Abstract
Caenorhabditis elegans gut granules are lysosome-related organelles with birefringent contents. mrp-4, which encodes an ATP-binding cassette (ABC) transporter homologous to mammalian multidrug resistance proteins, functions in the formation of gut granule birefringence. mrp-4(−) embryos show a delayed appearance of birefringent material in the gut granule but otherwise appear to form gut granules properly. mrp-4(+) activity is required for the extracellular mislocalization of birefringent material, body-length retraction, and NaCl sensitivity, phenotypes associated with defective gut granule biogenesis exhibited by embryos lacking the activity of GLO-1/Rab38, a putative GLO-1 guanine nucleotide exchange factor GLO-4, and the AP-3 complex. Multidrug resistance protein (MRP)-4 localizes to the gut granule membrane, consistent with it playing a direct role in the transport of molecules that compose and/or facilitate the formation of birefringent crystals within the gut granule. However, MRP-4 is also present in oocytes and early embryos, and our genetic analyses indicate that its site of action in the formation of birefringent material may not be limited to just the gut granule in embryos. In a search for genes that function similarly to mrp-4(+), we identified WHT-2, another ABC transporter that acts in parallel to MRP-4 for the formation of birefringent material in the gut granule.
ATP-binding cassette (ABC) transporters comprise a large family of membrane-associated proteins that couple ATP hydrolysis with substrate translocation (Dassa 2003). In eukaryotic cells, ABC transporters typically function in the export of material from the cytoplasm or cytoplasmic leaflet of a cellular membrane (Higgins 1992). ABC proteins transport a variety of substrates including toxic compounds, peptides, amino acids, lipids, ions, heme, and sugars. Consequently, their activities are implicated in a variety of important physiological and disease processes. ABC-transporter function often underlies multidrug resistance to therapeutic drugs used to treat cancer and viral diseases, and mutations in 14 of the 48 ABC transporters in humans are associated with specific diseases (Borst and Elferink 2002; Kaminski et al. 2006). Thus, elucidating the functions of ABC transporters in basic cellular processes is essential for a clear understanding of organismal development and physiology.
The soil nematode Caenorhabditis elegans has emerged as a model system to identify and analyze ABC-transporter functions in the context of a simple multicellular animal. The C. elegans genome encodes 61 ABC transporters with representatives in all eight subfamilies identified in Drosophila and humans (Sheps et al. 2004; Zhao et al. 2007). Functions for 18 C. elegans ABC transporters have been defined through genetic studies. Like humans, C. elegans ABC transporters are involved in a wide range of processes including apoptotic cell corpse removal (Wu and Horvitz 1998), dauer formation (Yabe et al. 2005), RNAi (Sundaram et al. 2006b), directed sperm motility (Kubagawa et al. 2006), as well as resistance to toxins (Broeks et al. 1995), heavy metals (Broeks et al. 1996; Vatamaniuk et al. 2005), and pathogens (Mahajan-Miklos et al. 1999).
Recently, two ABC transporters, PGP-2 and MRP-4, were identified as functioning in the endolysosomal system of C. elegans intestinal cells (Nunes et al. 2005; Schaheen et al. 2006; Schroeder et al. 2007). PGP-2 is associated with the gut granule, a cell-type-specific lysosome-related organelle that functions in fat storage, where it plays an essential role in its biogenesis (Schroeder et al. 2007). pgp-2(−) embryos improperly form gut granules and mislocalize birefringent material normally found only within the gut granule into the intestinal lumen. Disrupting the function of mrp-4 suppresses the formation of enlarged, fat-containing, late endosomal/lysosomal compartments formed in the intestinal cells of cup-5(−) mutant embryos (Schaheen et al. 2006). CUP-5, which is orthologous to human mucolipin-1, functions in trafficking from late endosomes to lysosomes (Treusch et al. 2004). The phenotype of mrp-4(−); cup-5(−) double mutants suggests a role for mrp-4 in the formation of gut granules.
Here we present an analysis of mrp-4(+) function in C. elegans embryos that define its role in gut granule formation. In addition, we identify another ABC transporter, WHT-2, as functioning similarly to MRP-4 during gut granule formation. We find that these two ABC transporters are not required for the biogenesis of gut granules per se, but instead promote the differentiation of these organelles by functioning in parallel to mediate their accumulation of birefringent material.
MATERIALS AND METHODS
C. elegans alleles and genetics:
N2 was used as the wild-type strain. All strains were grown at 22° and cultured as described (Brenner 1974). Mutant alleles are listed by chromosome and references are listed at http://www.wormbase.org:
LGI: apt-6(ok429), dpy-5(e61), haf-4(gk420), haf-4(ok1042), pgp-2(kx48), rrf-1(pk1417)
LGII: wht-4(ok1007)
LGIII: mrp-8(ok1360), wht-1(tm688), wht-3(ok927), wht-6(ok882), wht-7(ok812), wht-9(ok1044)
LGIV: fat-1(wa9), fat-2(wa17), fat-3(ok1126), fat-3(wa22), fat-4(ok958), fat-4(wa14), fat-6(tm331), mIs11[myo-2∷gfp, pes-1∷gfp, gut∷gfp], rme-2(b1008), rsd-2(pk3307), wht-5(ok806)
LGV: dpy-11(e224), cft-1(ok1180), fat-7(wa36), glo-4(ok623)
LGX: apt-7(tm920), glo-1(zu437), mrp-1(pk89), mrp-3(ok955), mrp-4(cd8), mrp-4(ok1095), mrp-6(ok1027), rsd-3(pk2013)
LG unknown: pwIs50[lmp-1∷gfp].
Using standard genetic techniques, the delayed accumulation of birefringent material in mrp-4(cd8) and mrp-4(ok1095) embryos was found to be recessive and shows a maternal affect. mrp-4(ok1095) was backcrossed to N2 prior to analysis. Presence of the mrp-4(ok1095) deletion was confirmed by PCR. Fosmid WRM0632cB05 was found to rescue the delayed accumulation of birefringent material of mrp-4(ok1095) embryos in one of three lines.
To create double mutants, mrp-4(ok1095) was mated into genetically marked (dpy-5 or dpy-11) Glo mutants to generate transheterozygous animals. Their Dpy progeny were allowed to self-fertilize. The embryonic progeny of the Dpy worms were scored for the delayed accumulation of birefringence. At least two independent double-mutant lines were isolated and in all cases they showed the same phenotype.
RNA interference was carried out by feeding, using clones from a C. elegans RNAi library (Geneservice, Cambridge, UK) as described by Kamath et al. (2003). The embryonic or larval progeny of L1–L2 stage animals placed upon RNAi plates were scored.
Microscopy:
Staining of embryonic and adult gut granules with acridine orange (Sigma, St. Louis) or Nile Red (Molecular Probes, Eugene, OR) was as described using a Zeiss Axioskop II plus microscope (Thornwood, NY) equipped with DIC, polarization, and fluorescence optics (Hermann et al. 2005; Schroeder et al. 2007). Endocytosis across the apical domain of intestinal cells was assessed with TRITC-BSA (Hermann et al. 2005). Autofluorescent material present within adult and embryonic gut cells was visualized with Zeiss 15 (Ex:BP586/12; EM:LP590) and Zeiss 2 (Ex:365; Em:LP420) filters, respectively. The appearance and localization of birefringent material was scored with polarization optics. In time-course experiments, embryonic length and the presence of birefringent material were scored every 45 min beginning 420 min after the first cell cleavage (1.5-fold stage) at 24°. In time-course experiments, glo-4(ok623) was linked to dpy-11(e224) and both apt-6(ok429) and pgp-2(kx48) were linked to dpy-5(e61). The dpy mutations did not affect the elongation or retraction of embryonic body length on their own, or the retraction exhibited by glo mutant embryos.
Embryos were fixed as described by Leung et al. (1999) and immunostained with affinity-purified rabbit MRP-4 (this work), FUS-1 (Kontani et al. 2005), PGP-2 (Schroeder et al. 2007), and mouse 3E6 GFP antisera (Qbiogene, Carlesbad, CA). MRP-4 was detected by incubating in primary antibodies at either 4° overnight or at 37° for 2 hr. In some experiments, MRP-4 or PGP-2 was localized in kxEx11[glo-1∷gfp] (Hermann et al. 2005), pwIs50[lmp-1∷gfp] (Treusch et al. 2004), pwIs72[vha-6∷rab-5∷gfp] (Hermann et al. 2005), pwIs170[vha-6∷rab-7∷gfp] (Chen et al. 2006), pwIs87[vha-6∷rme-1∷gfp] (Hermann et al. 2005), or bIs33[rme-8∷gfp] (Zhang et al. 2001) containing embryos. Colocalization was scored in pretzel-stage embryos by analyzing the extent of overlap between MRP-4- or PGP-2-stained and GFP-stained structures in merged images. Adult intestinal cells were fixed in methanol at −20° for 10 min, postfixed in acetone at −20° for 10 min, and immunostained with anti-PGP-2 antibodies overnight at 4° using published procedures (Shaham 2006). Adult gonads were prepared for staining as described by Shaham (2006) with the following modifications: the primary fix was for 10 min in 1% paraformaldehyde, with the addition of a 5-min methanol postfix at −20°, and all rinses were done with 1X PBS + 20μm BSA.
The presence, number, and morphology of RAB-5∷GFP, RAB-7∷GFP, and LMP-1∷GFP fusion protein-containing organelles within intestinal cells were assessed in living F33E2.4(RNAi), mrp-4(RNAi), and wht-2(RNAi) pretzel-stage embryos. GFP-stained organelles were scored in mrp-4(RNAi) embryos lacking birefringent material in their intestinal cells.
MRP-4 antibodies:
Affinity-purified antibodies recognizing MRP-4 were generated by Bethyl Laboratories (Montgomery, TX). Peptides corresponding to amino 1MEPNLQAKLKGIDAFC-cys16 and carboxy 1157cys-ILGARKAMSYFESNRNSC1137 terminal MRP-4 sequences were synthesized, coupled to KLH, and used for immunization. Antibodies were affinity purified using agarose-linked MRP-4 peptides. The specificity of the antisera was confirmed using mrp-4(RNAi) and mrp-4(cd8) embryos. The anti-amino terminal MRP-4 antisera was used in the studies presented here; however, identical results were observed using the anti-carboxy terminal MRP-4 antisera.
Generation of mrp-4∷gfp:
The transcriptional reporter mrp-4∷gfp was constructed using PCR fusion (Hobert 2002). A 2.1-kb sequence 5′ of the start of mrp-4 was amplified using p279 5′-AATAGATGTTTTACCGACACCTGGATCAC-3′ and p280 5′-CAGTGAAAAGTTCTTCTCCTTTACTCATCCGTATCTTCTCTCCTTATTTCGACCG-3′. gfp-nls was amplified from pPD95.67 (Addgene) using p269 5′-ATGAGTAAAGGAGAAGAACTTTTCACTG-3′ and p266 5′-AAGGGCCCGTACGGCCGACTAGTAGG-3′. The two PCR products were fused using p281 5′-TGAGTGCTGCCATTTTGTTCTGAATAT-3′ and p267 5′-GGAAACAGTTATGTTTGGTATATTGGG-3′. The resulting mrp-4∷gfp product was coinjected at 4.5 ng/μl with pRF4[Rol-6D] at 100 ng/μl into wild type. kxEx24[pmrp-4∷gfp; Rol6D] was used for the analysis presented here and three other independently derived lines showed the same GFP expression pattern.
NaCl sensitivity:
For analysis of NaCl sensitivity, embryos were cloned to NGM plates with 50 mm NaCl (standard conditions) or 300 mm NaCl and scored after 48 hr for living larvae. Worms were considered dead if they did not move when prodded by a platinum wire. glo-4(ok623) was linked to dpy-11(e224) and both apt-6(ok429) and pgp-2(kx48) were linked to dpy-5(e61). The dpy mutations did not effect NaCl sensitivity. The results are an average of four independent trials. P-values were calculated using a heteroscedastic t-test.
RESULTS
The appearance of birefringent material in gut granules is delayed in mrp-4(−) embryos:
Birefringent material, identified due to its ability to rotate plane-polarized light, is apparent within gut granules starting at the bean stage of embryogenesis and persists through hatching (Laufer et al. 1980). These mid- to late stages of C. elegans embryonic development, when birefringent material is present, can be distinguished by the body morphology of the embryo as it elongates from a ball of cells to fourfold length relative to the eggshell prior to hatching (Sulston et al. 1983). Currently, the molecular identity of the birefringent material(s) present within the gut granule is unknown; however, the formation and localization of gut granule birefringence is commonly used as a marker of gut cell differentiation (Laufer et al. 1980) and can be used to monitor the biogenesis of gut granules (Hermann et al. 2005).
glo-1(+) is necessary for the biogenesis of gut granules and the proper formation and localization of birefringent material in embryonic intestinal cells (Hermann et al. 2005). In the course of molecularly identifying glo-1(+), we carried out an RNAi screen of predicted genes contained on the cosmid R07B1, which rescues glo-1(−). mrp-4(RNAi) resulted in a partial phenocopy of glo-1(−); the intestinal cells of most mrp-4(RNAi) embryos lacked birefringent material (Table 1). However, four glo-1(−) alleles did not contain alterations in the coding sequence of mrp-4 (not shown). Instead, the glo-1(−) phenotype results from mutations in a Rab GTPase-encoding gene located adjacent to mrp-4 (Hermann et al. 2005).
TABLE 1.
Analysis of birefringent gut granules
| Genotype | % of embryos lacking birefringent material in intestinal cells (n) | % of larvae lacking birefringent material in intestinal cells (n) |
|---|---|---|
| Wild typea | 0 (>100) | 0 (>100) |
| ABC transporters | ||
| mrp-4(cd8) | 93 (99) | 20 (46) |
| mrp-4(ok1095) | 96 (164) | 12 (182) |
| mrp-4(RNAi) | 93 (390) | 14 (227) |
| wht-2(RNAi) | 52 (673) | 5 (137) |
| Double mutants | ||
| mrp-4(cd8); wht-2(RNAi) | 97 (107) | 81 (89) |
| mrp-4(ok1095); wht-2(RNAi) | 100 (109) | 89 (61) |
| Mosaic RNAi | ||
| rrf-1(pk1471)a | 0 (96) | 0 (47) |
| rrf-1(pk1471); mrp-4(RNAi) | 53 (45) | 36 (22) |
| rrf-1(pk1471); wht-2(RNAi) | 3 (119) | 0 (26) |
| rsd-2(pk3307)a | 0 (84) | 0 (45) |
| rsd-2(pk3307); mrp-4(RNAi) | 0 (63) | 0 (40) |
| rsd-2(pk3307); wht-2(RNAi) | 0 (62) | 0 (24) |
| rsd-3(pk2013)a | 0 (88) | 0 (40) |
| rsd-3(pk2013); mrp-4(RNAi) | 45 (107) | 11 (28) |
| rsd-3(pk2013); wht-2(RNAi) | 0 (88) | 0 (40) |
All strains were grown at 22°. Threefold and later stage embryos or L1–L2-stage larvae were scored for the presence of birefringent material in intestinal cells using polarization microscopy. n, the number of embryos or larvae scored.
The same results were seen when grown on RNAi plates expressing double-stranded RNA against F33E2.4, a gene not required for the formation of birefringent gut granules.
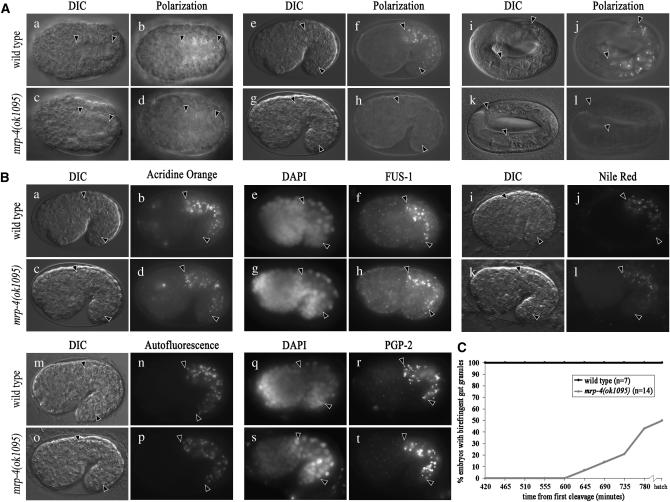
To confirm that mrp-4(+) activity was required for the formation of birefringent material in embryonic gut cells we analyzed embryos containing loss-of-function mutations in mrp-4. Both mrp-4(ok1095) and mrp-4(cd8) (Schaheen et al. 2006) embryos contain differentiated intestinal cells that lacked birefringent material in bean and 1.5-fold-stage embryos (Figure 1A, c–h, and data not shown). The majority of pretzel-stage mrp-4(−) embryos lacked birefringent material (Figure 1A, k and l, and Table 1). In a mixed population, a minority of pretzel-stage mrp-4(−) embryos contained birefringent intestinal granules and most newly hatched mrp-4(−) animals contained birefringent material in the intestinal cells at the first larval stage (L1) (Table 1). The presence of birefringent material exclusively in late-stage embryos suggested that the appearance of birefringent material was delayed in mrp-4(−) embryos. We examined the appearance of birefringent material in individual mrp-4(ok1095) (Figure 1C) and mrp-4(cd8) (not shown) embryos and found that this was indeed the case. We therefore conclude that mrp-4 is necessary for the accumulation of birefringent material within embryonic gut granules.
Figure 1.—
mrp-4 is required for the accumulation of birefringent material within embryonic gut granules. (A) mrp-4(−) embryos often lack birefringent, gut granule-associated material. Using polarization microscopy, birefringent contents of gut granules were visible in wild-type embryos (b, f, and j). In contrast, birefringent puncta were lacking in the intestinal primordium of all bean-stage (d), 1.5-fold-stage (h), and nearly all pretzel-stage (l) mrp-4(−) embryos. (B) mrp-4(−) embryos contain gut granules. Wild-type and mrp-4(−) 1.5-fold-stage embryos displayed acridine orange stained and FUS-1 (V-ATPase subunit) containing gut granules (a–h). Gut granules are additionally characterized by the presence of Nile Red stained fat (j), autofluorescence (n), and the ABC transporter PGP-2 (r). All of these markers were unaltered in mrp-4(−) embryos (l, p, and t). (C) Late-stage wild-type embryos always contained birefringent gut granule material. In contrast, the accumulation of birefringent material was delayed in mrp-4(−) embryos. The intestinal primordium is located between the solid arrowheads in A and B. C. elegans embryos are ∼50 μm long.
mrp-4 encodes a member of the multidrug resistance protein (MRP)/ABCC ABC transporter family. MRP family members were first identified in mammalian systems due to their drug export activity mediating multidrug resistance. However, it is clear that MRP transporters also mediate the movement of a variety of materials across cellular membranes including glutathione conjugates, bile salts, and nucleotides (Borst and Elferink 2002; Deeley et al. 2006; Kruh and Belinsky 2003). While the transport specificity of C. elegans MRPs are unclear, they are known to be necessary for heavy metal resistance (Broeks et al. 1996), dauer formation (Yabe et al. 2005), and RNAi (Sundaram et al. 2006a). C. elegans mrp-4 has recently been shown to contribute to the cellular defects and embryonic lethality resulting from loss of cup-5 function (Schaheen et al. 2006). CUP-5, a C. elegans lysosomal protein functionally orthologous to human mucolipin-1 (Fares and Greenwald 2001; Hersh et al. 2002), regulates a terminal step of lysosomal trafficking (Treusch et al. 2004). cup-5(−) embryos likely mislocalize MRP-4 within the endocytic pathway where its transport activity leads to enlarged, nonfunctional late endosomal compartments that contribute to the embryonic lethality of cup-5(−) (Schaheen et al. 2006).
MRP-4, like other transporters within the ABCC family, is predicted to contain three membrane-spanning and two ATPase domains (supplemental Figure 1 at http://www.genetics.org/supplemental/). mrp-4(ok1095) encodes an in-frame deletion predicted to remove the first ATPase domain and the third membrane-spanning domain of MRP-4 (supplemental Figure 1). As the activity of both ATPase domains are required for the activity of other MRP transporters (Gao et al. 1996; Zhu et al. 1997), mrp-4(ok1095) is likely to be a strong loss-of-function or null allele. The mrp-4(cd8) allele results in a premature stop codon prior to any of the domains known to be required for MRP function and therefore is a likely null allele (Schaheen et al. 2006). As both alleles disrupt MRP-4 expression (see Figure 3) and we have been unable to detect major phenotypic differences between mrp-4(ok1095), mrp-4(cd8), or mrp-4(RNAi) (Table 1) we conclude that they each result in a strong to complete loss-of-function phenotype. In our studies of MRP-4 function, we focused our analysis on the mrp-4(ok1095) allele.
Figure 3.—
Localization and expression of MRP-4. (A–D) Anti-MRP-4 and anti-GFP antibody staining of a 1.25-fold-stage embryo expressing the gut granule localized protein GLO-1∷GFP. White arrows denote examples of MRP-4 and GLO-1∷GFP colocalization to gut granules. Anti-MRP-4 antibody staining of wild-type (E), mrp-4(−) (F–H), and glo mutant (I–L) 1.5-fold-stage embryos is shown. In A–L intestinal cells are located between the black arrowheads. (M–O) MRP-4 does not colocalize with LMP-1∷GFP in wild-type embryos (white arrowheads). (P–R) Many (white arrows), but not all (white arrowheads), of the MRP-4 puncta colocalize with LMP-1∷GFP in intestinal cells of glo-1(−) pretzel-stage embryos. The anti-MRP-4 signal has been increased in glo-1(−) embryos relative to wild type to aid in detection and analysis. (S–Z) Anti-MRP-4 staining of a wild-type and mrp-4(cd8) adult gonads and two-cell-stage embryos. Organelles containing MRP-4 are present in oocytes (marked by brackets in S, T, W, and Z) and early-stage embryos in wild type and are lacking in mrp-4(cd8).
Formation of gut granules in mrp-4(−) embryos and adults:
On the basis of the delay in the appearance of birefringent material, MRP-4 could be required either for the formation of gut granules or, alternatively, for the differentiation of these organelles by specifically promoting the accumulation of birefringent material inside them. Gut granules are acidified organelles that contain subunits of the V-ATPase, fat, autofluorescent material and the ABCB family protein PGP-2 (Laufer et al. 1980; Clokey and Jacobson 1986; Hermann et al. 2005; Schroeder et al. 2007). mrp-4(ok1095) and mrp-4(cd8) (not shown) embryos contained organelles with gut granule characteristics that were similar in number and morphology to wild type (Figure 1B). mrp-4(ok1095) adults similarly contained gut granules (supplemental Figure 2 at http://www.genetics.org/supplemental/). We examined mrp-4(RNAi) embryos expressing the early endocytic marker RAB-5∷GFP and the late endocytic markers RAB-7∷GFP and LMP-1∷GFP (Chen et al. 2006). In all cases, these markers appeared normal in mrp-4(RNAi) pretzel-stage embryos (data not shown), indicating that MRP-4 does not play an essential role in forming these compartments. Together these data indicate that MRP-4 is required for the accumulation of material inside gut granules and not for the biogenesis of these organelles.
mrp-4(+) activity is required for multiple phenotypes associated with the loss of glo gene function:
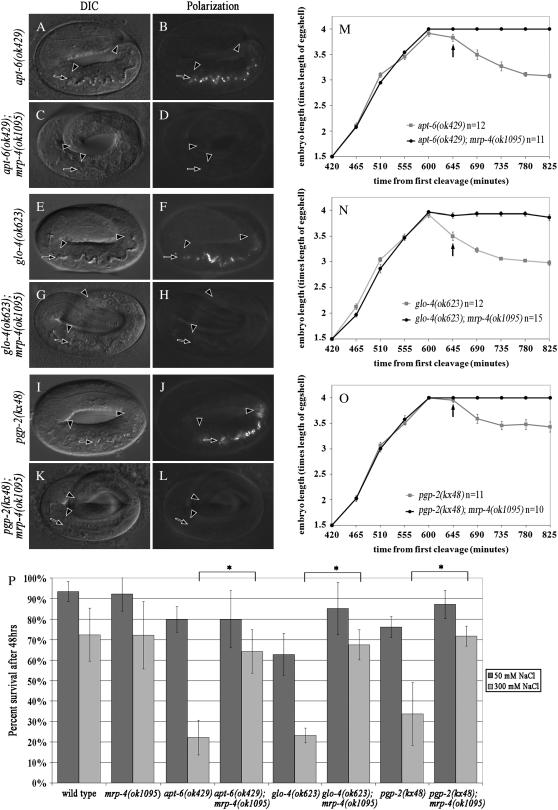
Disrupting the function of seven different glo genes (apt-6, apt-7, glo-1, glo-2, glo-3, glo-4, and pgp-2) results in the mislocalization of birefringent material into the intestinal lumen of late-stage embryos, likely as a consequence of defects in gut granule biogenesis (Hermann et al. 2005; Schroeder et al. 2007). To determine whether the accumulation of birefringent material in the intestinal lumen requires mrp-4(+) activity we performed mrp-4(RNAi) on five different glo mutants and scored for the presence of birefringent material in the intestinal lumen. In addition, we analyzed the localization of birefringent material in the embryonic intestine of apt-6(ok429); mrp-4(ok1095), glo-4(ok623); mrp-4(ok1095), and pgp-2(kx48); mrp-4(ok1095) double mutants. In all cases, disrupting mrp-4(+) activity led to a significant reduction in the number of late-stage embryos that mislocalized birefringent material into the intestinal lumen (Figure 2, A–L, and Table 2). Therefore, the extracellular mislocalization of birefringent material exhibited by mutants defective in gut granule formation is mediated by the activity of mrp-4(+).
Figure 2.—
mrp-4(−) suppresses glo phenotypes. The glo mutant's apt-6(−), glo-4(−), and pgp-2(−) mislocalize birefringent material into the intestinal lumen of pretzel-stage embryos (B, F, and J). glo mutants lacking the activity of mrp-4 typically did not contain birefringent material in the lumen of pretzel-stage embryos (D, H, and L). In A–L the intestinal lumen is marked with a solid arrow and intestinal cells are located between the solid arrowheads. apt-6(−), glo-4(−), and pgp-2(−) embryos elongated to 4-fold in length prior to retracting to 3- to 3.5-fold (M–O). Retraction typically coincided with the appearance of birefringent material in the intestinal lumen (arrow denotes average time of appearance). In M–O the length of individual embryos was monitored every 45 min starting at the 1.5-fold stage, and the bars represent the 95% confidence interval. (P) The survival of apt-6(−), glo-4(−), and pgp-2(−) mutants placed as embryos on 50 mm or 300 mm NaCl plates after 48 hr. The survival of mrp-4(−) double mutants was substantially higher on 300 mm NaCl media than the single glo mutants (*P < .01, t-test). Bars represent the 95% confidence limit.
TABLE 2.
mrp-4(−) and wht-2(−) modify the mislocalization of birefringence exhibited by glo mutants
| Genotype | % of embryos lacking birefringent material in intestinal cells | % of embryos containing birefringent material in the intestinal lumen | n |
|---|---|---|---|
| Wild typea | 0 | 0 | 45 |
| glo-1(zu437)a | 100 | 15 | 97 |
| glo-1(zu437); mrp-4(RNAi) | 100 | 6 | 32 |
| glo-1(zu437); wht-2(RNAi) | 100 | 13 | 134 |
| glo-4(ok623)a | 100 | 53 | 108 |
| glo-4(ok623); mrp-4(RNAi) | 100 | 4 | 26 |
| glo-4(ok623); mrp-4(ok1095)b | 100 | 0 | 200 |
| glo-4(ok623); wht-2(RNAi) | 100 | 53 | 103 |
| pgp-2(kx48)ac | 0 | 0 | 130 |
| pgp-2(kx48); mrp-4(RNAi) | 90 | 0 | 77 |
| pgp-2(kx48); mrp-4(ok1095)b | 98 | 0 | 246 |
| pgp-2(kx48); wht-2(RNAi) | 96 | 57 | 141 |
| pgp-2(kx48); mrp-4(ok1095); wht-2(RNAi)b | 100 | 11 | 35 |
| apt-6(ok429)a | 0 | 16 | 120 |
| apt-6(ok429); mrp-4(RNAi) | 100 | 0 | 25 |
| apt-6(ok429); mrp-4(ok1095)b | 100 | 0 | 107 |
| apt-6(ok429); wht-2(RNAi) | 100 | 58 | 153 |
| apt-6(ok429); mrp-4(ok1095); wht-2(RNAi)b | 100 | 15 | 34 |
| apt-7(tm920)a | 1 | 35 | 97 |
| apt-7(tm920); mrp-4(RNAi) | 66 | 10 | 39 |
| apt-7(tm920); wht-2(RNAi) | 22 | 58 | 71 |
All strains were grown at 22°. Later than twofold-stage embryos were scored for the presence of birefringent material in intestinal cells and in the intestinal lumen using polarization microscopy. n, the number of embryos scored.
Strains were grown on RNAi plates expressing double-stranded RNA against F33E2.4, a gene not required for the formation of birefringent gut granules.
Strains contained a linked dpy-5(e61) or dpy-11(e224) marker that did not affect the presence of localization of birefringent material.
Zero percent and 23% (n = 117) of pgp-2(kx48) embryos derived from adults grown on regular NGM plates lacked birefringent material in intestinal cells and contained birefringent material in the intestinal lumen, respectively.
Coincident with the appearance of birefringent material in the intestinal lumen, the body length of glo embryos retracts 15–25% (Figure 2, M–O) (Hermann et al. 2005). This relationship suggests that the presence of birefringent material in the intestinal lumen of the glo mutants results in body-length retraction. We examined this possibility by comparing body-length dynamics of single glo(−) and double glo(−); mrp-4(ok1095) mutant embryos, the latter of which do not mislocalize birefringent material into the intestinal lumen. Notably, mrp-4(ok1095) fully suppressed the retraction phenotype of three different glo mutants (Figure 2, M–O).
The postembryonic body length of C. elegans is maintained by high internal turgor pressure and exposure to hyperosmotic stress leads to rapid body-length retraction (Lamitina et al. 2004). It is likely that internal turgor pressure also maintains body length in late-stage, fully elongated embryos (Priess and Hirsh 1986). The extra-embryonic mislocalization of birefringent material into the intestinal lumen could result in hyperosmotic stress, reduced internal turgor pressure, and thus body-length retraction of glo embryos. To examine whether newly hatched glo larvae were hyperosmotically sensitive we exposed them to 300 mm NaCl and measured their survival after 48 hr. apt-6(ok429), glo-4(ok623), and pgp-2(kx48) animals were much more sensitive to high levels of NaCl than wild type (Figure 2P). The majority of glo larvae became paralyzed and unresponsive to touch after hatching on 300 mm NaCl plates, while wild-type worms were only modestly affected. The sensitivity to high NaCl conditions strongly suggests that the glo mutants are hyperosmotically stressed at hatching.
To determine whether the sensitivity of the glo mutants to high NaCl was due to the activity of mrp-4(+), we characterized the survival of apt-6(ok429); mrp-4(ok1095), glo-4(ok623); mrp-4(ok1095), and pgp-2(kx48); mrp-4(ok1095) double mutants on 300 mm NaCl plates. We found that the survival of the glo(−); mrp-4(ok1095) double mutants was similar to wild type (Figure 2P). Together these data show that mrp-4(+) activity is necessary for the mislocalization of birefringence, retraction, and NaCl sensitivity exhibited by the glo mutants. These data are also consistent with our interpretation that MRP-4, unlike other GLO proteins, functions in the formation or accumulation of birefringent material in gut granules as opposed to the biogenesis of gut granules themselves.
MRP-4 is associated with gut granules in embryos:
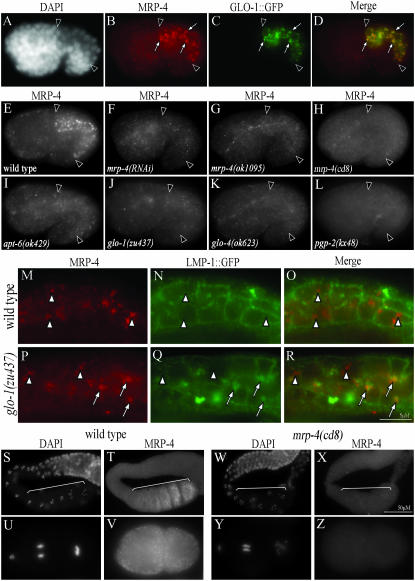
Analysis of reporter constructs containing mrp-4 promoter sequences have shown that mrp-4 is exclusively expressed in mid–late embryonic and adult intestinal cells (Zhao et al. 2004; Schaheen et al. 2006). Genes involved in gut granule biogenesis (glo-1 and pgp-2) are expressed soon after specification of intestinal precursors, and represent early markers of intestinal differentiation (Hermann et al. 2005; Schroeder et al. 2007). To determine when mrp-4 expression first initiates in the intestinal primordium, we analyzed the transcriptional activity of a 2.1-kb mrp-4 promoter fragment containing all of the sequences between the translational start codons of mrp-4 and the 5′ upstream gene, glo-1, by fusing it to gfp. Similar to prior studies, we found expression of mrp-4∷gfp in the intestinal cells of mid-stage embryos and adults (supplemental Figure 3, C–F, at http://www.genetics.org/supplemental/). Embryonic expression of mrp-4∷gfp was first detected in the two intestinal precursors during gastrulation (supplemental Figure 3, A and B), similar to what has been seen for glo-1 and pgp-2. This observation supports a role for mrp-4 during the initial events controlling the differentiation of the gut granule.
We investigated how directly MRP-4 might function in the accumulation of birefringent material by analyzing the subcellular localization of MRP-4 in embryonic intestinal cells. A carboxy-terminal GFP-tagged MRP-4∷GFP fusion protein has been localized to endosomal compartments within C. elegans embryonic intestinal cells (Schaheen et al. 2006). As we found that the MRP-4∷GFP fusion protein did not restore birefringent material in the intestinal cells of mrp-4(ok1095) or mrp-4(cd8) embryos (not shown), we generated and localized MRP-4 with anti-MRP-4 antibodies. Within embryonic intestinal cells, abundant punctate organelles were stained by the anti-MRP-4 antisera (Figure 3E). This staining pattern was not present in mrp-4(ok1095), mrp-4(cd8), or mrp-4(RNAi) embryos (Figure 3, F–H), confirming the specificity of the antisera. We compared the localization of anti-MRP-4 staining to the gut granule localized Rab GLO-1∷GFP (Hermann et al. 2005) in embryos and found that MRP-4 was associated with gut granules from early bean stage through hatching (Figure 3, A–D, and data not shown).
To determine where MRP-4 is localized and possibly functioning when gut granule biogenesis is blocked or altered, we examined the subcellular distribution of MRP-4 in glo(−) embryos. All of the glo(−) mutants displayed greatly diminished staining of intestinal cells by anti-MRP-4 antisera (Figure 3, I–L, and data not shown). The detectable MRP-4 in the glo(−) intestinal primordia localized to weakly stained punctate structures that appeared smaller than wild-type gut granules (Figure 3, E and I–L). This decrease in MRP-4 staining may be due to the mistrafficking of MRP-4 to conventional lysosomes where it is degraded.
We therefore investigated the identity of the organelles containing MRP-4 in glo-1(−) pretzel-stage embryos. We compared the distribution of MRP-4 relative to GFP fusion proteins that in wild-type are localized to early endosomes (RAB-5), recycling endosomes (RME-1), and late endosomes/lysosomes (LMP-1, RAB-7, RME-8) (Grant et al. 2001; Zhang et al. 2001; Chen et al. 2006). MRP-4 showed little colocalization with any of the markers in wild type consistent with its localization to gut granules (supplemental Table II at http://www.genetics.org/supplemental/). However, in glo-1(−) embryos, the weakly staining MRP-4 colocalized with LMP-1∷GFP and to a lesser extent with RAB-7∷GFP. In wild-type embryos 10% of MRP-4 containing puncta colocalized with LMP-1∷GFP (Figure 3, M–O and supplemental Table II at http://www.genetics.org/supplemental/). Whereas in glo-1(−) embryos, 59% of MRP-4 containing puncta colocalized with LMP-1∷GFP (Figure 3, P–R, and supplemental Table II). MRP-4 containing puncta colocalized with RAB-7∷GFP in wild-type embryos 4% of the time. However, 20% of MRP-4 containing puncta colocalized with RAB-7∷GFP in glo-1(−) (supplemental Table II).
We also investigated whether another gut granule-associated ABC transporter, PGP-2 (Schroeder et al. 2007), was mislocalized in glo-1(−) embryos. Similar to MRP-4, PGP-2 rarely colocalized with any of the early or late endosomal markers in wild-type embryos (supplemental Table II at http://www.genetics.org/supplemental/), however, PGP-2 was mislocalized to late endosomal compartments marked by LMP-1∷GFP and RAB-7∷GFP, in glo-1(−) embryos (supplemental Table II). We never saw mislocalization of MRP-4 or PGP-2 to the plasma membrane of intestinal cells in glo-1(−) embryos. This suggests that the birefringent material in the intestinal lumen of glo-1(−) embryos is not due to MRP-4 or PGP-2 activities at the plasma membrane.
mrp-4(+) activity in the germline facilitates the accumulation of birefringent material in gut granules of embryos:
In addition to being expressed in adult and embryonic intestinal cells (Zhao et al. 2004; Schaheen et al. 2006), mrp-4 mRNA is found in the C. elegans hermaphrodite germline (Reinke et al. 2004). As mrp-4(+) is likely to be functional in the adult germline (Kubagawa et al. 2006), we examined the contributions of adult and embryonic mrp-4(+) function in the formation of birefringent material localized to the gut granule. In genetic tests we found that mrp-4(+) activity in the hermaphrodite parent was sufficient for the formation of birefringent granules in mrp-4(−) embryos (supplemental Table III at http://www.genetics.org/supplemental/). In contrast, a single copy of mrp-4(+) in embryos derived from mrp-4(−) mothers was not sufficient for the formation of birefringent material (supplemental Table III). These results point to the adult as a possible site of mrp-4(+) function in gut granule differentiation.
To identify the tissue(s) in which adult mrp-4(+) activity supports gut granule differentiation, we carried out a series of mrp-4(RNAi) experiments in mutants that are permissive for RNAi in specific adult tissues. rrf-1(pk1417) mutants are permissive for RNAi in the adult germline but are defective in somatic RNAi (Sijen et al. 2001). The majority of mrp-4(RNAi); rrf-1(pk1417) embryos were defective in the formation of birefringent intestinal material (Table 1), suggesting that mrp-4(+) function in the germline is necessary for the differentiation of gut granules. However, some mrp-4(RNAi); rrf-1(pk1417) animals contained birefringent intestinal granules either due to residual activity of MRP-4 in adult tissues after RNAi or to some requirement of MRP-4 in embryonic tissues. We next utilized C. elegans rsd-2 and rsd-3 mutants, which have been reported to be competent for uptake of the RNAi trigger by, and RNAi in, intestinal cells but that are defective in the spread of RNAi to the adult germline (Tijsterman et al. 2004). We performed mrp-4(RNAi) in both rsd mutant backgrounds and found conflicting results. mrp-4(RNAi) in rsd-2(pk3307) did not promote the loss of gut granule birefringence while mrp-4(RNAi) in rsd-3(pk2013) animals resulted in a substantial number of embryos lacking birefringent material in intestinal cells (Table 1). We addressed the possibility that rsd-3(−) embryos were permissive for germline or embryonic RNAi of mrp-4, by analyzing the expression of MRP-4 in rsd-3(−); mrp-4(RNAi) adult gonads and 1.25-fold-stage intestinal cells. MRP-4 appeared to be expressed at normal levels in both tissues (not shown). However, at present we cannot rule out the possibility that subtle changes in MRP-4 expression underlies the phenotype in rsd-3(−); mrp-4(RNAi) backgrounds. On the basis of our results we conclude that mrp-4(+) function in the germline is necessary for gut granule differentiation. Our data do not preclude the possibility that mrp-4(+) function is similarly required in the adult intestine.
We examined whether the MRP-4 protein is present in the adult germline or early embryos prior to specification of intestinal precursors where it could function in processes necessary for the formation of birefringent gut granule-associated material. MRP-4 was detected on punctate organelles distributed throughout the oocyte cytoplasm (Figure 3, S and T). MRP-4-containing organelles of similar morphology were present in embryonic blastomeres from the 1-cell through the 100-cell stage of early embryogenesis (Figure 3, U and V, and data not shown). We currently do not know the identity of the compartments containing MRP-4 in the gonad or early embryos. Due to the presence of the MRP-4 protein in embryos prior to the initiation of embryonic transcription (Edgar et al. 1994), MRP-4 and possibly its corresponding mRNA must be stored in the oocyte and transferred to the embryos. Therefore, the maternal function of mrp-4(+) in gut granule differentiation might simply result from the stable inheritance of maternally derived MRP-4 which functions at the embryonic gut granule.
Analysis of ABCC transporter function in gut granule differentiation:
The appearance of birefringent material in gut granules of late-stage mrp-4(−) embryos (Figure 1C) indicates that factors in addition to MRP-4 function in the accumulation of birefringent material within the gut granule. We investigated whether other ABC transporters most similar to MRP-4 might play this role. Mutations in and/or RNAi against seven of the eight other ABCC subfamily transporters encoded by the C. elegans genome did not result in any defects in the accumulation of intestinal birefringence (supplemental Table I at http://www.genetics.org/supplemental/). To test for functional redundancy within the ABCC family, we analyzed double mutants between mrp-4 and seven other ABCC subfamily members. Since the majority of mrp-4(−) late-stage embryos lacked birefringent gut granules, whereas L1–L2-stage mrp-4(−) typically contained birefringent material in the intestine (Table 1), we scored for genetic interactions between mrp-4(−) and other ABCC genes in early stage larvae. While none of the double mutants showed a dramatic loss of birefringent material from gut granules (supplemental Table I), mrp-4(−); mrp-3(−) animals were sterile, a phenotype not displayed by either single mutant. As the loss of gut granules and associated birefringence does not lead to sterility (Hermann et al. 2005), this phenotype likely results from a genetic interaction disrupting a non-gut-granule-related process in the germline. Together, these data indicate that of the ABCC transporters analyzed, only mrp-4 plays a significant role in the formation of birefringent material associated with the gut granule.
The WHT-2 ABCG transporter functions in gut granule differentiation:
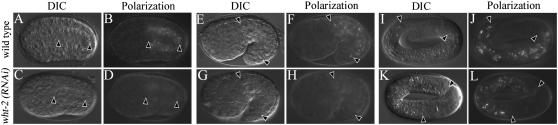
Recently, mrp-4(+) function has been implicated in the directed migration of sperm to the oocyte. The role of mrp-4(+) in this process was apparent only when the activity of an ABCG subfamily member wht-2 was disrupted by RNAi (Kubagawa et al. 2006). We therefore examined whether any ABCG transporters acted on their own or redundantly with MRP-4 in the formation of gut granule birefringence. Of the 9 ABCG-encoding genes in the C. elegans genome, we identified only wht-2 as playing a role in this process (supplemental Table I at http://www.genetics.org/supplemental/). wht-2(RNAi) results in a delayed appearance of birefringent material (Figure 4), similar to what was seen when mrp-4(+) activity was disrupted (Table 1). wht-2(RNAi); mrp-4(−) double-mutant larvae show a dramatic loss of birefringent material from intestinal cells not seen in either single mutant. This observation suggests that wht-2(+) and mrp-4(+) have distinct functions in gut granule differentiation.
Figure 4.—
The accumulation of birefringent material within embryonic gut granules is delayed in wht-2(RNAi) embryos. wht-2(RNAi) bean- and 1.5-fold-stage embryos lacked birefringent, gut granule-associated material (A–H). In contrast, birefringent puncta were present within the intestinal cells of many pretzel-stage wht-2(RNAi) embryos (I–L). Intestinal cells are located between the solid arrowheads.
The sperm motility defect seen in mrp-4(RNAi); wht-2(RNAi) animals resembles the phenotype resulting from mutations in genes involved in the production or transport of polyunsaturated fatty acids (Kubagawa et al. 2006). Interestingly, disrupting genes required for these processes did not result in a loss of birefringent material from embryonic gut granules (supplemental Table I at http://www.genetics.org/supplemental/).
We carried out a series of studies aimed at understanding the role of wht-2 in gut granule formation. Like mrp-4(−), wht-2(RNAi) embryos contained organelles with gut granule characteristics that were similar in number and morphology to wild type (supplemental Figure 4 at http://www.genetics.org/supplemental/). In addition, the appearance of RAB-5∷GFP, RAB-7∷GFP, and LMP-1∷GFP was unaltered in pretzel-stage wht-2(RNAi) embryos (data not shown). mrp-4(cd8); wht-2(RNAi) embryos did not display defects in gut granule biogenesis (data not shown). Thus the mrp-4(+) and wht-2(+) genes function in gut granule differentiation and do not appear to be necessary for gut granule biogenesis.
We next investigated the functional similarities between mrp-4 and wht-2. Like mrp-4, wht-2 is expressed in the intestine and its transcript is enriched in oocytes (Reinke et al. 2004; Zhao et al. 2004). wht-2(RNAi) in rrf-1 and rsd backgrounds showed that wht-2 was required in the germline but not the adult intestine or other adult somatic tissues for the accumulation of birefringent material in gut granules (Table 1). This is in contrast to mosaic mrp-4(RNAi) experiments that suggest a role for mrp-4 outside the adult germline (Table 1). Interestingly, when we examined the requirement of wht-2 in the extracellular accumulation of birefringent material, we found that wht-2(RNAi) affected the Glo phenotype differently than mrp-4(−). mrp-4(+) is required for the accumulation of birefringent material in the intestinal lumen of glo-1(−), glo-4(−), pgp-2(−), apt-6(−), and apt-7(−) embryos (Table 2). In contrast, wht-2(RNAi) did not alter the mislocalization of birefringent material in glo-1(−) and glo-4(−) mutants (Table 2). Additionally, wht-2(RNAi) greatly enhanced the proportion of embryos that mislocalized birefringent material into the intestinal lumen in pgp-2(−), apt-6(−), and apt-7(−) embryos (Table 1). Consistent with mrp-4(+) and wht-2(+) having opposing activities in glo mutants, mrp-4(−); wht-2(RNAi); pgp-2(−) and mrp-4(−); wht-2(RNAi); apt-6(−) triple mutants appeared most similar to the pgp-2(−) or apt-6(−) single mutants (Table 1). The phenotype of the triple mutants also suggests that mechanisms independent of both mrp-4 and wht-2 can mediate the accumulation of birefringent material in the embryonic intestinal lumen.
DISCUSSION
ABC transporter function in lysosomal differentiation:
Gut granules are lysosome-related organelles present within the intestinal cells of C. elegans embryos and adults (Clokey and Jacobson 1986; Hermann et al. 2005). One of the most distinguishing features of embryonic gut granules is their birefringent contents (Laufer et al. 1980), which are not found in any other lysosomal compartment in C. elegans. Therefore, the process of constructing gut granules likely involves cell-type-specific mechanisms that mediate their differentiation and specialization. Our work demonstrates that gut granule differentiation requires the activity of the ABC transporters MRP-4 and WHT-2. Inhibiting mrp-4(+) or wht-2(+) gene function results in the delayed formation of birefringent contents within the intestinal primordium (Figures 1 and 4). These genes are required for the differentiation of the gut granule rather than its biogenesis, as mrp-4(−) and wht-2(−) embryos display autofluorescent, acidified, and fat-, V-ATPase-, and PGP-2-containing gut granules (Figure 1, and supplemental Figure 4 at http://www.genetics.org/supplemental/). From an analysis of embryos containing mutations or RNAi against 49 of 61 predicted ABC transporter genes in C. elegans (this work; Schroeder et al. 2007; and L. K. Schroeder, A. L. Lawrenson and G. J. Hermann, unpublished data), we think it likely that within the C. elegans ABC superfamily, mrp-4 and wht-2 have unique functions in gut granule differentiation.
Our genetic analyses indicate that mrp-4 and wht-2 each function in gut granule differentiation, as either mutant shows a similar delay in the appearance of birefringent gut granule material (Figures 1 and 4). However, mrp-4(−); wht-2(RNAi) double mutants show a more severe loss of gut granule birefringence than either single mutant (Table 1), indicating that even though they function similarly, these genes act independently to promote gut granule differentiation. Similar genetic interactions between mrp-4 and wht-2 have also been seen in their control of directed sperm migration (Kubagawa et al. 2006). One possible explanation of the functional relationship between MRP-4 and WHT-2 is that these proteins transport the same substrates, as has been shown for Ycf1 and Btp1p, two ABCC transporters that function in the differentiation of the lysosome-like vacuole in yeast. These proteins act independently in the transport of material into the vacuole lumen, but interact functionally due to having overlapping substrate specificities (Sharma et al. 2002). It is likely that MRP-4 and WHT-2 function as membrane transporters due to their conservation of sequences essential for transport activity and overall similarity to well-characterized ABC transport proteins (Sheps et al. 2004). However, due to the lack of clear orthology between either of these proteins and mammalian ABC transporters whose substrate specificity has been well characterized (Sheps et al. 2004), it is not possible to accurately predict the type of molecules transported by MRP-4 or WHT-2.
Even without knowing the identity of the substrate transported by MRP-4, our studies of MRP-4 suggest two possibilities for how it might function during gut granule differentiation. MRP-4 might promote gut granule differentiation by directly transporting molecules into the gut granule that are required for the formation of birefringence. In support of this idea, we identified MRP-4 as a gut granule-associated protein (Figure 3). Furthermore, MRP-4 localizes to gut granules immediately prior to and during the early bean stage of embryogenesis (not shown), the time at which birefringent material first becomes observable in the gut granule (Bossinger and Schierenberg 1992). As many other ABC superfamily members are associated with late endosomal/lysosomal compartments in specific metazoan cell types (Mackenzie et al. 2000; Yamano et al. 2001; Zhou et al. 2001; Jedlitschky et al. 2004), differential expression and localization of ABC transporters might be a conserved mechanism mediating the differentiation of lysosomes, many of which exhibit highly specialized functions (Raposo et al. 2007). For example, ABCA3 functions at the lamellar body to facilitate lipid import and the biogenesis of lung surfactant (Yamano et al. 2001; Cheong et al. 2006), ABCB4 likely functions at platelet dense granules to facilitate the uptake of ADP, a key signal in platelet aggregation (Jedlitschky et al. 2004), and the ABCG subfamily proteins White and Scarlet both function at the Drosophila pigment granule to facilitate the uptake of eye color pigments (Mackenzie et al. 2000).
In addition to being localized to gut granules in mid- and late-stage embryos, MRP-4 is also associated with organelles in oocytes and early embryos (Figure 3). We currently do not know the identity of these compartments, however the presence of MRP-4 at these stages suggests that its activity in gut granule differentiation is not restricted to being localized at the gut granule. In support of this, the maternal effect exhibited by mrp-4(−) (supplemental Table III at http://www.genetics.org/supplemental/) and mosaic mrp-4(RNAi) experiments (Table 1) indicate that mrp-4(+) functions in the adult germline, and possibly the adult intestine for the differentiation of the gut granule. For example, MRP-4 might function in these tissues to mediate the transport of substrates that ultimately become, or are required for the formation of birefringent material in embryonic gut granules.
MRP-4 function in the adult is also implicated in the production of a signal-directing sperm migration (Kubagawa et al. 2006). As other genes regulating sperm migration, such as fat-2, fat-3, and rme-2 (Kubagawa et al. 2006), do not show defects in gut granule differentiation (supplemental Table I at http://www.genetics.org/supplemental/), we think it possible that mrp-4 functions in adult tissues to independently control sperm behavior and gut granule differentiation. This could result from MRP-4 mediating the transport of two distinct substrates, a characteristic common to many mammalian ABCC transporters (Deeley et al. 2006).
The Glo phenotype:
Our studies of MRP-4 and WHT-2 provide new insight into the phenotypes associated with the loss of gut granules in C. elegans. In prior work we identified seven different glo genes that when mutated give rise to embryos that mislocalize birefringent material into the embryonic intestinal lumen (Hermann et al. 2005; Schroeder et al. 2007). On the basis of their identity and predicted molecular functions, it is likely that these genes function in distinct steps and processes required for the biogenesis and/or maintenance of gut granules. It is therefore surprising that each of these mutants exhibits body-length retraction (Figure 2, and Hermann et al. 2005) prior to, and NaCl sensitivity after, hatching (Figure 2). Remarkably, in each of the glo mutants that we have examined, mrp-4(+) activity is required for all three phenotypes they exhibit (Figure 2 and Table 2). While it is possible that mrp-4(−) suppresses each of these phenotypes independently, we believe that the role of mrp-4(+) in promoting the mislocalization of birefringent material into the intestinal lumen of the glo mutants is the primary cause of body-length retraction and NaCl sensitivity. In the embryonic intestinal lumen, material assembled into birefringent crystals is likely to be in equilibrium with a high concentration of noncrystalline material, the latter of which could induce hypertonic stress. The resulting decrease in hydrostatic pressure, as the embryo loses water into the intestinal lumen, would lead to body-length retraction and sensitivity to high osmotic conditions at hatching.
In contrast to mrp-4, disrupting the function of wht-2 enhanced or did not alter the mislocalization of birefringent material in glo mutants (Table 2). Moreover, in pgp-2(−) and apt-6(−) backgrounds mrp-4 and wht-2 have opposing activities (Table 2). The striking difference between wht-2 and mrp-4 function in the glo backgrounds might result from these proteins being mislocalized to different subcellular compartments when gut granule biogenesis is disrupted. If one compartment is permissive for release of birefringent material into the intestinal lumen while the other is not, then competition for transport of a common substrate would result in opposing activities.
In glo-1(−) embryos, two gut granule-associated ABC transporters, MRP-4 and PGP-2 became mislocalized to late endosomal/lysosomal compartments, marked by RAB-7∷GFP and LMP-1∷GFP (Figure 3, and supplemental Table II at http://www.genetics.org/supplemental/). It is likely that the MRP-4 and PGP-2 that get mislocalized to these late endosomes/lysosomes are degraded, thus resulting in the observed decrease in the number and intensity of their localization in glo-1(−) embryos. Furthermore, since we did not detect MRP-4 or PGP-2 on the plasma membrane of glo-1(−) embryos, we think that the late endosomal/lysosomal-localized MRP-4 results in the accumulation of material necessary for the formation of birefringent crystals in these compartments. Subsequent trafficking to, or fusions of these compartments with the plasma membrane might result in the release of this birefringent material into the intestinal lumen (Van Der Goot and Gruenberg 2006; Luzio et al. 2007). It remains to be determined where WHT-2 is localized in glo-1(−) embryos, which possibly could account for the different effects of mrp-4(−) and wht-2(−) on the Glo phenotype. More generally, the observation that mrp-4(+) activity contributes to multiple phenotypes resulting from defects in genes controlling gut granule biogenesis, suggests that some symptoms of human diseases with altered lysosome-related organelle biogenesis, such as Hermansky–Pudlak syndrome, might similarly result from the mislocalization of specific ABC transporters.
Birefringent contents of the gut granule:
The presence of birefringent organelles within intestinal cells of soil nematodes, such as C. elegans, has been known for over a century (Chitwood and Chitwood 1974). On the basis of its initial identification in Rhabditis species the birefringent material was termed rhabditin (Cobb 1914); however, the identity of this material has yet to be determined. The birefringent nature of the material that accumulates in the gut granule indicates that it assembles into a crystalline or liquid crystalline structure. The ability to form crystals in vivo is exhibited by many different biological molecules including proteins, lipids, cholesterol, amino acids, and heme (Finzi and Dunlap 2001). As gut granules stain with markers that accumulate in hydrophobic environments (Schroeder et al. 2007), and the material forming birefringent crystals is likely to be very concentrated and therefore among the most abundant molecules in the lumen of the gut granule, we believe it is hydrophobic in nature.
Birefringent granules appear in the intestinal primordium at the same time that yolk, a complex of lipids and lipoproteins (Sharrock et al. 1990; Kubagawa et al. 2006), redistributes to the intestinal primordium (Bossinger and Schierenberg 1992). The coincidence of yolk accumulation and the appearance of birefringent material in the intestinal primordium suggest that yolk-derived components such as lipoproteins, lipids, or cholesterol could accumulate and form birefringent crystals in the gut granule. For this to be the case, only a fraction of the typical levels of yolk would need to be necessary for the formation of wild-type birefringence, as rme-2(−) embryos, which contain substantially reduced yolk (Grant and Hirsh 1999), generate birefringence normally (supplemental Table I at http://www.genetics.org/supplemental/). The material forming birefringent crystals is unlikely to be cholesterol or a cholesterol derivative, as C. elegans contains extremely low levels of sterols (Entchev and Kurzchalia 2005) and none of the Glo mutants are sensitive to low cholesterol growth conditions (E. Currie and G. J. Hermann, unpublished results). Ultimately, the molecular identification of the birefringent material will suggest candidate substrates for both MRP-4 and WHT-2 and in the long term could provide a model system to study human diseases like Niemann-Pick (Elleder et al. 1983), Fabry (Kolter and Sandhoff 2006), amyloidosis (Garcia-Garcia et al. 1999), and cystinosis (Gahl et al. 2002), whose pathology is associated with the accumulation of birefringent material in lysosomes.
Acknowledgments
We are grateful to Hanna Fares, Barth Grant, Kenji Kontani, Joel Rothman, and Jennifer Watts for sharing strains and antibodies. We thank Hanna Fares for comments on the manuscript and members of the Hermann, Lycan, Reiness, and Binford labs for helpful discussions. Julie Engle, Theresa Romeyn, and Melody Rynerson initially observed the suppression of the Glo phenotypes by mrp-4(−) while students in BIO361 at Lewis & Clark College. Some strains were provided by the Caenorhabditis Genetics Center, the C. elegans Knockout Consortium, and the National Bioresource Project for C. elegans. This work was supported by grants from the National Science Foundation (MCB-0314332), the Merck Institute for Science Education, and the Rogers Summer Research Program.
References
- Borst, P., and R. O. Elferink, 2002. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 71: 537–592. [DOI] [PubMed] [Google Scholar]
- Bossinger, O., and E. Schierenberg, 1992. Transfer and tissue-specific accumulation of cytoplasmic components in embryos of Caenorhabditis elegans and Rabditis dolichura: in vivo analysis with a low-cost signal enhancement device. Development 114: 317–330. [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeks, A., B. Gerrard, R. Allikmets, M. Dean and R. H. Plasterk, 1996. Homologues of the human multidrug resistance genes MRP and MDR contribute to heavy metal resistance in the soil nematode Caenorhabditis elegans. EMBO J. 15: 6132–6143. [PMC free article] [PubMed] [Google Scholar]
- Broeks, A., H. W. Janssen, J. Calafat and R. H. Plasterk, 1995. A P-glycoprotein protects Caenorhabditis elegans against natural toxins. EMBO J. 14: 1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. C., P. J. Schweinsberg, S. Vashist, D. P. Mareiniss, E. J. Lambie et al., 2006. RAB-10 is required for endocytic recycling in the Caenorhabditis elegans intestine. Mol. Biol. Cell 17: 1286–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong, N., M. Madesh, L. W. Gonzales, M. Zhao, K. Yu et al., 2006. Functional and trafficking defects in ATP binding cassette A3 mutants associated with respiratory distress syndrome. J. Biol. Chem. 281: 9791–9800. [DOI] [PubMed] [Google Scholar]
- Chitwood, B. G., and M. B. Chitwood, 1974. Introduction to Nematology, pp. 106–107. University Park Press, Baltimore.
- Clokey, G. V., and L. A. Jacobson, 1986. The autofluorescent “lipofuscin granules” in the intestinal cells of Caenorhabditis elegans are secondary lysosomes. Mech. Ageing Dev. 35: 79–94. [DOI] [PubMed] [Google Scholar]
- Cobb, N. A., 1914. Rhabditin: contribution to a science of nematology. J. Parisitol. 1: 40–41. [Google Scholar]
- Dassa, E., 2003. Phylogenetic and functional classification of ABC (ATP-binding cassette) systems, pp. 3–35 in ABC Transporters From Bacteria to Man, edited by I. B. Holland, S. P. C. Cole, K. Kuchler and C. F. Higgins. Elsevier Science, San Diego.
- Deeley, R. G., C. Westlake and S. P. Cole, 2006. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol. Rev. 86: 849–899. [DOI] [PubMed] [Google Scholar]
- Edgar, L. G., N. Wolf and W. B. Wood, 1994. Early transcription in Caenorhabditis elegans embryos. Development 120: 443–451. [DOI] [PubMed] [Google Scholar]
- Elleder, M., J. Hrodek and J. Cihula, 1983. Niemann-Pick disease: lipid storage in bone marrow macrophages. Histochem. J. 15: 1065–1077. [DOI] [PubMed] [Google Scholar]
- Entchev, E. V., and T. V. Kurzchalia, 2005. Requirement of sterols in the life cycle of the nematode Caenorhabditis elegans. Semin. Cell Dev. Biol. 16: 175–182. [DOI] [PubMed] [Google Scholar]
- Fares, H., and I. Greenwald, 2001. Regulation of endocytosis by CUP-5, the Caenorhabditis elegans mucolipin-1 homolog. Nat. Genet. 28: 64–68. [DOI] [PubMed] [Google Scholar]
- Finzi, L., and D. D. Dunlap, 2001. Polarized light microscopy, in Encyclopedia of Life Sciences. John Wiley & Sons, New York (http://www.els.net).
- Gahl, W. A., J. G. Thoene and J. A. Schneider, 2002. Cystinosis. N. Engl. J. Med. 347: 111–121. [DOI] [PubMed] [Google Scholar]
- Gao, M., D. W. Loe, C. E. Grant, S. P. Cole and R. G. Deeley, 1996. Reconstitution of ATP-dependent leukotriene C4 transport by Co-expression of both half-molecules of human multidrug resistance protein in insect cells. J. Biol. Chem. 271: 27782–27787. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia, M., Argiles, A. Gouin-Charnet, M. Durfort, J. Garcia-Valero et al., 1999. Impaired lysosomal processing of beta2-microglobulin by infiltrating macrophages in dialysis amyloidosis. Kidney Int. 55: 899–906. [DOI] [PubMed] [Google Scholar]
- Grant, B., and D. Hirsh, 1999. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 10: 4311–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, B., Y. Zhang, M.-C. Paupard, S. X. Lin, D. Hall et al., 2001. Evidence that RME-1, a conserved C. elegans EH domain protein, functions in endocytic recycling. Nat. Cell Biol. 3: 573–579. [DOI] [PubMed] [Google Scholar]
- Hermann, G. J., L. K. Schroeder, C. A. Hieb, A. M. Kershner, B. M. Rabbitts et al., 2005. Genetic analysis of lysosomal trafficking in Caenorhabditis elegans. Mol. Biol. Cell 16: 3273–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh, B. M., E. Hartwig and H. R. Horvitz, 2002. The Caenorhabdits elegans mucolipin-like gene cup-5 is essential for viability and regulates lysosomes in multiple cell types. Proc. Natl. Acad. Sci. USA 99: 4355–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, C. F., 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8: 67–113. [DOI] [PubMed] [Google Scholar]
- Hobert, O., 2002. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32: 728–730. [DOI] [PubMed] [Google Scholar]
- Jedlitschky, G., K. Tirschmann, L. E. Lubenow, H. K. Nieuwenhuis, J. W. Akkerman et al., 2004. The nucleotide transporter MRP4 (ABCC4) is highly expressed in human platelets and present in dense granules, indicating a role in mediator storage. Blood 104: 3603–3610. [DOI] [PubMed] [Google Scholar]
- Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin, et al., 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237. [DOI] [PubMed] [Google Scholar]
- Kaminski, W. E., A. Piehler and J. J. Wenzel, 2006. ABC A-subfamily transporters: structure, function and disease. Biochim. Biophys. Acta 1762: 510–524. [DOI] [PubMed] [Google Scholar]
- Kolter, T., and K. Sandhoff, 2006. Sphingolipid metabolism diseases. Biochim. Biophys. Acta 1758: 2057–2079. [DOI] [PubMed] [Google Scholar]
- Kontani, K., I. P. G. Moskowitz and J. H. Rothman, 2005. Repression of cell-cell fusion by components of the C. elegans vacuolar ATPase complex. Dev. Cell 8: 787–794. [DOI] [PubMed] [Google Scholar]
- Kruh, G. D., and M. G. Belinsky, 2003. The MRP family of drug efflux pumps. Oncogene 22: 7537–7552. [DOI] [PubMed] [Google Scholar]
- Kubagawa, H. M., J. L. Watts, C. Corrigan, J. W. Edmonds, E. Sztul et al., 2006. Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat. Cell Biol. 8: 1143–1148. [DOI] [PubMed] [Google Scholar]
- Lamitina, S. T., R. Morrison, G. W. Moeckel and S. Strange, 2004. Adaptation of the nematode Caenorhabditis elegans to extreme osmotic stress. Am. J. Physiol. Cell Physiol. 286: C785–C791. [DOI] [PubMed] [Google Scholar]
- Laufer, J. S., P. Bazzicalupo and W. B. Wood, 1980. Segregation of developmental potential in early embryos of Caenorhabditis elegans. Cell 19: 569–577. [DOI] [PubMed] [Google Scholar]
- Leung, B., G. J. Hermann and J. R. Priess, 1999. Organogenesis of the Caenorhabditis elegans intestine. Dev. Biol. 216: 114–134. [DOI] [PubMed] [Google Scholar]
- Luzio, J. P., P. R. Pryor and N. A. Bright, 2007. Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8: 622–632. [DOI] [PubMed] [Google Scholar]
- Mackenzie, S. M., A. J. Howells, G. B. Cox and G. D. Ewart, 2000. Sub-cellular localisation of the White/Scarlet ABC transporter to pigment granule membranes within the compound eye of Drosophila melanogaster. Genetica 108: 239–252. [DOI] [PubMed] [Google Scholar]
- Mahajan-Miklos, S., M. W. Tan, L. G. Rahme and F. M. Ausubel, 1999. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96: 47–56. [DOI] [PubMed] [Google Scholar]
- Nunes, F., M. Wolf, J. Hartmann and R. J. Paul, 2005. The ABC transporter PGP-2 from Caenorhabditis elegans is expressed in the sensory neuron pair AWA and contributes to lysosome formation and lipid storage within the intestine. Biochem. Biophys. Res. Commun. 338: 862–871. [DOI] [PubMed] [Google Scholar]
- Priess, J. R., and D. I. Hirsh, 1986. Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev. Biol. 117: 156–173. [DOI] [PubMed] [Google Scholar]
- Raposo, G., M. S. Marks and D. F. Cutler, 2007. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr. Opin. Cell Biol. 19: 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke, V., I. S. Gil, S. Ward and K. Kazmer, 2004. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development 131: 311–323. [DOI] [PubMed] [Google Scholar]
- Schaheen, L., G. Patton and H. Fares, 2006. Suppression of the cup-5 mucolipidosis type IV-related lysosomal dysfunction by the inactivation of an ABC transporter in C. elegans. Development 133: 3939–3948. [DOI] [PubMed] [Google Scholar]
- Schroeder, L. K., S. Kremer, M. J. Kramer, E. Currie, E. Kwan et al., 2007. Function of the Caenorhabditis elegans ABC transporter PGP-2 in the biogenesis of a lysosome-related fat storage organelle. Mol. Biol. Cell 18: 995–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham, S., 2006. Wormbook: methods in cell biology, in Wormbook, edited by The Caenorhabditiselegans Research Community. http://www.wormbook.org.
- Sharma, K. G., D. L. Mason, G. Liu, P. A. Rea, A. K. Bachhawat et al., 2002. Localization, regulation, and substrate transport properties of Bpt1p, a Saccharomyces cerevisiae MRP-type ABC transporter. Eukaryot. Cell 1: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock, W. J., M. E. Sutherlin, K. Leske, T. K. Cheng and T. Y. Kim, 1990. Two distinct yolk lipoprotein complexes from Caenorhabditis elegans. J. Biol. Chem. 265: 14422–14431. [PubMed] [Google Scholar]
- Sheps, J. A., S. Ralph, Z. Zhao, D. L. Baillie and V. Ling, 2004. The ABC transporter gene family of Caenorhabditis elegans has implications for the evolutionary dynamics of multidrug resistance in eukaryotes. Genome Biol. 5: R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijen, T., J. Fleenor, F. Simmer, K. L. Thijssen, S. Parrish et al., 2001. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107: 465–476. [DOI] [PubMed] [Google Scholar]
- Sulston, J. E., E. Schierenberg, J. G. White and J. N. Thomson, 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100: 64–119. [DOI] [PubMed] [Google Scholar]
- Sundaram, P., B. Echalier, W. Han, D. Hull and L. Timmons, 2006. a ATP-binding cassette transporters are required for efficient RNA interference in Caenorhabditis elegans. Mol. Biol. Cell 17: 3678–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram, P., B. Echalier, W. Han, D. Hull and L. Timmons, 2006. b ATP-binding cassette transporters are required for efficient RNA interference in Caenorhabditis elegans. Mol. Biol. Cell 17: 3678–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijsterman, M., R. C. May, F. Simmer, K. L. Okihara and R. H. Plasterk, 2004. Genes required for systemic RNA interference in Caenorhabditis elegans. Curr. Biol. 14: 111–116. [DOI] [PubMed] [Google Scholar]
- Treusch, S., S. Knuth, S. A. Slaugenhaupt, E. Goldin, B. D. Grant et al., 2004. Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis. Proc. Natl. Acad. Sci. USA 13: 4483–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goot, F. G., and J. Gruenberg, 2006. Intra-endosomal membrane traffic. Trends Cell Biol. 16: 514–521. [DOI] [PubMed] [Google Scholar]
- Vatamaniuk, O. K., E. A. Bucher, M. V. Sundaram and P. A. Rea, 2005. CeHMT-1, a putative phytochelatin transporter, is required for cadmium tolerance in Caenorhabditis elegans. J. Biol. Chem. 280: 23684–23690. [DOI] [PubMed] [Google Scholar]
- Wu, Y. C., and H. R. Horvitz, 1998. The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell 93: 951–960. [DOI] [PubMed] [Google Scholar]
- Yabe, T., N. Suzuki, T. Furukawa, T. Ishihara and I. Katsura, 2005. Multidrug resistance-associated protein MRP-1 regulates dauer diapause by its export activity in Caenorhabditis elegans. Development 132: 3197–3207. [DOI] [PubMed] [Google Scholar]
- Yamano, G., H. Funahashi, O. Kawanami, L. X. Zhao, N. Ban et al., 2001. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Lett. 508: 221–225. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., B. Grant and D. Hirsh, 2001. RME-8, a conserved J-domain protein, is required for endocytosis in Caenorhabditis elegans. Mol. Biol. Cell 12: 2011–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z., J. A. Sheps, V. Ling, L. L. Fang and D. L. Baillie, 2004. Expression analysis of ABC transporters reveals differential functions of tandemly duplicated genes in Caenorhabditis elegans. J. Mol. Biol. 344: 409–417. [DOI] [PubMed] [Google Scholar]
- Zhao, Z., J. H. Thomas, N. Chen, J. A. Sheps and D. L. Baillie, 2007. Comparative genomics and adaptive selection of the ATP-binding-cassette gene family in Caenorhabditis species. Genetics 175: 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, C., L. Zhao, N. Inagaki, J. Guan, S. Nakajo et al., 2001. Atp-binding cassette transporter ABC2/ABCA2 in the rat brain: a novel mammalian lysosome-associated membrane protein and a specific marker for oligodendrocytes but not for myelin sheaths. J. Neurosci. 21: 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Q., H. Sun and M. S. Center, 1997. Functional analysis of the nucleotide binding domains of the multidrug resistance protein (MRP). Oncol. Res. 9: 229–236. [PubMed] [Google Scholar]