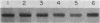Abstract
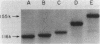
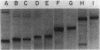
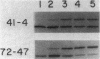
Five classes of MalE-LacZ hybrid proteins have previously been characterized. These proteins differ in the amount of the maltose-binding protein (MBP) that is attached to beta-galactosidase. Although none of these proteins is secreted into the periplasm, the four larger classes of hybrid proteins, those that include an intact MBP signal peptide, are inserted into the cytoplasmic membrane, suggesting that the secretion process has at least been initiated. In this study, we demonstrated that some portion of the four larger hybrid proteins can be translocated across the cytoplasmic membrane, thus permitting processing of the signal peptide. We have found that hybrid proteins that include only a small portion of the mature MBP are inefficiently recognized as exported proteins, and translocation and processing of these appear to be relatively slow, posttranslational events. In marked contrast, hybrid proteins that include a substantial portion of the mature MBP are efficiently recognized, and translocation and processing of these occur very rapidly, possibly cotranslationally. Our results complement other studies and very strongly suggest a role for the mature MBP in the export process.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankaitis V. A., Bassford P. J., Jr Regulation of adenylate cyclase synthesis in Escherichia coli: studies with cya-lac operon and protein fusion strains. J Bacteriol. 1982 Sep;151(3):1346–1357. doi: 10.1128/jb.151.3.1346-1357.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis V. A., Rasmussen B. A., Bassford P. J., Jr Intragenic suppressor mutations that restore export of maltose binding protein with a truncated signal peptide. Cell. 1984 May;37(1):243–252. doi: 10.1016/0092-8674(84)90320-9. [DOI] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Emr S. D., Silhavy T. J., Beckwith J., Beduelle H., Clément J. M., Hedgpeth J., Hofnung M. The genetics of protein secretion in Escherichia coli. Methods Cell Biol. 1981;23:27–38. doi: 10.1016/s0091-679x(08)61489-2. [DOI] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Silhavy T. J., Beckwith J. R. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J Bacteriol. 1979 Jul;139(1):19–31. doi: 10.1128/jb.139.1.19-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P., Beckwith J. Escherichia coli mutants accumulating the precursor of a secreted protein in the cytoplasm. Nature. 1979 Feb 15;277(5697):538–541. doi: 10.1038/277538a0. [DOI] [PubMed] [Google Scholar]
- Bedouelle H., Bassford P. J., Jr, Fowler A. V., Zabin I., Beckwith J., Hofnung M. Mutations which alter the function of the signal sequence of the maltose binding protein of Escherichia coli. Nature. 1980 May 8;285(5760):78–81. doi: 10.1038/285078a0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Silhavy T. J., Bassford P. J., Jr, Shuman H. A., Beckwith J. R. Sites within gene lacZ of Escherichia coli for formation of active hybrid beta-galactosidase molecules. J Bacteriol. 1979 Jul;139(1):13–18. doi: 10.1128/jb.139.1.13-18.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Goodman J. M., Wickner W. T. Procoat, the precursor of M13 coat protein, requires an electrochemical potential for membrane insertion. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4669–4673. doi: 10.1073/pnas.77.8.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Bassford P. J., Jr Localization and processing of outer membrane and periplasmic proteins in Escherichia coli strains harboring export-specific suppressor mutations. J Biol Chem. 1982 May 25;257(10):5852–5860. [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Purification, structure, and properties of hybrid beta-galactosidase proteins. J Biol Chem. 1983 Dec 10;258(23):14354–14358. [PubMed] [Google Scholar]
- Herrero E., Jackson M., Bassford P. J., Sinden D., Holland I. B. Insertion of a MalE beta-galactosidase fusion protein into the envelope of Escherichia coli disrupts biogenesis of outer membrane proteins and processing of inner membrane proteins. J Bacteriol. 1982 Oct;152(1):133–139. doi: 10.1128/jb.152.1.133-139.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Ito K., Beckwith J. R. Role of the mature protein sequence of maltose-binding protein in its secretion across the E. coli cytoplasmic membrane. Cell. 1981 Jul;25(1):143–150. doi: 10.1016/0092-8674(81)90238-5. [DOI] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Different exported proteins in E. coli show differences in the temporal mode of processing in vivo. Cell. 1981 Jul;25(1):151–157. doi: 10.1016/0092-8674(81)90239-7. [DOI] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Processing in vivo of precursor maltose-binding protein in Escherichia coli occurs post-translationally as well as co-translationally. J Biol Chem. 1981 Mar 10;256(5):2504–2507. [PubMed] [Google Scholar]
- Koshland D., Botstein D. Evidence for posttranslational translocation of beta-lactamase across the bacterial inner membrane. Cell. 1982 Oct;30(3):893–902. doi: 10.1016/0092-8674(82)90294-x. [DOI] [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Mutations in a new gene, secB, cause defective protein localization in Escherichia coli. J Bacteriol. 1983 Apr;154(1):253–260. doi: 10.1128/jb.154.1.253-260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Synthesis of exported proteins by membrane-bound polysomes from Escherichia coli. Eur J Biochem. 1977 May 2;75(1):43–53. doi: 10.1111/j.1432-1033.1977.tb11502.x. [DOI] [PubMed] [Google Scholar]
- Randall L. L. Translocation of domains of nascent periplasmic proteins across the cytoplasmic membrane is independent of elongation. Cell. 1983 May;33(1):231–240. doi: 10.1016/0092-8674(83)90352-5. [DOI] [PubMed] [Google Scholar]