinities of the membrane anchors. Similarly, specific interactions between the ectodomain of lipid-anchored GPI-APs in the outer leaflet of the plasma membrane can organize different GPI-APs into distinct microdomains.
From these recent studies, a consensus is emerging for non-activated cells that lipid rafts are dynamic, heterogeneous, nanoscale entities that determine the lipid environments of proteins and thereby enhance or regulate protein–protein interactions. The localization of a few proteins in rafts can confer upon these proteins subtly different propensities for interactions with other proteins. Raft size and composition is likely to depend on the activation state, such as in immune cells where activation by means of clustering receptors can cause coalescence of the small, heterogeneous, highly dynamic nano-structures into larger, more stable rafts that can serve as platforms for signaling. Thus, rafts can shift the balance in favor of particular intermolecular interactions to enhance the signaling efficiency. However, large-scale membrane polarization, such as that which occurs for T cells at the immunological synapse, depends on membrane protein and cytoskeletal reorganization. Lipid rafts possibly initiate and probably contribute to, but do not dictate, the compositional organization of such larger signaling domains (72). The lipid raft hypothesis has catalyzed a renaissance in our interest and understanding of plasma membrane structure and function. Nevertheless, as in many areas of cell biology, much remains to be learned about how membrane heterogeneity participates in a wide range of cell processes.
Acknowledgments
Supported by National Institutes of Health grant AI22449 and in part by National Science Foundation-Nanoscale Interdisciplinary Research Team grant DMR-0404195 and the Nanobiotechnology Center (ECS-9876771).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jain MK. Introduction to Biological Membranes. New York: John Wiley & Sons; 1988. [Google Scholar]
- 2.Singer SJ, Nicholson GL. The fluid mosaic model of the structure of cell membrane. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 3.Simons K, Ikonen E. Functional rafts in cell membrane. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 4.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 5.Brown DA, London E. Structure and function of sphingolipid and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 6.Silvius JR. Role of cholesterol in lipid raft formation: lessons from lipid model systems. Biochim Biophys Acta. 2001;1610:174–183. doi: 10.1016/s0005-2736(03)00016-6. [DOI] [PubMed] [Google Scholar]
- 7.Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 8.Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:351–378. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 9.Pierce SK. Lipid rafts and B-cell activation. Nat Rev Immunol. 2002;2:96–105. doi: 10.1038/nri726. [DOI] [PubMed] [Google Scholar]
- 10.Holowka D, Gosse JA, Hammond AT, Han X, Sengupta P, Smith NL, Wagenknecht-Wiesner A, Wu M, Young RM, Baird B. Lipid segregation and IgE receptor signaling: a decade of progress. Biochim Biophys Acta. 2005;1746:252–259. doi: 10.1016/j.bbamcr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Kabouridis PS. Lipid rafts in T cell receptor signaling. Mol Membr Biol. 2006;23:49–57. doi: 10.1080/09687860500453673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 13.Salaun C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manes S, Viola A. Lipid rafts in lymphocyte activation and migration. Mol Membr Biol. 2006;23:59–69. doi: 10.1080/09687860500430069. [DOI] [PubMed] [Google Scholar]
- 15.Manes S, del Real G, Martinez-A C. Pathogens: raft hijackers. Nat Rev Immunol. 2003;3:557–568. doi: 10.1038/nri1129. [DOI] [PubMed] [Google Scholar]
- 16.Lafont F, van der Goot FG. Bacterial invasion via lipid rafts. Cell Microbiol. 2005;7:613–620. doi: 10.1111/j.1462-5822.2005.00515.x. [DOI] [PubMed] [Google Scholar]
- 17.Korlach J, Schwille P, Webb WW, Feigenson GW. Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc Natl Acad Sci USA. 1999;96:8461–8466. doi: 10.1073/pnas.96.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Almeida RF, Fedorov A, Prieto M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys J. 2003;85:2406–2416. doi: 10.1016/s0006-3495(03)74664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veatch SL, Keller SL. Seeing spots: complex phase behavior in simple membranes. Biochim Biophys Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich C, Volovyk ZN, Levi M, Thompson NL, Jacobson K. Partitioning of Thy-1, GM1, and cross-linked phospholipid analogs into lipid rafts reconstituted in supported model membrane monolayers. Proc Natl Acad Sci USA. 2001;98:10642–10647. doi: 10.1073/pnas.191168698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, Jacobson K, Gratton E. Lipid rafts reconstituted in model membranes. Biophys J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samsonov AV, Mihalyov I, Cohen FS. Characterization of cholesterol-sphingomyelin domains and their dynamics in bilayer membranes. Biophys J. 2001;81:1486–1500. doi: 10.1016/S0006-3495(01)75803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammond AT, Heberle FA, Baumgart T, Holowka D, Baird B, Feigenson GW. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc Natl Acad Sci USA. 2004;102:6320–6325. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silvius JR. Fluorescence energy transfer reveals microdomain formation at physiological temperatures in lipid mixtures modeling the outer leaflet of the plasma membrane. Biophys J. 2003;85:1034–1045. doi: 10.1016/S0006-3495(03)74542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott R, Szleifer I, Schick M. Phase diagram of a ternary mixture of cholesterol and saturated and unsaturated lipids calculated from a microscopic model. Phys Rev Lett. 2006;96:98–101. doi: 10.1103/PhysRevLett.96.098101. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Feigenson GW. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys J. 1999;76:2142–2157. doi: 10.1016/S0006-3495(99)77369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McConnell HM, Radhakrishnan A. Condensed complexes of cholesterol and phospholipids. Biochim Biophys Acta. 2003;1610:159–173. doi: 10.1016/s0005-2736(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 28.McConnell HM, Vrljic M. Liquid-liquid immiscibility in membranes. Annu Rev Biophys Biomol Struct. 2003;32:469–492. doi: 10.1146/annurev.biophys.32.110601.141704. [DOI] [PubMed] [Google Scholar]
- 29.Pandit SA, Jacobsson E, Scott HL. Simulation of the early stages of nano-domain formation in mixed bilayers of sphingomyelin, cholesterol, and dioleylphosphatidylcholine. Biophys J. 2004;87:3312–3322. doi: 10.1529/biophysj.104.046078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Almeida RF, Loura LM, Fedorov A, Prieto M. Lipid rafts have different sizes depending on membrane composition: a time-resolved fluorescence resonance energy transfer study. J Mol Biol. 2005;346:1109–1120. doi: 10.1016/j.jmb.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 31.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder R, London E, Brown D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci USA. 1994;91:12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed SN, Brown DA, London E. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. Biochemistry. 1997;36:10944–10953. doi: 10.1074/jbc.273.2.1150. [DOI] [PubMed] [Google Scholar]
- 34.Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol. 1998;164:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 35.Fridriksson EK, Shipkova PA, Sheets ED, Holowka D, Baird B, McLafferty FW. Quantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometry. Biochemistry. 1999;38:8056–8063. doi: 10.1021/bi9828324. [DOI] [PubMed] [Google Scholar]
- 36.Gidwani A, Holowka D, Baird B. Fluorescence anisotropy measurements of lipid order in plasma membranes and lipid rafts from RBL-2H3 mast cells. Biochemistry. 2001;40:12422–12429. doi: 10.1021/bi010496c. [DOI] [PubMed] [Google Scholar]
- 37.Ge M, Gidwani A, Brown HA, Holowka D, Baird B, Freed JH. Ordered and disordered phases coexist in plasma membrane vesicles of RBL-2H3 mast cells: An ESR study. Biophys J. 2003;85:1278–1288. doi: 10.1016/S0006-3495(03)74563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheets ED, Holowka D, Baird B. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcεRI and their association with detergent-resistant membranes. J Cell Biol. 1999;145:877–887. doi: 10.1083/jcb.145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys J. 2002;83:2693–2701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heerklotz H, Szadkowska H, Anderson T, Seelig J. The sensitivity of lipid domains to small perturbations demonstrated by the effect of Triton. J Mol Biol. 2003;329:793–799. doi: 10.1016/s0022-2836(03)00504-7. [DOI] [PubMed] [Google Scholar]
- 42.Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci USA. 2003;100:13964–13969. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pichler H, Riezman H. Where sterols are required for endocytosis. Biochim Biophys Acta. 2004;1666:51–61. doi: 10.1016/j.bbamem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Baumgart T, Hammond A, Sengupta P, Hess S, Holowka D, Baird B, Webb WW. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc Natl Acad Sci USA. 2006;104:3165–70. doi: 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner MS, Sens P, Socci ND. Nonequilibrium raftlike membrane domains under continuous recycling. Phys Rev Lett. 2005;95:1683–1701. doi: 10.1103/PhysRevLett.95.168301. [DOI] [PubMed] [Google Scholar]
- 46.Sharma P, Verma R, Sarasji RC, K Ira, G Gousset, Rao Krishnamoorthy M, Mayor S. Nanoscale Organization of Multiple GPI-Anchored Proteins in Living Cell Membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- 47.Prior IA, Muncke C, Parton RG, Hancock JF. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J Cell Biol. 2003;160:165–170. doi: 10.1083/jcb.200209091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plowman SJ, Muncke C, Parton RG, Hancock JF. H-ras, K-ras, and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton. Proc Natl Acad Sci USA. 2005;102:15500–15505. doi: 10.1073/pnas.0504114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson BS, Steinberg SL, Liederman K, Pfeiffer JR, Surviladze Z, Zhang J, Samelson LE, Yang L, Kotula JM, Oliver JM. Markers for detergent-resistant lipid rafts occupy distinct and dynamic domains in native membranes. Mol Biol Cell. 2004;15:2580–2592. doi: 10.1091/mbc.E03-08-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swamy MJ, Ciani L, Ge M, Holowka D, Baird B, Freed JH. Coexisting domains in the plasma membranes of live cells characterized by spin-label ESR spectroscopy. Biophys J. 2006;90:4452–4465. doi: 10.1529/biophysj.105.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayor S, Maxfield FR. Insolubility and redistribution of GPI-anchored proteins at the cell surface after detergent treatment. Mol Biol Cell. 1995;6:929–944. doi: 10.1091/mbc.6.7.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao M, Mukherjee S, Maxfield FR. Cholesterol depletion induces large scale domain segregation in living cell membranes. Proc Natl Acad Sci USA. 2001;98:13072–13077. doi: 10.1073/pnas.231377398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Subczynski WK, Kusumi A. Dynamics of raft molecules in the cell and artificial membranes: approaches by pulse EPR spin labeling and single molecule optical microscopy. Biochim Biophys Acta. 2003;1610:231–243. doi: 10.1016/s0005-2736(03)00021-x. [DOI] [PubMed] [Google Scholar]
- 54.Pralle A, Keller P, Florin EL, Simons K, Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheets ED, Lee GM, Simson R, Jacobson K. Transient confinement of a glycosylphosphatidylinositol-anchored protein in the plasma membrane. Biochemistry. 1997;36:12449–12458. doi: 10.1021/bi9710939. [DOI] [PubMed] [Google Scholar]
- 56.Dietrich C, Yang B, Fujiwara T, Kusumi A, Jacobson K. Relationship of lipid rafts to transient confinement zones detected by single particle tracking. Biophys J. 2002;82:274–282. doi: 10.1016/S0006-3495(02)75393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ritchie K, Ino R, Fujiwara T, Murase K, Kusumi A. The fence and picket structure of the plasma membrane of live cells as revealed by single molecule techniques. Mol Membr Biol. 2003;5:626–632. doi: 10.1080/0968768021000055698. [DOI] [PubMed] [Google Scholar]
- 58.Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- 59.Saffman PG, Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci USA. 1975;72:3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vrljic M, Nishimura SY, Brasselet B, Moerner WE, McConnell HM. Translational diffusion of individual class II MHC membrane proteins in cells. Biophys J. 2002;83:2681–2692. doi: 10.1016/S0006-3495(02)75277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vrljic M, Nishimura SY, Moerner WE, McConnell HM. Cholesterol depletion suppresses the translational movement of class II major histocompatibility complex proteins in the plasma membrane. Biophys J. 2005;88:334–347. doi: 10.1529/biophysj.104.045989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kenworthy AK, Nichols BJ, Remmert CL, Hendrix GM, Kumar M, Zimmerberg J, Lippincott-Schwartz J. Dynamics of putative raft-associated proteins at the cell surface. J Cell Biol. 2004;165:735–746. doi: 10.1083/jcb.200312170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kusumi A, Suzuki K. Toward understanding the dynamics of membrane-raft-based molecular interactions. Biochim Biophys Acta. 2005;1746:234–251. doi: 10.1016/j.bbamcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Field KA, Holowka D, Baird B. FcεRI-mediated recruitment of p53/56lyn to detergent-resistant membrane domains accompanies cellular signaling. Proc Natl Acad Sci U S A. 1995;92:9201–9205. doi: 10.1073/pnas.92.20.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Field KA, Holowka D, Baird B. Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J Biol Chem. 1997;272:4276–4280. doi: 10.1074/jbc.272.7.4276. [DOI] [PubMed] [Google Scholar]
- 66.Young RM, Zheng X, Holowka D, Baird B. Reconstitution of regulated phosphorylation of FcepsilonRI by a lipid raft-excluded protein-tyrosine phosphatase. J Biol Chem. 2005;280:1230–1235. doi: 10.1074/jbc.M408339200. [DOI] [PubMed] [Google Scholar]
- 67.Larson DR, Gosse JA, Holowka DA, Baird BA, Webb WW. Temporally resolved interactions between antigen-stimulated IgE receptors and Lyn kinase on living cells. J Cell Biol. 2005;171:527–536. doi: 10.1083/jcb.200503110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu M, Holowka D, Craighead HG, Baird B. Visualization of plasma membrane compartmentalization with patterned lipid bilayers. Proc Natl Acad Sci. 2004;101:13798–13803. doi: 10.1073/pnas.0403835101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nicolau DV, Jr, Burrage K, Parton RG, Hancock JF. Identifying optimal lipid raft characteristics required to promote nanoscale protein-protein interactions on the plasma membrane. Mol Cell Biol. 2006;26:313–323. doi: 10.1128/MCB.26.1.313-323.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 72.Saito T, Yokosuka T. Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr Opin Immunol. 2006;18:305–313. doi: 10.1016/j.coi.2006.03.014. [DOI] [PubMed] [Google Scholar]


