Abstract
The Drosophila dec-1 gene produces three proproteins required for female fertility and eggshell assembly. The three proproteins are distinguished by their C termini. Fc106, the most abundant proprotein, is cleaved within the vitelline membrane to three mature derivatives in a developmentally regulated manner. To define sequences within fc106 that are critical for its function, we created wild-type and mutant versions of an fc106 cDNA transgene. The functional consequences of the mutations were assessed in dec-14, a female-sterile splicing mutant that does not produce the fc106 isoform. The fertility of dec-14 females was restored by the introduction of either a wild-type transgene or a transgene bearing a C-terminal deletion that included fc106-specific sequences. Surprisingly, the removal of internal coding sequences created an aberrant DEC-1 proprotein that induced female sterility when introduced into wild-type flies. Dominant female sterility was not associated with larger deletions that included the fc106 N terminus, suggesting that abnormal juxtaposition of N- and C-terminal sequences in the aberrant proprotein interfered with endogenous DEC-1 proteins. Changes in the fractionation behavior of the endogenous fc106 C-terminal derivative, s60, and morphological changes in the endochorion in response to expression of the aberrant proprotein support this interpretation.
EXTRACELLULAR matrices are complex molecular networks that not only provide protective functions but also serve as reservoirs for bioactive molecules (Aumailley and Gayraud 1998). Matrices assemble and function in complex microenvironments. Despite extensive knowledge of the components of several matrices, little is known about how matrices assemble in vivo. The Drosophila eggshell provides an opportunity to study the assembly of a complex extracellular architecture in vivo in a system that features temporal and spatial resolution. The eggshell is a specialized extracellular matrix that forms between the oocyte and overlying follicle cells during the later stages of oogenesis, stages 8–14. Largely proteinaceous, the eggshell is a highly organized multilayer structure that displays regional and radial complexity. In mature stage 14 egg chambers the eggshell consists of five morphologically distinct layers: an oocyte proximal vitelline membrane, a wax layer, a crystalline inner chorionic layer, a tripartite endochorion, and an outer, amorphous exochorion (Margaritis 1985). Several vitelline membrane and endochorion structural components have been identified (Waring 2000). Protein null mutants for three major structural components, DEC-1, s36, and sV23 (Digan et al. 1979; Bauer and Waring 1987; Savant and Waring 1989), have been recovered in genetic screens for sterile females. Assembly defects manifested at the morphological level are associated with the s36 and dec-1 mutants. An organized tripartite endochorion fails to organize in the absence of s36 (Digan et al. 1979) while the endochorion collapses into the underlying vitelline membrane in DEC-1 protein null mutants (Bauer and Waring 1987). The identification of regions and motifs within the sV23 and DEC-1 proteins that are essential for eggshell assembly and function have been the focus of recent studies (Badciong et al. 2001; Mauzy-Melitz and Waring 2003; Manogaran and Waring 2004).
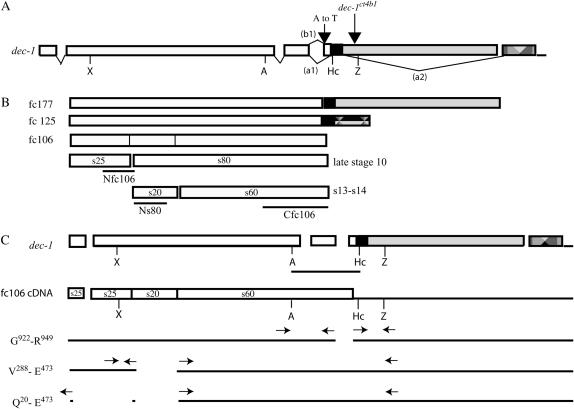
The dec-1 gene produces three alternatively spliced transcripts that encode three proproteins: fc106, fc125, and fc177 (106, 125, and 177 kDa in size, respectively). In wild-type egg chambers fc106 accumulates at ∼10 times the level of fc177 or fc125. Although the proproteins have distinct C termini, there is extensive overlap in their sequences. Aside from its six C-terminal amino acids, all of the fc106 proprotein is contained in the larger fc125 and fc177 proproteins (Waring et al. 1990 and Figure 1B). Despite the overlap, all three proproteins appear to provide distinct and essential functions. Selective removal of fc177 or fc125 by the introduction of premature proprotein-specific termination codons showed both minor proproteins are required for female fertility (Mauzy-Melitz and Waring 2003). While eggshell morphology appeared normal in fc125-deficient females, in the absence of fc177 the tripartite endochorion failed to form. A continuous electron-dense aggregate was observed in the endochorion layer rather than the characteristic floor, pillar, and roof morphology. The fc106 proprotein is absent in the dec-1 splicing mutant dec-14. Homozygous dec-14 females produce fc177 at wild-type levels and fc125 accumulates at ∼10 times its normal level (Waring et al. 1990). Although dec-14 females are sterile, the characteristic tripartite ultrastructure of the endochorion is maintained (Komitopoulou et al. 1983). Females heterozygous for dec-14 and dec-1ct4b1, a deficiency that breaks within the dec-1 gene (Hawley and Waring 1988; Figure 1A), are fertile. A single aberrant dec-1 mRNA that includes the entire fc106 open reading frame is transcribed from dec-1ct4b1 and fc106 and its C-terminal derivatives, s80 and s60, are produced at wild-type levels (Waring et al. 1990). The fertility of dec-14/dec-1ct4b1 females suggests that like fc125 and fc177, the fc106 proprotein and/or its derivatives provide distinct and essential functions.
Figure 1.—
The dec-1 gene, proteins, and mutant transgene constructs. (A) The dec-1 gene. Open reading frames (ORFs) are denoted by rectangles. Sans the six C-terminal amino acids, the entire fc106 ORF (open rectangles, 950 amino acids) is present in the minor DEC-1 proproteins, fc125 and fc177. The solid rectangle represents a small ORF shared by fc125 and fc177; the fc177-specific ORF is shaded; the patterned rectangle denotes the fc125-specific ORF. The third intron contains two alternative 3′ splice acceptor sites. The (b1) pathway is used for fc106 mRNA; pathway (a1) is used for fc125 and fc177 mRNAs. The fc106- specific ORF is terminated in exon 4 after 18 bases. The fc177-specific ORF is removed from the fc106 and fc125 mRNAs via the (a2) splicing pathway. The large downward arrow shows the location of the dec1ct4b1 breakpoint within the dec-1 gene. The small downward arrow represents the AG → TG dinucleotide change at the fc106-specific splice acceptor site in the dec-14 allele. Key restriction sites used in constructing the fc106 cDNA transgene and its mutated derivatives included X (XhoI), A (ApaI), Hc (HincII), and Z (XbaI). (B) DEC-1 proproteins and processing of fc106. The three DEC-1 proproteins fc177, fc125, and fc106 with ORF designations as in A are shown. The timing and regions of fc106 that are separated by cleavage (s25, s80, s20, and s60) are indicated. The open reading frames used to produce the DEC-1 antisera used in this study (Nfc106, Ns80, and Cfc106) are indicated below the cleaved derivatives. (C) Construction of the dec-1 fc106 cDNA transgene and its mutant derivatives. The dec-1 gene is shown at the top; the line below the ApaI–HincII region indicates sequences that were derived from an fc106 cDNA (Hawley and Waring 1988). The coding region (rectangles) and 3′-UTR (line) of the fc106 cDNA transgene are shown below the dec-1 gene. Coding regions that were deleted (spaces) in the mutant fc106 cDNA transgenes and the positions of the primer pairs used in their construction are indicated (the forward vector primer used for the 5′-PCR fragment of the Q20–E473 construct is not shown). All of the transgenes contained ∼2 kb of 5′-flanking and 1 kb of 3′-flanking dec-1 DNA in addition to the regions shown.
The DEC-1 proproteins are secreted from the follicle cells into the assembling vitelline membrane during stages 9–12. The proproteins are cleaved in a stage-specific manner within the vitelline membrane, forming at least six products (Noguerón and Waring 1995) with distinct spatial distributions in the mature egg chamber (Nogueron et al. 2000). For example, fc106 accumulates in the vitelline membrane during stages 9 and 10 and is cleaved to N-terminal (s25) and C-terminal (s80) derivatives during late stage 10 (Figure 1B). As the egg chamber matures, s80 is cleaved to a 20-kDa N-terminal product, s20, and a 60-kDa C-terminal product, s60. In late stage 14 egg chambers both s25 and s60 are detected in the vitelline membrane and endochorion layers; s25 also localizes within the crystalline inner chorionic layer. In contrast, s20 does not appear to be a structural component of the mature eggshell. Shortly after its biogenesis, s20 is detected in membrane-bound vesicles within the oocyte.
As a first step toward identifying biologically significant regions within the DEC-1 proteins we did an interspecies comparison of dec-1 genes that revealed its rapidly evolving nature (Badciong et al. 2001). Despite extensive divergence at the amino acid level, the dec-1 genes from Drosophila virilis and D. melanogaster were functionally interchangeable as both rescued the aberrant eggshell morphology and sterility of DEC-1 protein null mutants. Focusing on evolutionarily conserved blocks, we have begun to mutate fc106 in an attempt to identify functionally significant sequences within this proprotein. Mutations were introduced via an fc106 cDNA transgene and the ability of the transgene to restore fertility in dec-14 females was used to test the effects of the mutations. While the removal of an evolutionarily conserved block at its C terminus was without consequence, the removal of the internal s20 region created a mutant fc106 transgene whose presence in wild-type females caused 100% sterility. Biochemical analyses showed the transgene harboring the internal deletion produced an aberrant proprotein that was secreted, became integrated into the eggshell, and was processed in the manner anticipated. While endogenous fc106 was processed normally, fractionation and ultrastructural studies suggested the presence of the aberrant proprotein altered either trafficking of endogenous s60 from the vitelline membrane to the endochorion or its interactions within the endochorion layer. Our results indicate that the fc106 proprotein plays a critical role in eggshell assembly beyond its role in regulating the biogenesis of its mature DEC-1 derivatives.
MATERIALS AND METHODS
Culture conditions and stocks:
The Oregon R, P2 wild-type strain was maintained in mass culture; all other stocks were maintained on standard yeast, cornmeal, molasses, and agar medium. The dec-1ct4b1 deficiency chromosome and the dec-1 mutant alleles, dec-129 (fs(1)410) and dec-14 (fs(1)1501), have been described previously (Bauer and Waring 1987; Hawley and Waring 1988). The dominantly marked, multiply inverted balancer chromosomes CyO; TM3, Ser and TM3, Sb were used for manipulations of the transgenic lines.
Construction of transgenes:
The dec-1 fc106 cDNA transgene consists of dec-1 genomic DNA except for a 260-bp ApaI–HincII fragment of cDNA origin (Figure 1C). The genomic ApaI– HincII fragment contains dec-1 intervening sequences IVS2 and -3. IVS3 contains alternative 3′ splice acceptor sites that distinguish splicing of the fc106 transcript (b1, Figure 1A) from the fc125 and fc177 transcripts (a1, Figure 1A; Waring et al. 1990). Utilization of the fc106 acceptor site results in the inclusion of 14 additional nucleotides in exon 4. The resultant frameshift leads to termination of the open reading frame 19 nucleotides downstream of the exon 3–exon 4 splice junction. An ApaI– HincII fragment of fc106 origin (0.49 kb, Figure 1C) was isolated from a partial fc106 cDNA clone (Hawley and Waring 1988) and exchanged with its 0.73-kb genomic counterpart in a 0.9-kb ApaI–XbaI genomic DNA subclone. After adding a 2 kb 5′ XhoI–ApaI genomic fragment, the resultant XhoI–XbaI fragment was excised and exchanged with its counterpart in a pCaSpeR 4 P-element dec-1 rescue vector that contained the dec-1 gene along with 1.9 and 1.0 kb of dec-1 5′- and 3′-flanking DNA, respectively.
Deletions within the dec-1 fc106 cDNA transgene (Figure 1C) were created using the PCR-based strategy described by Hughes and Andrews (1996). Two primer pairs were used to amplify 5′ and 3′ fragments that flanked the desired deletions. SphI restriction sites were engineered into the 5′ ends of all primers that abutted the deleted regions.
The conditions used for all PCR reactions were 94° for 1 min, 60° for 1 min, and 72° for 1 min using 2.5 units of Taq polymerase (Promega, Madison, WI) for 31 cycles. For the V288–E473 deletion, the fc106 cDNA transgene was used as template: a 518-bp 5′-PCR fragment was amplified with a forward primer positioned 8–24 bases 5′ of the XhoI site (5′ TGGCCGGATGATGCGACG 3′) and a reverse primer that abutted the V288 codon and included an engineered SphI site (5′ CTTGCATGCCTTACCTCGGAAATGTCGGAG 3′). The V288–E473 3′-PCR fragment (1.93 kb) was amplified with a forward primer that abutted the E473 codon (5′ ACGTGAGCATGCAGATGGAGAGCGAGAAGG 3′) and a reverse primer positioned 3–24 bases 3′ of the XbaI site (5′ TCCCGAAGTTCTACTAGAAACC 3′). The 5′- and 3′-PCR fragments were subcloned individually into a pGEM-T vector (Promega). Following excision, the 5′ XhoI–SphI and 3′ SphI–ApaI fragments were subcloned sequentially into a pGEM-11Z vector. The XhoI–ApaI deletion subclone was digested with SphI, ectopic SphI bases in the 3′ overhangs were removed with Klenow fragment, and the resulting blunt ends were ligated. Following transformation, the XhoI–ApaI deletion fragment was excised from the plasmid and exchanged with its wild-type counterpart in an XhoI–XbaI dec-1 fc106 cDNA subclone. The XhoI–XbaI deletion fragment was then excised and exchanged with its counterpart in the pCaSpeR 4–dec-1 fc106 cDNA transgene plasmid described earlier. For the C-terminal deletion (ΔG922–R949) a 367-bp 5′-PCR product was generated with a forward primer positioned 4–25 nucleotides 5′ of the ApaI site (5′ GAACCCCCAGTCTGTCCAGCAG 3′) and a reverse primer abutting the G922 codon (5′ CTGGCATGCTGACTTTGATACGAATTGACTG 3′). A 281-bp 3′-PCR product was amplified with a forward primer that included the terminal Q950 codon (5′ CTGGCATGCAATAAACCCGAAGCAACCAGGCG 3′) and the reverse primer used in the construction of the 3′-PCR fragment for the ΔV288–E473 deletion described above. An ApaI–XbaI deletion subclone was created in pGEM-7Z, exchanged with its counterpart in the XhoI–XbaI dec-1 fc106 cDNA subclone, and introduced into the dec-1 fc106 cDNA transgene essentially as described above.
A different cloning scheme was used to construct the ΔQ20–E473 deletion since the XhoI site in the dec-1 coding sequence was absent in this transgene (Figure 1C). Using a 2.5-kb NcoI dec-1 subclone in pGEM-5Z as template, a 2.3-kb 5′-PCR fragment was generated using a forward vector primer (5′ CTCCCATATGGTCGACCTGCAGGCG 3′) and a reverse dec-1 primer (5′ GCTGCATGCCTTACCTCGGAAATGTCGGATCCGGCAACCTGGACGACAAGAAGCGC) that abutted the nucleotides encoding Q20 and included a 5′ tail consisting of coding sequence for the first seven amino acids of s80 (S281–R287) as well as a synthetic SphI site. Following subcloning into a pGEM-T vector, a NotI site in the polylinker region was used to excise a 2.2-kb NotI–SphI fragment that included ∼2 kb of dec-1 5′-flanking DNA. The 3′ SphI–XbaI fragment used to create the ΔV288–E473 transgene was excised from a pGEM-T subclone and the NotI–SphI and SphI–XbaI fragments were subcloned in succession into a modified pSP73 vector (NotI site inserted into the polylinker region between the HindIII and XhoI sites). Following removal of the ectopic SphI bases as described above, the ∼4-kb NotI–XbaI fragment was excised and exchanged with its counterpart in a NotI–KpnI dec-1 fragment that had been subcloned into a modified CaSpeR 4 vector (XbaI polylinker site removed). The NotI–KpnI subclone is analogous to the pCaSpeR 4 dec-1 rescue vector described earlier, but is missing the nucleotides that encode amino acids Q20–A274 (Mauzy-Melitz 2001). The wild-type dec-1 rescue vector could not be used for the NotI–XbaI exchange because the dec-1 nucleotides that encode amino acids A272 and A273 form a NotI site. The dec-1 fc106 transgene with the Q20–E473 deletion thus includes ∼2 kb of 5′-flanking DNA, the 5′-UTR, nucleotides that encode the putative signal sequence M1–G19, the N terminus of s80 (S281–R287), the s60 coding region (Q474–Q950), and the remainder of the dec-1 gene plus ∼1 kb of 3′-flanking DNA. The precision of all of the deletions described was verified by DNA sequencing.
Recombinant pCaSpeR 4 plasmid DNAs were purified and coinjected with a helper plasmid into w*/w* preblastoderm embryos as previously described (Mauzy-Melitz and Waring 2003). Transformants were recovered in the G1 generation, chromosomal linkages of the transgenes were determined, and homozygous transgene stocks were created and maintained.
Construction of females with multiple dec-1 transgenes:
The dec-1 gene is located in region 7C3 of the X chromosome. A wild-type dec-1 transgene integrated on the second chromosome was used to supply extra copies of the dec-1 gene. A single copy of this autosomal dec-1 transgene is sufficient to rescue the sterility of DEC-1 null mutant females. White females (w*, dec-1+) with two copies of the dec-1 transgene and a Ser-marked third chromosome balancer (w*, dec-1+/w*, dec-1+; P[w+, dec-1+]/P[w+, dec-1+]; TM3, Ser/+) were mated to males carrying the mutant dec-1 transgene fc106 ΔV288–E473 in trans to a Sb-marked TM3 balancer third chromosome (w*, dec-1+; +/CyO; P[w+, fc106ΔV288-E473]/TM3, Sb. Female progeny carrying single copies of the wild-type and mutant dec-1 transgenes were selected for analysis (w*, dec-1+/w*, dec-1+; P[w+, dec-1+]/CyO; P[w+, fc106ΔV288-E473]/TM3, Ser). To generate females with two extra copies of the wild-type dec-1 gene and one copy of the fc106ΔV288–E473 transgene, F1 males from the previous cross (w*, dec-1+; P[w+, dec-1+]/CyO; P[w+, fc106ΔV288-E473]/TM3, Ser) were mated with w*, dec-1+/w*, dec-1+; P[w+, dec-1+]/CyO; TM3, Sb/+ females. Female progeny with two copies of the wild-type dec-1 transgene gene and one copy of the fc106ΔV288–E473 mutant transgene were selected for analysis (w*, dec-1+/w*, dec-1+; P[w+, dec-1+]/P[w+, dec-1+]; P[w+, fc106ΔV288–E473]/TM3, Sb).
Biochemical fractionation:
Stage 14 egg chambers (20) from wild-type and transformant females were resuspended in 100 μl of Tris-buffered saline (TBS: 50 mm Tris, pH 7.4, 150 mm NaCl) in the absence or the presence of 5% β-mercaptoethanol (BME) and disrupted in a Kontes dounce homogenizer (B-type pestle). Samples disrupted in the presence of BME were subjected to centrifugation at 15,000 × g for 5 min and separated into reduced pellet (PR) and supernatant (SR) fractions. Samples disrupted in the absence of the reducing agent were centrifuged at 10,000 × g for 5 min and separated into pellet (P1) and supernatant (S1) fractions. The pellet was resuspended in TBS containing 5% BME, incubated at room temperature for 30 min, recentrifuged for 5 min at 15,000 × g, and separated into reduced pellet (P2R) and supernatant (S2R) fractions. Following the addition of Laemmli sample buffer (Laemmli 1970) with 5% BME, all samples were heated to 95° for 3 min. Proteins in the supernatant and pellet fractions were subjected to SDS–PAGE and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA) for 50 min at 35 V using a Bio-Rad Trans-Blot apparatus with plate electrodes. The three DEC-1 antisera Nfc106, Ns80, and Cfc106 and the sV23 and s36 antisera used in this study have been described previously (Noguerón and Waring 1995; Pascucci et al. 1996). Antigen–antibody complexes were visualized by enhanced chemical luminescence following the addition of HRP-conjugated goat anti-rabbit secondary antibodies as previously described (Manogaran and Waring 2004). For reprobing, nitrocellulose membranes were placed in Tris-buffered saline containing 0.7% β-mercaptoethanol and 2% SDS for 30 min at room temperature. The stripped blots were rinsed, blocked, incubated with antiserum, and processed as above. For developmental Western blots, egg chambers separated according to stages were resuspended directly in Laemmli sample buffer containing 5% BME. When indicated, a mini-Protean 3 electrophoresis cell and a mini Trans-Blot electrophoretic transfer cell (Bio-Rad) were used for protein separation and transfer.
Egg-laying and fertility tests:
Zero- to 2-day-old flies were collected and placed in vials with a wet yeast paste for 2–3 days. Well-fed flies were then transferred to egg-collection chambers (1 female and 2 males per chamber). Construction of the egg-collection chamber was patterned after that described by Acosta and Goni (2000). The conical base of a Blue Max Jr. 15-ml polystyrene conical tube (17-mm diameter Falcon tubes) was cut and removed. The tube was inverted and the plastic cap was filled with agar–apple juice medium (3 and 30%, respectively) containing a fungal inhibitor (methyl 4-hydroxybenzoate at 0.5 mg/ml). After solidification, a thin layer of fresh yeast paste was painted onto the agar surface with a fine-haired brush. Flies were placed in the tube and the tube was sealed with a cotton plug. The tubes were placed in a humid environment (80–90%) for ∼24 hr at ambient temperature (23°–25°). At the end of the collection period the plastic caps were removed and the number of eggs in each cap was counted with a stereomicroscope. The caps were returned to the humid environment and the number of larvae that hatched in each cap in the ensuing 48-hr period was recorded.
Morphological analysis:
Stage 14 egg chambers were collected from hand-dissected ovaries, fixed overnight at 4° with 2% glutaraldehyde in 0.1 m sodium cacodylate, pH 7.4, postfixed in 1% osmium tetroxide for 2 hr, dehydrated, and infiltrated with Spurr resin. Thin sections were stained with lead citrate and uranyl acetate and viewed with a Hitachi H-600 transmission electron microscope (University of Wisconsin-Milwaukee Imaging Facility).
RESULTS
An fc106 cDNA transgene can rescue the sterility of dec-14 females:
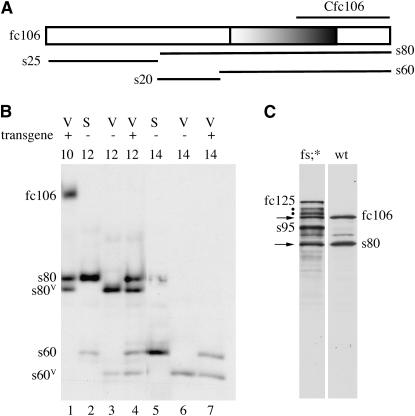
The extensive sequence overlap between fc106 and the minor fc125 and fc177 proproteins (Figure 1B) in essence precludes introducing fc106-specific mutations into the dec-1 gene. To engineer mutations only into fc106, an fc106 cDNA transgene driven by dec-1 regulatory sequences was created. To quantitate fc106 production by the cDNA transgene, the transgene was introduced into flies (w*/w*) that produced functional electrophoretic variants of the DEC-1 proteins (Mauzy-Melitz and Waring 2003). The positions of the standard forms of the fc106 C-terminal derivatives, s80 and s60, are shown in lanes 2 and 5 of Figure 2B; the positions of the variant forms are shown in lanes 3 and 6. The increased mobility of both variant fc106 C-terminal derivatives (s80V and s60V, lanes 3 and 6) suggests the variant forms have a smaller s60 region. A large portion of the s60 region consists of tandem repeats (Figure 2A). A spontaneous deletion within these repeats is a likely explanation for the variant isoforms. Egg chambers from w*/w* flies carrying two copies of the dec-1 fc106 cDNA transgene displayed both the standard and the variant forms of s80 and s60 (lanes 1, 4, and 7). The similar intensities of the standard and variant forms (lanes 4 and 7) indicate that DEC-1 products from the dec-1 fc106 cDNA transgene accumulate at wild-type levels.
Figure 2.—
C-terminal derivatives from the fc106 cDNA transgene accumulate at wild-type levels. (A) Schematic of fc106 proprotein and its processed derivatives (see Figure 1B) highlighting tandem copies of a 26-amino-acid motif rich in methionine (15%) and glutamine (39%) (Waring et al. 1990) located within the s60 region. (B) Staged egg chambers from w*/w* (V), Oregon R P2 strain (S), or w*/w* females carrying the fc106 cDNA transgene (V) (+) were disrupted in Laemmli sample buffer containing 5% β-mercaptoethanol. Proteins were separated by SDS–PAGE and the Western blot was incubated with the Cfc106 antiserum (Figure 2A). The positions of the standard and variant (V) forms of fc106 and its C-terminal derivatives, s80 and s60, are indicated at the left. The stages of the egg chambers are shown at the top; lane numbers are at the bottom. (C) Stage 10 egg chambers from Oregon R females (wt) and dec-14 females with two copies of the fc106 cDNA transgene (fs;*) disrupted and analyzed as in B using a Mini-Protean electrophoresis cell for protein separation. The positions of fc125, its processing intermediates (.), and its C-terminal cleavage product, s95, are shown on the left; fc106 and its C-terminal cleavage product, s80, are shown on the right. The arrows denote fc106 and s80 derived from the fc106 cDNA transgene.
To test whether the dec-1 fc106 cDNA transgene provides requisite fc106 functions, the transgene was introduced into homozygous dec-14 females via a series of genetic crosses. In dec-14 egg chambers fc125 is overexpressed and fc177 accumulates at wild-type levels. While the absence of fc106 is the likely cause of the dec-14 female-sterile phenotype, the 10-fold increase in fc125 accumulation may be problematic. If overproduction of fc125 is inconsequential and if the dec-1 fc106 cDNA is fully functional, then dec-14 females carrying the dec-1 fc106 cDNA transgene should be fertile. Figure 2C shows that fc106 and s80 accumulate at levels comparable to fc125 and its C-terminal derivative, s95, in stage 10 egg chambers from dec-14 females carrying two copies of the dec-1 fc106 cDNA trangene. The overexpression of fc125 and its derivatives in dec-14 egg chambers is underscored by the absence of signals in these size ranges in extracts from an equivalent number of wild-type stage 10 egg chambers. Homozygous dec-14 females carrying two copies of the fc106 cDNA transgene laid eggs that hatched at ∼60% (39/66) the frequency of the wild-type control (dec-14/FMO) (Table 1). To determine whether mutations in other genes linked to the dec-14 mutation affected the hatching rate, the transgene was also tested in a heteroallelic combination of dec-14 and dec-129, a DEC-1 protein null allele. Although a few larvae hatched from dec-14/dec129 eggs (2%), wild-type hatching frequencies were observed after the addition of one (fc106/TM3, 74%) or two copies (fc106/fc106, 78%) of the fc106 cDNA transgene (Table 1). These data show that the infertility of dec-14 females is due to the absence of fc106.
TABLE 1.
Rescue of dec-14 female sterility by dec-1 fc106 transgenes
| Genotype | No. females | Eggs laid | Larvae | % hatching |
|---|---|---|---|---|
| dec-14/FMO | 8 | 699 | 461 | 66 |
| dec-14/dec-14; fc106/fc106 | 5 | 183 | 72 | 39 |
| dec-14/dec-129; fc106/fc106 | 4 | 335 | 260 | 78 |
| dec-14/dec-129; fc106/TM3 | 3 | 249 | 184 | 74 |
| dec-14/dec-129 | 5 | 386 | 9a | 2 |
| dec-14/dec-14; fc106ΔG922–R949/fc106ΔG922–R949 | 2 | 135 | 52 | 38 |
| dec-14/dec-129; fc106ΔG922–R949/fc106ΔG922–R949 | 3 | 132 | 75 | 56 |
| dec-14/dec-129; fc106ΔG922–R949/TM3 | 2 | 125 | 81 | 64 |
| dec-14/dec-14; fc106ΔQ20–E473/fc106ΔQ20–E473 | 5 | 205 | 2 | 0.9 |
| w*/w* | 5 | 386 | 281 | 84 |
| w*/w*; fc106ΔV288–E473/+ | 5 | 256 | 0 | 0 |
One conditioned female of the designated genotype and two white (w*/w*) males were placed in egg collection chambers. The number of females tested, the total number of eggs laid in a 24-hr period, the total number of larvae recovered, and the hatching percentages are indicated. Either dec-14/FMO or w*/w* females were used as wild-type controls. The percentage of eggs that hatched in the wild-type controls was variable, likely reflecting day-to-day differences in the environmental conditions as well as the number of unfertilized eggs.
Of the nine larvae recovered, seven were from eggs laid by a single female, one larva was recovered from each of two females, and no larvae were recovered from eggs laid by three females.
s25 and s20 N-terminal derivatives accumulate at wild-type levels in dec-14 egg chambers:
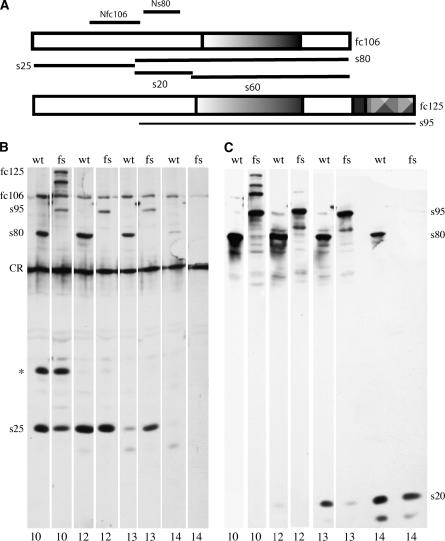
The sterility of dec-14 females in the absence of fc106 suggests that the fc106 proprotein per se, one or more of its cleaved derivatives, or both, are essential for proper eggshell assembly. The fc106 N-terminal derivatives, s25 and s20, are cleaved from sequences within the region of overlap between fc106 and fc125 (M1–R944) (Figure 3A). The fc106 and fc125 proproteins are synthesized and processed during the same stages of egg chamber development. If their N termini are processed similarly, then s25 may accumulate at wild-type levels in dec-14 egg chambers. Similarly, s20 may accumulate at wild-type levels in dec-14 stage 14 egg chambers if s95 is cleaved like s80. To determine if s25 and s20 accumulate in dec-14 egg chambers, antisera directed against N-terminal sequences were used to follow processing of fc125 and s95. An antiserum directed against an open reading frame that included 140 amino acids from the s25 region and the first 11 amino acids of s80 (Nfc106, Figure 3A) was used to detect fc125 N-terminal cleavage products; an antiserum directed against a 132-amino-acid open reading frame in the s20 region (Ns80) was used to detect s95 N-terminal cleavage products. As shown in Figure 3B, the N-terminal cleavage products from fc125 in dec-14 egg chambers are indistinguishable from the products produced by fc106 in wild-type egg chambers. In wild-type stage 10 egg chambers fc106, s80, a transient DEC-1 related product in the 40-kDa size range, and a stable derivative migrating in the 25-kDa region (s25) were detected by the Nfc106 antiserum. On the basis of its lack of reactivity with the Ns80 antibody (Noguerón and Waring 1995 and Figure 3C) the 40-kDa species appears to be an N-terminal processing intermediate. Conceptual translation of the N-terminal region (Q20–A280) predicts a protein of ∼27 kDa. Anomalous migration of the N-terminal product on SDS gels (∼40-kDa size range) is not unexpected given the slow migration (∼130-kDa size range) of its 106-kDa precursor, fc106. The stable species migrating in the 25-kDa range, s25, likely represents a smaller, processed derivative of the cleaved N-terminal region. Similar N-terminal derivatives were apparent in stage 10 dec-14 egg chambers, suggesting that the sterility of dec-14 females is not due to the absence of s25. In late-stage egg chambers s25 becomes insoluble. This is reflected in the reduction/loss of the s25 signal in late-stage egg chambers. The Ns80 antiserum (Figure 3C) showed that s20 accumulates in both wild-type and dec-14 egg chambers during stages 13 and 14. Taken together these data show that fc125, like fc106, is a source of s25 and s20 and that both accumulate at wild-type levels in dec-14 egg chambers. This suggests that the absence of fc106 sequences in the form of s60 or its precursors (fc106, s80) is responsible for the sterility of dec-14 females.
Figure 3.—
N-terminal processing of the fc125 DEC-1 proprotein. (A) A schematic of fc106, fc125, and their cleaved derivatives (s80, s25, s20, s60, and s95) along with the positions of the open reading frames used to raise the Nfc106 and Ns80 antisera. (B and C) Lanes from Western blots of SDS-soluble proteins from wild-type (wt) or dec-14 mutant egg chambers (fs). (B) Proteins recognized by the Nfc106 antiserum. (C) Lanes shown in A stripped and reincubated with the Ns80 antiserum. (B) The positions of fc125 and its C-terminal derivative s95 and fc106 and its C-terminal derivative s80 are indicated to the left. CR denotes non-DEC-1 related cross-reacting material that is present in DEC-1 protein null mutants (not shown). A transient N-terminal derivative (*) and s25 were recognized by the Nfc106 antiserum. (C) Positions of the C-terminal s95 and s80 derivatives and the N-terminal s20 derivative recognized by the Ns80 antiserum are shown to the right. Egg chamber stages are indicated at the bottom of each lane.
The fc106-specific C terminus is not required for its function:
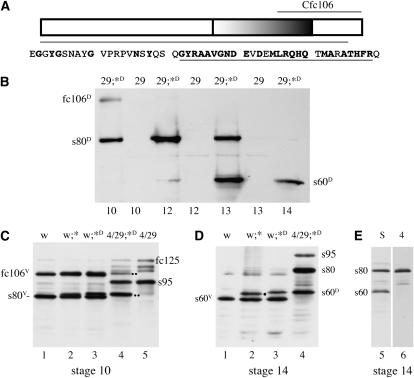
As a first step toward determining features of the fc106 proprotein that are critical for its function, fc106-specific amino acids were deleted from the fc106 cDNA transgene. Fc106-specific amino acids fall within a larger block of 28 evolutionarily conserved C-terminal amino acids (Badciong et al. 2001 and Figure 4A). A small internal deletion encompassing these C-terminal residues was created, fc106ΔG922–R949. To facilitate unambiguous identification of fc106ΔG922–R949 and its derivatives, autosomally linked transgenes were crossed into DEC-1 null mutants (dec-129). Figure 4B shows that in homozygous dec-129 mutants carrying the fc106ΔG922–R949 transgene fc106-like and s80-like proteins accumulate in stage 10 egg chambers. Like its wild-type counterpart, the 80-kDa product is cleaved to an s60-like derivative during stages 13 and 14. These data suggest that processing of fc106 is not dependent upon the presence of either these conserved residues or other DEC-1 proproteins. When the fc106ΔG922–R949 transgene was introduced into homozygous dec-14 females, hatching rates similar to those with the fc106 cDNA transgene were observed (Table 1). Hatching rates approaching wild type (∼60%) were seen when either one copy or two copies of the fc106ΔG922–R949 transgene were crossed into dec-129/dec-14 females (Table 1). Accumulation of fc106ΔG922–R949 derivatives in the dec-129/dec-14 background was verified by Western blot analysis (Figure 4, C and D). In stage 10 egg chambers the intensities of the fc106ΔG922–R949 derivatives were comparable to those of their fc125 counterparts (Figure 4C) despite the loss of potential epitopes in fc106ΔG922–R949. Paradoxically, in stage 14 egg chambers the s60-like fc106ΔG922–R949 derivative migrated slightly more slowly than s60 from the intact fc106 cDNA transgene. Anticipating that the removal of 28 amino acids would create a faster-migrating species, this result was unexpected. The anomalous migration may reflect differences in the ability of different parts of s60 to bind SDS. If the G922–R949 region binds more SDS than other parts of the molecule, its removal may create a slower species by reducing the charge-to-mass ratio. Alternatively, the deletion may cause utilization of a cryptic s80 cleavage site. In either case, the data suggest that neither the fc106-specific amino acids nor the highly conserved block of amino acids at the C terminus of fc106 are critical to its function. This does not exclude a role for these sequences in the dec-1 gene, however. The nucleotides that encode these amino acids may be critical for alternative splicing of the dec-1 transcripts, or the highly conserved amino acids in this region may be important for the function of the fc125 and/or fc177 proproteins and/or their derivatives.
Figure 4.—
Fc106 accumulates and is processed in the absence of its evolutionarily conserved C terminus. (A) A schematic of fc106. The truncated line below the schematic indicates the region present in the fc106ΔG922–R949 transgene. The sequence shows the 50 C-terminal amino acids of fc106. Amino acids that are identical in the D. melanogaster and D. virilis fc106 proproteins are in boldface type; the amino acids deleted in fc106ΔG922–R949 are underlined. The Cfc106 antiserum (above the schematic) was used in B–E. (B) Western blot of SDS-soluble proteins from staged egg chambers isolated from homozygous DEC-1 null females (dec-129) in the absence (29) or presence of the fc106ΔG922–R949 transgene (29;*D). The positions of fc106-, s80-, and s60-like derivatives (fc106D, s80D, and s60D) are indicated; egg chamber stages are shown at the bottom of the lanes. (C–E) Western blots of SDS-soluble proteins from stage 10 (C) and 14 (D and E) egg chamber proteins resolved using a Mini-Protean electrophoresis cell system. In C the positions of the variant DEC-1 forms fc106V and s80V produced by homozygous white females (w) are shown (lane 1) along with fc125 and s95 produced by the dec-14 allele in heterozygous dec-14/dec-129 (4/29) females (lane 5). A wild-type fc106 cDNA transgene (*) or a mutant fc106ΔG922–R949 transgene (*D) was introduced into white (lanes 2 and 3) or dec-14/dec-129 females (lane 4) as indicated. The positions of the fc106ΔG922–R949 fc106-like and s80-like derivatives are indicated (..) in lane 4. (D) A Western blot showing DEC-1 proteins in stage 14 egg chambers. The genotypes of the females are as described in C. The positions of variant s60 (s60V) (lane 1), s60 from the fc106 cDNA transgene (.) (lane 2), and s60 produced from fc106ΔG922–R949 (s60D) (lanes 3 and 4) are indicated. In lane 4, s80 represents a C-terminal product produced by cleavage of s95 (see E, lane 6) and perhaps some s80-like derivative from fc106ΔG922–R949 that has yet to be cleaved. (E) Western blot of stage 14 egg chambers from Oregon R P2-strain females (S) showing the positions of the standard forms of the s80 and s60 DEC-1 proteins (lane 5) and from homozygous dec-14 females (4) showing the cleaved C-terminal derivative of s95 that migrates in the 80-kDa size range (lane 6).
An fc106ΔV288–E473 transgene induces female sterility in wild-type flies:
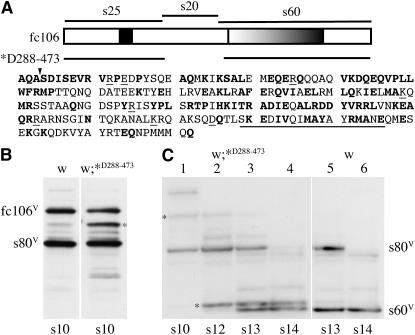
Comparative evolutionary analyses have proven useful for revealing highly conserved amino acid sequences that are important for the structure and function of several proteins. Since the removal of the fc106-specific amino acids was not detrimental to its function, other evolutionary conserved aspects of fc106 were considered as targets for mutagenesis. Previous studies showed that despite extensive sequence divergence between the D. virilis and D. melanogaster dec-1 homologs, the genes were functionally interchangeable. Evolutionarily conserved features of fc106 include the alanine–proline-rich central region within the s25 region, the highly charged nature of the s20 region, and the 26-amino-acid repeat, RQWS/TEE/DQAKI/AQQ, in s60 (Badciong et al. 2001 and Figure 5).
Figure 5.—
Processing of endogenous fc106 and fc106ΔV288–E473 derivatives in wild-type egg chambers carrying the fc106ΔV288–E473 transgene. (A) A schematic of fc106 highlighting an alanine/proline-rich segment in the s25 region and the tandem repeat in the s60 region. The discontinuity in the line below the schematic indicates the region that was deleted in the fc106ΔV288–E473 transgene (*D288–473). The amino acids in the s20 region are shown at the bottom; those that are identical in D. melanogaster and D. virilis are in boldface type. Amino acids previously deleted in a dec-1 mutant transgene (ΔS456–E473, Badciong et al. 2001) are underlined. The downward arrowhead denotes the beginning of s80 (S281). (B and C) Western blots incubated with the Cfc106 antiserum depicted in Figure 1. (B) Western blot of SDS-soluble proteins of stage 10 egg chambers from white females (w) and white flies carrying the fc106ΔV288–E473 transgene (w;*D288–473); proteins were separated using the Mini-Protean electrophoresis cell. The positions of the variant forms of fc106 and s80 are indicated. An aberrant fc106 proprotein produced by the fc106ΔV288–E473 transgene is indicated (*). (C) Developmental Western blot of SDS-soluble proteins from flies designated as in B. The positions of variant forms of s80 and s60 are indicated; the positions of the fc106ΔV288–E473 proprotein and its s60-like C-terminal derivative are indicated (*). Egg chamber stages are shown at the bottom of each lane.
The s20 region is not retained in the eggshell after its cleavage from s80 in late-stage egg chambers. Therefore a potential role for s20 sequences in eggshell assembly is likely to occur in the context of its precursors, which include fc106, fc125, s80, and s95. By removing s20 sequences from the fc106 cDNA transgene and crossing the mutant transgene into dec-14 females the role of s20 sequences in its fc106 and s80 precursors can be investigated.
The s20 region heads the s80 DEC-1 protein. On the basis of N-terminal amino acid sequence analysis (Waring et al. 1990), s80 begins at S281 within a block of evolutionary conserved amino acids (Figure 5A). The site at which s80 is cleaved to produce s60 and s20 has not been determined. On the basis of the sizes of the cleaved byproducts and the evolutionary conservation of s80 processing (D. melangaster and D. virilis), the putative cleavage site was predicted to fall within a block of conserved amino acids (K457–E473). Surprisingly a dec-1 transgene with an internal deletion of the nucleotides that included these amino acids (ΔS456–E473, Figure 5A) was functionally indistinguishable from a wild-type dec-1 transgene (Badciong et al. 2001). Since dec-1 function was not perturbed in the ΔS456–E473 transgene, the s20 deletion was terminated at E473 (Figure 5A), reasoning that hypothetical s60 functions would also be preserved in an fc106ΔV288–E473 transgene. To retain cleavage of the s25 region, the conserved block of amino acids surrounding the fc106 cleavage site (A278–R287) was included in the fc106ΔV288–E473 transgene. Figure 5B shows an aberrant DEC-1 proprotein in the expected size range accumulating in stage 10 egg chambers from white females carrying the fc106ΔV288–E473 transgene. As shown in the developmental Western blot in Figure 5C, an s60-like derivative distinct from endogenous s60V appeared in stage 12 egg chambers. Two DEC-1 species in the 60-kDa size range were observed in stages 13 and 14 as endogenous s80V was processed to s60V. These data suggest that the timing and processing of endogenous fc106V and s80V are not altered by the presence of the mutant proteins. Processing of the aberrant fc106ΔV288–E473 proprotein may be delayed, however. Since the fc106 cleavage site was included in the fc106ΔV288–E473 transgene, cleavage of the mutant proprotein as well as endogenous fc106V was expected in late stage 10 egg chambers (Figure 5, B and C). In the pooled stage 10 egg chambers shown in Figure 5, B and C (w;*D288–473), the intensity of the s80V signal is greater than that of its fc106V precursor. In marked contrast, accumulation of the s60-like derivative from fc106ΔV288–E473 is minimal.
Despite normal processing and accumulation of the endogenous DEC-1 derivatives, wild-type females carrying the fc106ΔV288–E473 transgene are sterile. Transformants representing four independent integration events were recovered in our screen. While progeny were routinely recovered from matings involving male transformants, female transformants never produced progeny, suggesting a dominant female-sterile effect. To confirm the negative effect of the mutant transgene, male transformants were mated with wild-type females. As anticipated, transformant male progeny were fertile and transformant female progeny were sterile. Table 1 shows no larvae hatched from 256 eggs laid by white* females carrying the fc106ΔV288–E473 transgene; this compares to the 84% hatching rate observed in its wild-type counterpart (w*/w*). To determine whether the sterility of wild-type females carrying one copy of the fc106ΔV288–E473 transgene (third chromosome) could be rescued by increasing the wild-type dec-1 copy number, one or two additional copies were introduced via wild-type dec-1 transgenes linked to the second chromosome. Neither a 3:1 nor a 4:1 wild-type dec-1:fc106ΔV288–E473 ratio was sufficient to restore fertility.
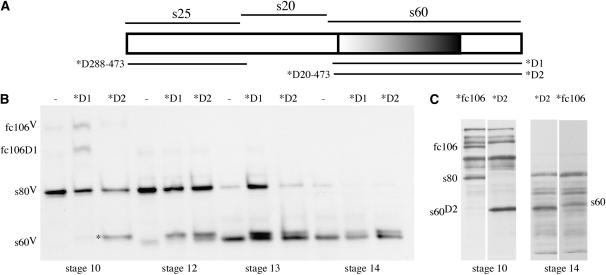
Although designed in principle to yield a functional s60 derivative like the ΔS456–E473 transgene, the N terminus of the fc106ΔV288–E473 s60-like product, as well as the timing of its appearance, is different from that of the ΔS456–E473 derivative. Cleavage of the s25 region from fc106ΔV288–E473 is expected to yield an s60-like derivative headed by the first seven amino acids of the s20 region (S281–R287). In addition, whereas s60 is normally cleaved from its precursor (s80) during stages 13 and 14, the cleaved fc106ΔV288–E473 derivative accumulates in stage 12 egg chambers. The negative effect of the fc106ΔV288–E473 transgene could be due to premature accumulation of the s60-like derivative and/or its abnormal N terminus. To investigate these possibilities an fc106ΔQ20–E473 transgene was created. This transgene includes the predicted signal sequence, the first seven amino acids of the s20 region (S281–R287), and the s60 region (Q474–Q951). After removal of the signal peptide an s60-like product identical to the predicted fc106ΔV288–E473 derivative is expected. The dec-1 gene is expressed during stages 9 and 10, and therefore the s60-like fc106ΔQ20–E473 protein will accumulate prematurely. In Figure 6B the accumulation of variant wild-type, fc106ΔV288–E473 (*D1), and fc106ΔQ20–E473 (*D2) DEC-1 products is compared. In late stage 10 egg chambers from white females carrying one copy of the fc106ΔQ20–E473 transgene (*D2) two prominent bands were observed, endogenous s80V and a species in the 60-kDa range that migrated similarly to the s60-like derivative from fc106ΔV288–E473 (see *D1, stages 12–14). The s60-like species from fc106ΔQ20–E473 was maintained as the egg chambers developed to stage 14. White (w*/w*) females containing one or two copies of the fc106ΔQ20–E473 transgene were fertile, suggesting that neither premature accumulation of the s60-like derivative nor its abnormal N terminus is responsible for the sterility induced by the fc106ΔV288–E473 transgene.
Figure 6.—
Expression of fc106ΔQ20–E473 in wild-type and dec-14 mutant egg chambers. (A) A schematic of fc106 with its major regions indicated above. The lines below the schematic indicate the regions that are present in the fc106ΔV288–E473 (*D288–473) and fc106ΔQ20–E473 (*D20–473) transgenes. The abbreviated nomenclature used in B and C is shown to the right. (B) Developmental Western blot of SDS-soluble proteins from wild-type (w/w) egg chambers in the absence (−) or presence of mutant (*D1 or *D2) fc106 cDNA transgenes. Egg chamber stages are indicated at the bottom of each set. The positions of fc106V, s80V, and s60V from the endogenous wild-type dec-1 gene as well as the fc106ΔV288–E473 proprotein (fc106D1) are indicated to the left; the asterisk denotes the s60-like product produced by the fc106ΔQ20–E473 (*D2) transgene. The mobility of this gene product is indistinguishable from that of the s60-like cleavage product of fc106ΔV288–E473 (*D1) (e.g., compare *D1 and *D2, stage 12). The blot was incubated with the Cfc106 antibody. (C) Expression of the full-length fc106 (*fc106) and *D2 transgenes in dec-14 egg chambers. SDS-soluble egg chamber proteins were resolved using a mini-protean electrophoresis cell system. The positions of *fc106 and *D2 transgene products (fc106, s80, and s60 and s60D2, respectively) are indicated against the background of unmarked dec-14 derivatives. The egg chamber stages are shown at the bottom; the blot was incubated with the Cfc106 antiserum.
While the fc106ΔQ20–E473 product did not interfere with the functions of the endogenous fc106 products, it was not able to rescue the sterility of dec-14 females. As shown in Table 1, dec-14 females with two copies of the fc106ΔQ20–E473 transgene laid eggs that failed to hatch (0.9% hatching rate). Western blot analysis of stage 10 egg chamber proteins (Figure 6C) verified that the s60D2 transgene product stably accumulates in dec-14 egg chambers. Although the s60D2 derivative has seven ectopic amino acids at its N terminus (S281–R287), it migrated faster than s60 from the fc106 cDNA transgene (Figure 6C, stage 14). This suggests that the endogenous s80 cleavage site lies within the regions deleted in the fc106ΔQ20–E473 and fc106ΔV288–E473 transgenes.
Alterations in the extractability of s60 in stage 14 egg chambers from females expressing the fc106ΔV288–E473 transgene:
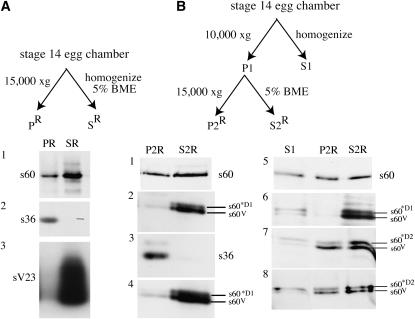
Biochemical fractionation and immunolocalization studies have shown that in wild-type stage 14 egg chambers s60 is present in both the vitelline membrane and the endochorion layers of the eggshell (Nogueron et al. 2000). Enriched eggshell fractions can be obtained by low-speed centrifugation of homogenates from stage 14 egg chambers. When a reducing agent is included in the homogenization buffer, only endochorion proteins are enriched in the low-speed pellet. When reducing agents are omitted, both vitelline membrane and endochorion proteins are selectively enriched in the pellet fraction. As shown in section 1 of Figure 7A, when wild-type stage 14 egg chambers were homogenized in the presence of a reducing agent s60 was recovered in both the supernatant (SR) and the pellet (PR) fractions, presumably reflecting its inclusion in both the vitelline membrane and the endochorion layers. As expected, s36, a major endochorion protein, was detected only in the pellet (Figure 7A, section 2), while sV23, a major vitelline membrane protein, was detected only in the BME-sensitive supernatant (Figure 7A, SR, section 3). In Figure 7B enriched eggshells were prepared in the absence of a reducing agent so that both vitelline membrane and endochorion proteins were recovered in the pellet (P1) fraction. The P1 pellet was resuspended in buffered saline containing 5% BME and centrifuged to yield BME-resistant pellet (P2R) and BME-sensitive supernatant (S2R) fractions. As expected, s60 from wild-type eggshells (P1) was recovered in both the BME-resistant (P2R) and the BME-sensitive (S2R) fractions (Figure 7B, section 1). When the enriched eggshell fraction (P1) from white females carrying the transgene fc106ΔV288–E473 (*D1) was resuspended in the reducing agent, both endogenous s60 (s60V) and the s60-like derivative of fc106ΔV288–E473 (s60*D1) were recovered almost exclusively in the BME-sensitive supernatant fraction (Figure 7B, S2R, sections 2 and 4). In contrast, the s36 endochorion protein was detected only in the BME-resistant fraction (Figure 7B, P2R, section 3). The relative distributions of the proteins in sections 1–4 of Figure 7B indicate anomalies in the behavior of not only s60*D1, but also endogenous s60 in eggshells derived from females carrying the fc106ΔV288–E473 transgene.
Figure 7.—
Fractionation of endogenous s60 is altered in females carrying the fc106ΔV288–E473 transgene. (A) Wild-type (Oregon R) stage 14 egg chambers homogenized in the presence of BME were separated into pellet (PR) and supernatant (SR) fractions by centrifugation at 15,000 × g. SDS-soluble proteins from both fractions (PR and SR) were resolved by SDS–PAGE. (1) The 60-kDa size range of a blot incubated with the DEC-1 Cfc106 antibody (Figure 1A). The position of the standard form of s60 is shown. (2) Blot in 1 stripped and reincubated with an s36 chorion protein antibody. Reactive proteins (s36) in the 36-kDa size range are shown. (3) Blot shown in 2 restripped and reincubated with an sV23 vitelline membrane protein antibody. Reactive proteins (sV23) in the 23-kDa size range are shown. (B) Stage 14 egg chambers homogenized in the absence of a reducing agent were separated by centrifugation at 10,000 × g into enriched eggshell pellet (P1) and supernatant (S1) fractions. The pellet fraction (P1) was resuspended in Tris-buffered saline and incubated with 5% BME. Second pellet (P2R) and supernatant (S2R) fractions were collected following centrifugation at 15,000 × g. (1–4) P2R and S2R fractions from stage 14 egg chambers. (1) A 60-kDa region of blot from wild-type (Oregon R) females incubated with the DEC-1 Cfc106 antibody; (2–4) fractions from wild-type (w/w) females carrying the fc106ΔV288–E473 (*D1) transgene. (2 and 4) A 60-kDa region of blots incubated with the Cfc106 antibody from independent experiments. The positions of the endogenous variant form of s60 (s60V) and the s60-like derivative from the *D1 transgene (s60*D1) are indicated. (3) A 36-kDa region of the blot shown in 2 after stripping and reincubation with the s36 antibody. (5–8) The 60-kDa region from Western blots showing S1, P2R, and S2R fractions incubated with the DEC-1 Cfc106 antibody: (5) fractions from wild-type (Oregon R) stage 14 egg chambers displaying the standard form of s60; (6) fractions from white (w/w) females carrying the fc106ΔV288–E473 (*D1) transgene; (7 and 8) fractions from white (w/w) females carrying the fc106ΔQ20–E473 (*D2) transgene. The blots in 7 and 8 are from independent experiments. In 6–8 the positions of the endogenous variant form of s60 (s60V) and the s60-like derivatives from the *D1 (s60*D1) and *D2 (s60*D2) transgenes are indicated.
The behavior of endogenous s60 (s60V) in egg chambers isolated from wild-type females, wild-type females with the fc106ΔV288–E473 transgene, and wild-type females with the fc106ΔQ20–E473 transgene is compared in Figure 7B, sections 5–8. The initial supernatants (S1) as well as the BME-sensitive supernatants (S2R) were analyzed to monitor s60 and s60-like molecules that failed to integrate into the eggshell and therefore were not recovered in the P1 fraction. The relatively small amounts of 60-kDa products in the S1 fractions (Figure 7B, sections 5–8) suggest that most endogenous s60 and s60-like transgene products become incorporated into large eggshell fragments. Consistent with our previous results, s60 was recovered in BME-resistant (P2R) and -sensitive (S2R) fractions in wild-type stage 14 egg chambers (Figure 7B, section 5), but only in the supernatant fractions when the fc106ΔV288–E473 transgene was present (Figure 7B, section 6). In contrast, endogenous s60 (s60V) and s60*D2 from the transgene were recovered in both BME-resistant (P2R) and BME-sensitive (S2R) fractions when eggshells were prepared from wild-type females carrying the fc106ΔQ20–E473 transgene (Figure 7B, sections 7 and 8). These data suggest that the fractionation of endogenous s60 is altered in the presence of fc106ΔV288–E473, but not fc106ΔQ20–E473, transgene products.
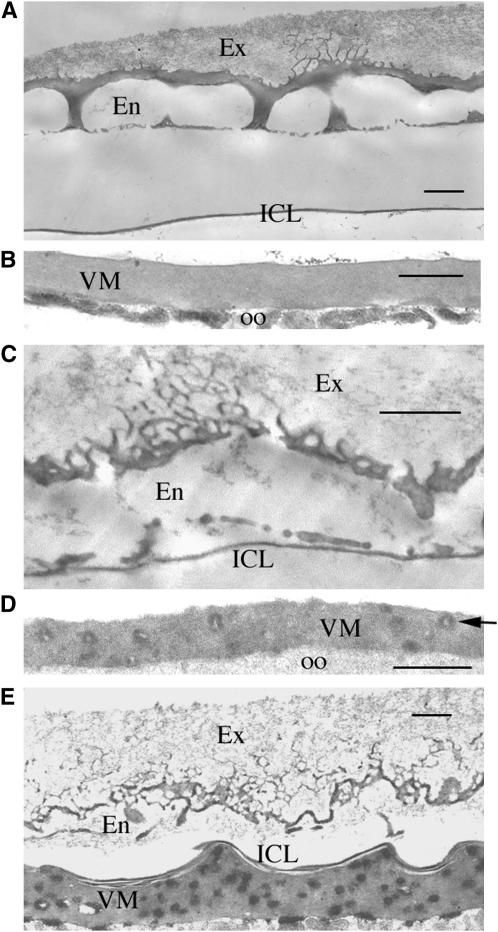
The absence of s60 molecules in the BME-resistant eggshell fraction (P2R) when fc106ΔV288–E473 is expressed suggests an anomaly in the BME-resistant endochorion layer. In wild-type stage 14 egg chambers the endochorion has a characteristic tripartite structure consisting of a reticular roof network, pillars, and a discontinuous floor (Figure 8A) while the oocyte proximal vitelline membrane has a uniform appearance (Figure 8B). In the DEC-1 protein null mutant dec-129 endochorion substructure is absent, electron dense material is dispersed throughout the vitelline membrane, and there is an abnormal association of the inner chorionic layer (ICL) with the vitelline membrane layer (Figure 8E and Mauzy-Melitz and Waring 2003). Although less dramatic than dec-129, eggshells from wild-type flies carrying the fc106ΔV288–E473 transgene showed abnormal morphology (Figure 8, C and D). Discontinuities in the reticular roof network were evident, the tripartite nature of the endochorion was disrupted, and the vitelline membrane layer was punctuated by abnormal accumulations. These data are consistent with abnormal endochorion assembly.
Figure 8.—
Transmission electron micrographs showing the main body of eggshells from late stage 14 egg chambers. (A) Nonproteinaceous exochorion (Ex), endochorion (En), and inner chorionic layer (ICL) from wild-type egg chambers. (B) Vitelline membrane (VM) and portion of underlying oocyte (oo) from wild-type egg chambers. (C and D) Eggshell layers from a white (w/w) female carrying the fc106ΔV288–E473 transgene with the exochorion, the endochorion, and the ICL shown in C and the vitelline membrane in D. The arrow points to an abnormal electron-dense accumulation within the vitelline membrane layer. (E) Eggshell from a DEC-1 null female (dec-129). Bars, 1 μm.
DISCUSSION
In this study mutant fc106 transgenes were created in which either a small block (29 C-terminal amino acids) or large regions (s20 and s25/s20) of the fc106 proprotein were removed. Structurally all of the deletions were tolerated as the mutant proteins were secreted from the cell and accumulated at wild-type levels. Of the eight mutant dec-1 transgenes studied to date, including this study (Badciong et al. 2001; Mauzy-Melitz and Waring 2003), DEC-1 mutant derivatives failed to accumulate only when the transcripts had a premature termination codon, suggesting that nonsense-mediated mRNA decay may be a factor in these cases (Singh and Lykke-Andersen 2003). In general, protein misfolding and intracellular retention do not appear to be problematic when expressing mutant DEC-1 proteins. This is consistent with the largely unstructured nature predicted for DEC-1 proteins.
In wild-type females fc106 is cleaved in a developmentally regulated manner, yielding s25, s20, and s60. The s25 N-terminal derivative and the s60 C-terminal derivative become part of the mature structure. In contrast, the internal derivative, s20, is rapidly taken up by the oocyte. To investigate its role in eggshell assembly, the s20 region was deleted from an fc106 cDNA transgene. The aberrant proprotein was secreted from the follicle cells and cleaved to s25 and an s60-like derivative. Surprisingly expression of the aberrant proprotein exerted a dominant-negative effect on eggshell assembly and female fertility.
Dominant-negative effects have been associated with several mutations in extracellular matrix proteins. Dominant mutations in fibrillar collagens have been implicated in a variety of human pathologies. In most cases the presence of an aberrant procollagen molecule prevents the formation of a stable triple helix, resulting in degradation of all three chains in a process referred to as “procollagen suicide.”
Mutations in the fibrillin-1 gene, associated with Marfan syndrome and related connective-tissue disorders, also show autosomal dominant inheritance. A significant number of these disease-causing mutations are cysteine substitutions that are predicted to cause misfolding. Recent studies using recombinant fragments of fibrillin-1 with missense mutations (C1117Y and C1129Y) showed that the mutant molecules were retained within the ER and thus exert their dominant-negative effects intracellularly. In contrast, a fibrillin-1 fragment with a G1127S mutation showed normal trafficking and was hypothesized to exert its dominant-negative effect extracellularly (Whiteman and Handford 2003).
Dominant collagen VI mutations have recently been shown to be a common cause of Ullrich congenital muscular dystrophy (Pan et al. 2003; Baker et al. 2005). Dominant mutations have been associated with in-frame deletions in the N-terminal region of the triple-helical domain. Although incorporated into tetramers and secreted, immunofluorescence labeling showed an almost complete absence of collagen VI microfibrils. The authors speculate that the inability of abnormal tetramers to align properly impairs the end-to-end association of tetramers needed for microfibril formation. In the absence of microfibril formation collagen VI protein accumulated aberrantly in the interstitial and perivascular space.
The fc106ΔV288–E473 transgene exerts its dominant-negative effect extracellularly. In wild-type egg chambers the fc106ΔV288–E473 proprotein is secreted during the stages of vitelline membrane formation and accumulates at levels consistent with the single-copy status of the transgene. Biochemical fractionation data indicate that the aberrant protein is not excluded from eggshell assembly. When egg chambers were disrupted with buffered saline the mutant s60 derivative was recovered in the low-speed pellet, indicative of its integration into the eggshell. The presence of the fc106ΔV288–E473 protein does not appear to disrupt processing of the endogenous DEC-1 proteins. Western blot analysis indicated timely and correct C-terminal processing of the endogenous fc106 and s80 precursors. In wild-type stage 14 egg chambers s60 was recovered in BME-sensitive and BME-resistant fractions. In egg chambers carrying the mutant transgene both endogenous s60 and the s60-like product from the transgene were recovered exclusively in the BME-sensitive fraction. The altered behavior of endogenous s60 in the presence of the mutant transgene is consistent with the formation of aberrant complexes containing wild-type and mutant DEC-1 proteins.
The persistence of the dominant-negative effect in the presence of two extra copies of the wild-type gene suggests that DEC-1 proteins may form multisubunit complexes. One copy of a wild-type DEC-1 transgene is sufficient to restore fertility in DEC-1 protein null mutant females. The sterility of wild-type females carrying the fc106ΔV288–E473 transgene suggests that <50% of the putative DEC-1 complexes that form are composed only of wild-type subunits. In wild-type egg chambers carrying one copy of the fc106ΔV288–E473 transgene and two copies of a wild-type transgene, ∼50% of hypothetical trimers and 40% of hypothetical tetramers are expected to be composed entirely of wild-type subunits. Since four wild-type copies of the DEC-1 gene were not sufficient to negate the dominant-negative effect of the fc106ΔV288–E473 transgene, it is likely that tetrameric or higher-order DEC-1 oligomeric complexes form.
How s60 fractionates in stage 14 eggshells appears to be dependent upon events that occur early in the assembly process. In wild-type egg chambers with the fc106ΔV288–E473 transgene the s60-like product appeared in stage 12 egg chambers; in egg chambers with the fc106ΔQ20–E473 transgene a similar s60-like derivative accumulated in stage 10 egg chambers. The early accumulation of the fc106ΔQ20–E473 s60-like derivative did not alter the fractionation of the endogenous s60 derivative or affect the fertility of wild-type females. These data suggest that the abnormal juxtaposition of s25 and s60 sequences in the fc106ΔV288–E473 proprotein in stage 10 egg chambers is responsible for both the altered fractionation of endogenous s60 and the female sterility.
The simplest interpretation of the data is that endogenous s60 interactions in the endochorion layer are disrupted when the aberrant DEC-1 proprotein fc106ΔV288–E473 is present. As shown in Figure 7, a substantial fraction of s60 is released to the soluble fraction when eggshell fragments are exposed to a reducing agent. Conceptual translation of the fc106 open reading frame shows a single cysteine residue in the C-terminal region of s60. This cysteine does not appear to be involved in the formation of intermolecular disulfide bridges since the SDS-electrophoretic profiles of soluble s60 run in the presence and the absence of a reducing agent are indistinguishable (G. Waring, unpublished results). In addition, despite being functionally interchangeable with its D. melanogaster counterpart, the D. virilis dec-1 gene does not have a cysteine residue in the s60 region (Badciong et al. 2001). These results suggest that the solubilization of s60 with a reducing agent is the result of the disruption of an underlying disulfide-based molecular network with which s60 interacts. As the recovery of the major vitelline membrane proteins in low-speed pellets is dependent upon intact disulfide bonds, the vitelline membrane is the likely origin of the BME-sensitive s60 fraction. The endochorion, a molecular network whose structural integrity is not dependent upon intermolecular disulfide bonds (Waring and Mahowald 1979; Mindrinos et al. 1980), is the likely origin of the BME-resistant s60 fraction. The absence of a BME-resistant s60 fraction and the morphological abnormalities observed in the eggshells from wild-type females with the fc106ΔV288–E473 transgene suggest that either trafficking of s60 from the vitelline membrane to the endochorion or interactions of s60 within the endochorion are disrupted.
How the aberrant fc106ΔV288–E473 proprotein exerts its effect on endogenous DEC-1 products is not known. The wild-type fc106 proprotein contains three distinct regions. The N-terminal s25 region is characterized by its acidic nature (pI ∼ 4) and high alanine and proline content (33%). The internal s20 region is basic (pI ∼ 10) and rich in charged amino acids (17% K/R; 14% E/D). The C-terminal s60 region consists largely of a repeating motif (26 amino acids) with a high glutamine (40%) content. Dominant-negative effects were not encountered when mutant dec-1 transgenes carrying a deletion of the s25 region either alone (Mauzy-Melitz 2001) or in combination with the s20 region (fc106ΔQ20–E473, this study) were introduced into wild-type flies. Thus it appears that the inclusion of the N-terminal s25 region is needed to elicit the dominant-negative effect. If fc106 proproteins associate laterally and if the hydrophobic N terminus is a significant driving force, then abnormal DEC-1 complexes may be created if the glutamine-rich repeating motif of fc106ΔV288–E473 becomes apposed to the highly charged s20 region of endogenous fc106. The abnormal apposition of these surfaces may disrupt the recruitment of proteins that are needed for later assembly events involving s60. Biochemical characterization of DEC-1-containing complexes in wild-type egg chambers in the absence and the presence of the fc106ΔV288–E473 transgene at different developmental stages should help elucidate the molecular basis of the dominant-negative effect of the fc106ΔV288–E473 proprotein and, along with localization studies, provide insights on interlayer trafficking and/or how s60 integrates and functions during endochorion morphogenesis.
Regardless of the mechanism, the ability to create mutant eggshell transgenes that cause dominant female sterility may have potential for insect population control (Robinson 2002; Markaki et al. 2004). With minimal effects on fitness beyond those associated with the transgene insertion site, X- and autosomally linked transgenes can be introduced into the target population via males. Male transmission of an X-linked transgene would render all F1 females sterile. Transgenes linked to an autosome would produce fertile transgene-bearing male progeny for additional population control. However, for single-insert lines, only half of the F1 females would inherit the transgene and become sterile.
Acknowledgments
We thank Debra Mauzy-Melitz for technical assistance with the electron microscopy and Heather Owen for use of the imaging facility at the University of Wisconsin-Milwaukee. Marquette University fellowship support to D.K.S. and support from the National Institutes of Health (1R15 GM62816-01) to G.L.W. are gratefully acknowledged.
References
- Acosta, M., and B. Goni, 2000. A technique for collecting and examining a large number of eggs. Dros. Inf. Serv. 83: 174–175. [Google Scholar]
- Aumailley, M., and B. Gayraud, 1998. Structure and biological activity of the extracellular matrix. J. Mol. Med. 76: 253–265. [DOI] [PubMed] [Google Scholar]
- Badciong, J. C., J. M. Otto and G. L. Waring, 2001. The functions of the multiproduct and rapidly evolving dec-1 eggshell gene are conserved between evolutionarily distant species of Drosophila. Genetics 159: 1089–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, N. L., M. Morgelin, R. Peat, N. Goemans, K. N. North et al., 2005. Dominant collagen VI mutations are a common cause of Ullrich congenital muscular dystrophy. Hum. Mol. Genet. 14: 279–293. [DOI] [PubMed] [Google Scholar]
- Bauer, B. J., and G. L. Waring, 1987. 7C female sterile mutants fail to accumulate early eggshell proteins necessary for later chorion morphogenesis in Drosophila. Dev. Biol. 121: 349–358. [DOI] [PubMed] [Google Scholar]
- Digan, M. E., A. C. Spradling, G. L. Waring and A. P. Mahowald, 1979. The genetic analysis of chorion morphogenesis in Drosophila melanogaster, pp. 171–181 in Eucaryotic Gene Regulation ICN-UCLA Symposium, edited by R. e. a. Axel. Academic Press, New York.
- Hawley, R. J., and G. L. Waring, 1988. Cloning and analysis of the dec-1 female-sterile locus, a gene required for proper assembly of the Drosophila eggshell. Genes Dev. 2: 341–349. [DOI] [PubMed] [Google Scholar]
- Hughes, M. J., and D. W. Andrews, 1996. Creation of deletion, insertion and substitution mutations using a single pair of primers and PCR. BioTechniques 20: 188–196. [DOI] [PubMed] [Google Scholar]
- Komitopoulou, K., M. Gans, L. Margaritis and F. C. Kafatos, 1983. Isolation and characterization of sex-linked female-sterile mutants in Drosophila melanogaster with special attention to eggshell mutants. Genetics 105: 897–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U. K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- Manogaran, A., and G. L. Waring, 2004. The N-terminal prodomain of sV23 is essential for the assembly of a functional vitelline membrane network in Drosophila. Dev. Biol. 270: 261–271. [DOI] [PubMed] [Google Scholar]
- Margaritis, L. H., 1985. Structure and physiology of the eggshell., pp. 153–230 in Comprehensive Insect Physiology, Biochemistry, and Pharmacology, edited by G. A. Kerkut and L. I. Gilbert. Pergamon, Elmsford, NY.
- Markaki, M., R. K. Craig and C. Savakis, 2004. Insect population control using female specific pro-drug activation. Insect. Biochem. Mol. Biol. 34: 131–137. [DOI] [PubMed] [Google Scholar]
- Mauzy-Melitz, D., 2001. Genetic dissection of eggshell assembly in D. melanogaster. Ph.D. Thesis, Marquette University, Milwaukee.
- Mauzy-Melitz, D., and G. L. Waring, 2003. fc177, a minor dec-1 proprotein, is necessary to prevent ectopic aggregation of the endochorion during eggshell assembly in Drosophila. Dev. Biol. 255: 193–205. [DOI] [PubMed] [Google Scholar]
- Mindrinos, M., W. H. Petri, V. K. Galanopoulos, M. F. Lombard and L. H. Margaritis, 1980. Crosslinking of the Drosophila chorion involves a peroxidase. Roux's Arch. 189: 187–196. [DOI] [PubMed] [Google Scholar]
- Noguerón, M. I., and G. L. Waring, 1995. Regulated processing of dec-1 eggshell proteins in Drosophila. Dev. Biol. 172: 272–279. [DOI] [PubMed] [Google Scholar]
- Nogueron, M. I., D. Mauzy-Melitz and G. L. Waring, 2000. Drosophila dec-1 eggshell proteins are differentially distributed via a multistep extracellular processing and localization pathway. Dev. Biol. 225: 459–470. [DOI] [PubMed] [Google Scholar]
- Pan, T. C., R. Z. Zhang, D. G. Sudano, S. K. Marie, C. G. Bonnemann et al., 2003. New molecular mechanism for Ullrich congenital muscular dystrophy: a heterozygous in-frame deletion in the COL6A1 gene causes a severe phenotype. Am. J. Hum. Genet. 73: 355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascucci, T., J. Perrino, A. P. Mahowald and G. L. Waring, 1996. Eggshell assembly in Drosophila: processing and localization of vitelline membrane and chorion proteins. Dev. Biol. 177: 590–598. [DOI] [PubMed] [Google Scholar]
- Robinson, A. S., 2002. Mutations and their use in insect control. Mutat. Res. 511: 113–132. [DOI] [PubMed] [Google Scholar]
- Savant, S. S., and G. L. Waring, 1989. Molecular analysis and rescue of a vitelline membrane mutant in Drosophila. Dev. Biol. 135: 43–52. [DOI] [PubMed] [Google Scholar]
- Singh, G., and J. Lykke-Andersen, 2003. New insights into the formation of active nonsense-mediated decay complexes. Trends Biochem. Sci. 28: 464–466. [DOI] [PubMed] [Google Scholar]
- Waring, G. L., 2000. Morphogenesis of the eggshell in Drosophila. Int. Rev. Cytol. 198: 67–108. [DOI] [PubMed] [Google Scholar]
- Waring, G. L., and A. P. Mahowald, 1979. Identification and time of synthesis of chorion proteins in Drosophila melanogaster. Cell 16: 599–607. [DOI] [PubMed] [Google Scholar]
- Waring, G. L., R. J. Hawley and T. Schoenfeld, 1990. Multiple proteins are produced from the dec-1 eggshell gene in Drosophila by alternative RNA splicing and proteolytic cleavage events. Dev. Biol. 142: 1–12. [DOI] [PubMed] [Google Scholar]
- Whiteman, P., and P. A. Handford, 2003. Defective secretion of recombinant fragments of fibrillin-1: implications of protein misfolding for the pathogenesis of Marfan syndrome and related disorders. Hum. Mol. Genet. 12: 727–737. [DOI] [PubMed] [Google Scholar]