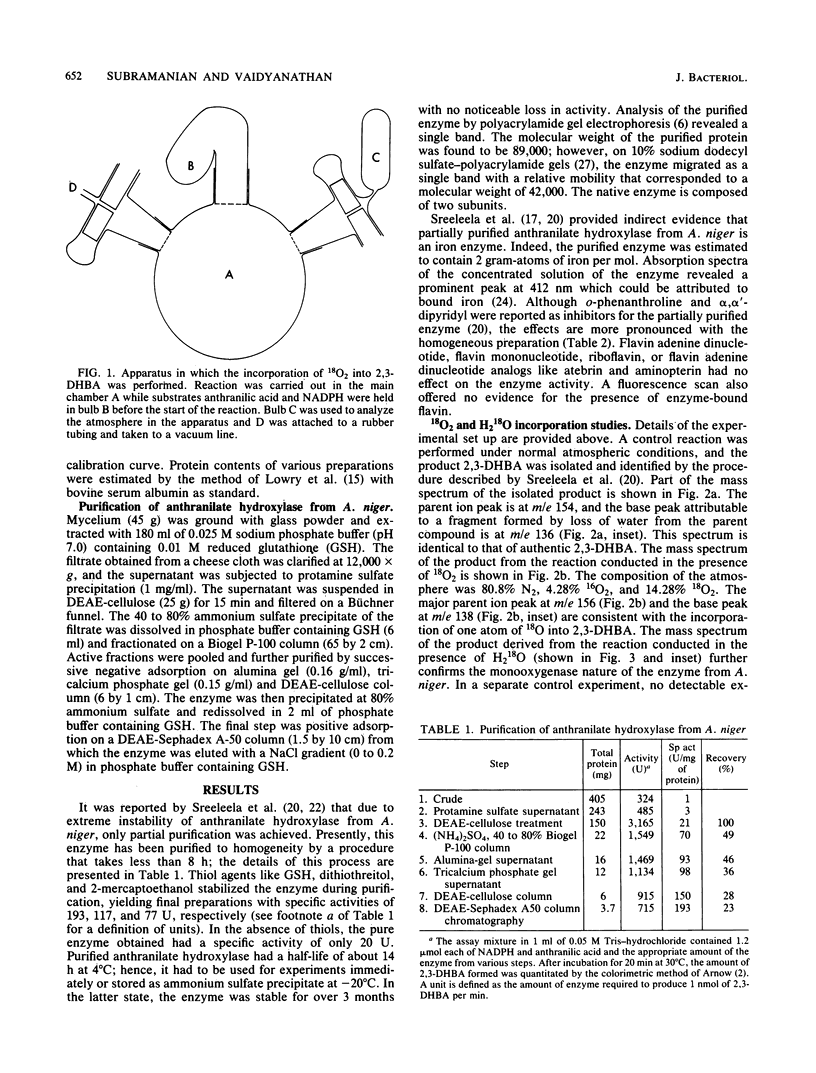
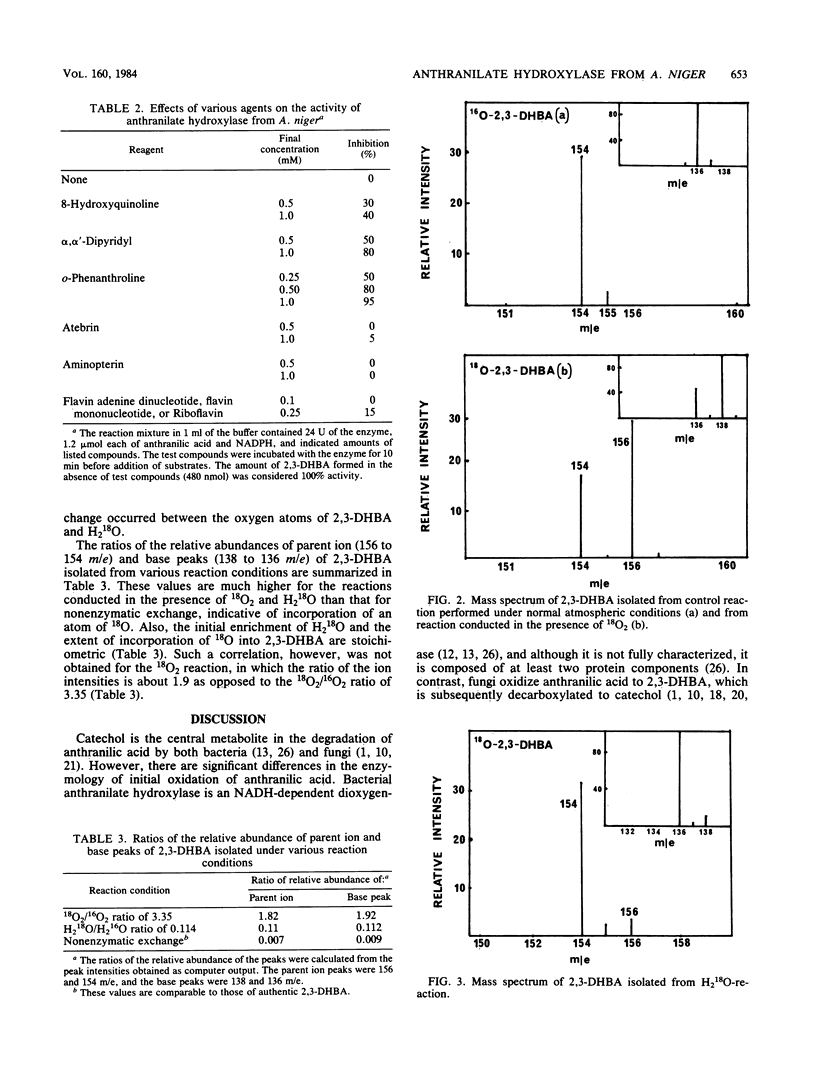
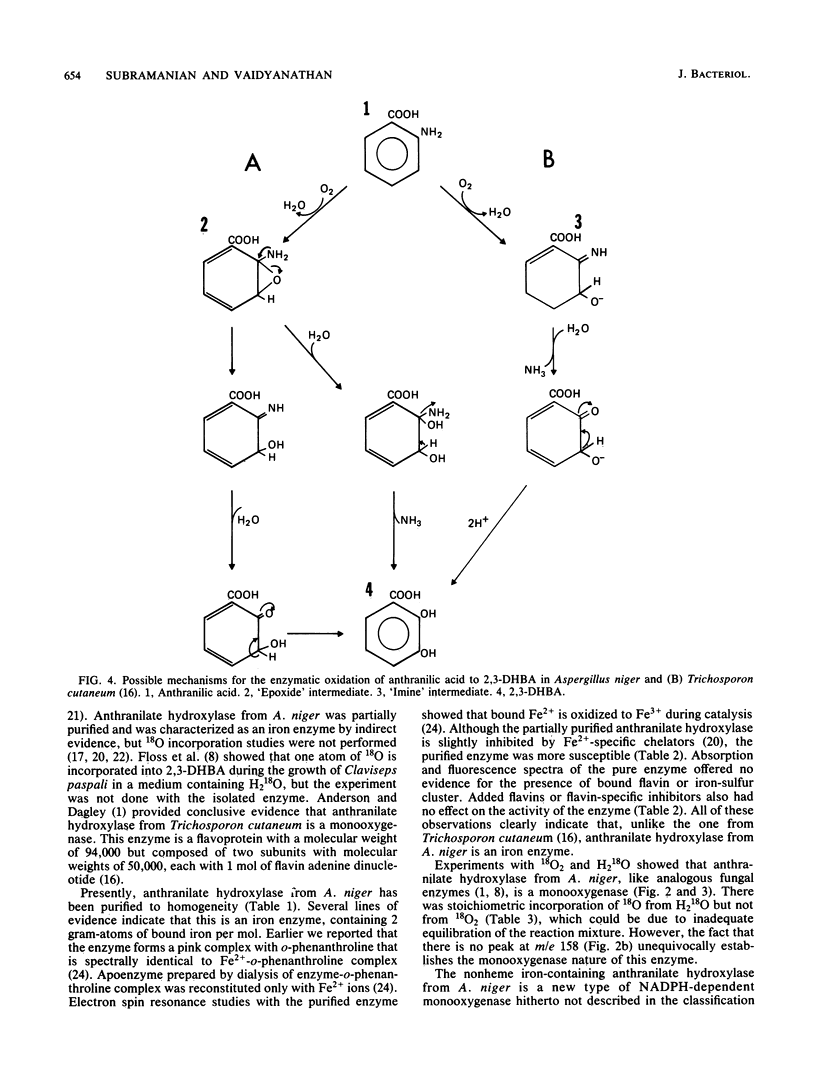
Abstract
Anthranilate hydroxylase from Aspergillus niger catalyzes the oxidative deamination and dihydroxylation of anthranilic acid to 2,3-dihydroxybenzoic acid. This enzyme has been purified to homogeneity and has a molecular weight of 89,000. The enzyme is composed of two subunits of 42,000 with 2 gram-atoms of nonheme iron per mol. Fe2+-chelators like alpha,alpha'-dipyridyl and o-phenanthroline are potent inhibitors of the enzyme activity. Absorption and fluorescence spectra of the enzyme offer no evidence for the presence of other cofactors like flavin. Flavins and flavin-specific inhibitors like atebrin have no effect on the activity of the enzyme. The enzyme incorporates one atom of oxygen each from 18O2 and H218O into the product 2,3-dihydroxybenzoic acid. Based on these studies, it is concluded that anthranilate hydroxylase from A. niger is a new type of NADPH-linked nonheme iron monooxygenase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Dagley S. Catabolism of tryptophan, anthranilate, and 2,3-dihydroxybenzoate in Trichosporon cutaneum. J Bacteriol. 1981 Apr;146(1):291–297. doi: 10.1128/jb.146.1.291-297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axcell B. C., Geary P. J. Purification and some properties of a soluble benzene-oxidizing system from a strain of Pseudomonas. Biochem J. 1975 Jan;146(1):173–183. doi: 10.1042/bj1460173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYRDE R. J., HARRIS J. F., WOODCOCK D. Fungal detoxication, the metabolism of -(2-naphthyloxy)-n-alkylcarboxylic acids by Aspergillus niger. Biochem J. 1956 Sep;64(1):154–160. doi: 10.1042/bj0640154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Ensley B. D., Gibson D. T., Laborde A. L. Oxidation of naphthalene by a multicomponent enzyme system from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1982 Mar;149(3):948–954. doi: 10.1128/jb.149.3.948-954.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floss H. G., Guenther H., Groeger D., Erge D. Origin of the oxygen atoms in the conversion of anthranilic acid to 2,3-dihydroxybenzoic acid by Claviceps paspali. Arch Biochem Biophys. 1969 Apr;131(1):319–320. doi: 10.1016/0003-9861(69)90138-6. [DOI] [PubMed] [Google Scholar]
- ICHIHARA A., ADACHI K., HOSOKAWA K., TAKEDA Y. The enzymatic hydroxylation of aromatic carboxylic acids; substrate specificities of anthranilate and benzoate oxidases. J Biol Chem. 1962 Jul;237:2296–2302. [PubMed] [Google Scholar]
- Kobayashi S., Kuno S., Itada N., Hayaishi O., Kozuka S., Oae S. O-18 studies on anthranilate hydroxylase--a novel mechanism of double hydroxylation. Biochem Biophys Res Commun. 1964 Aug 11;16(6):556–561. doi: 10.1016/0006-291x(64)90192-5. [DOI] [PubMed] [Google Scholar]
- Kumar R. P., Streeleela N. S., Rao P. V., Vaidyanathan C. S. Anthranilate hydroxylase from Aspergillus niger: evidence for the participation of iron in the double hydroxylation reaction. J Bacteriol. 1973 Mar;113(3):1213–1216. doi: 10.1128/jb.113.3.1213-1216.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- RAMSAY W. N. M. The determination of iron in blood plasma or serum. Biochem J. 1953 Jan;53(2):227–231. doi: 10.1042/bj0530227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P. V., Moore K., Towers G. H. The conversion of tryptophan to 2,3-dihydroxybenzoic acid and catechol by Aspergillus niger. Biochem Biophys Res Commun. 1967 Sep 27;28(6):1008–1012. [PubMed] [Google Scholar]
- Rao S., Sreeleela N. S., Premkumar R., Vaidyanathan C. S. Anthranilic acid hydroxylase from Aspergillus niger. Biochem Biophys Res Commun. 1968 Apr 19;31(2):193–196. [PubMed] [Google Scholar]
- Sreeleela N. S., SubbaRao P. V., Premkumar R., Vaidyanathan C. S. A new anthranilic acid hydroxylase from Aspergillus niger. Purification and properties. J Biol Chem. 1969 May 10;244(9):2293–2298. [PubMed] [Google Scholar]
- Subramanian V., Liu T. N., Yeh W. K., Gibson D. T. Toluene dioxygenase: purification of an iron-sulfur protein by affinity chromatography. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1131–1139. doi: 10.1016/0006-291x(79)91998-3. [DOI] [PubMed] [Google Scholar]
- Subramanian V., Sugumaran M., Vaidyanathan C. S. Anthranilate hydroxylase, an iron enzyme, from Aspergillus niger. Indian J Biochem Biophys. 1979 Dec;16(6):370–374. [PubMed] [Google Scholar]
- TANIUCHI H., HATANAKA M., KUNO S., HAYAISHI O., NAKAJIMA M., KURIHARA N. ENZYMATIC FORMATION OF CATECHOL FROM ANTHRANILIC ACID. J Biol Chem. 1964 Jul;239:2204–2211. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamaguchi M., Fujisawa H. Purification and characterization of an oxygenase component in benzoate 1,2-dioxygenase system from Pseudomonas arvilla C-1. J Biol Chem. 1980 Jun 10;255(11):5058–5063. [PubMed] [Google Scholar]


