Abstract
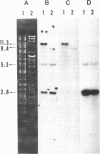
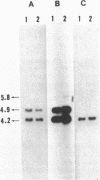
By using cloned Rhizobium meliloti nodulation (nod) genes and nitrogen fixation (nif) genes, we found that the genes for both nodulation and nitrogen fixation were on a plasmid present in fast-growing Rhizobium japonicum strains. Two EcoRI restriction fragments from a plasmid of fast-growing R. japonicum hybridized with nif structural genes of R. meliloti, and three EcoRI restriction fragments hybridized with the nod clone of R. meliloti. Cross-hybridization between the hybridizing fragments revealed a reiteration of nod and nif DNA sequences in fast-growing R. japonicum. Both nif structural genes D and H were present on 4.2- and 4.9-kilobase EcoRI fragments, whereas nifK was present only on the 4.2-kilobase EcoR2 fragment. These results suggest that the nif gene organizations in fast-growing and in slow-growing R. japonicum strains are different.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Better M., Lewis B., Corbin D., Ditta G., Helinski D. R. Structural relationships among Rhizobium meliloti symbiotic promoters. Cell. 1983 Dec;35(2 Pt 1):479–485. doi: 10.1016/0092-8674(83)90181-2. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Kallas T., Rebière M. C., Rippka R., Tandeau de Marsac N. The structural nif genes of the cyanobacteria Gloeothece sp. and Calothrix sp. share homology with those of Anabaena sp., but the Gloeothece genes have a different arrangement. J Bacteriol. 1983 Jul;155(1):427–431. doi: 10.1128/jb.155.1.427-431.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza K., Fuhrmann M., Hahn M., Regensburger B., Hennecke H. In Rhizobium japonicum the nitrogenase genes nifH and nifDK are separated. J Bacteriol. 1983 Aug;155(2):915–918. doi: 10.1128/jb.155.2.915-918.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser H. H., Bohlool B. B., Hu T. S., Weber D. F. Fast-growing rhizobia isolated from root nodules of soybean. Science. 1982 Mar 26;215(4540):1631–1632. doi: 10.1126/science.215.4540.1631. [DOI] [PubMed] [Google Scholar]
- Masterson R. V., Russell P. R., Atherly A. G. Nitrogen fixation (nif) genes and large plasmids of Rhizobium japonicum. J Bacteriol. 1982 Nov;152(2):928–931. doi: 10.1128/jb.152.2.928-931.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R. K., Schilperoort R. A., Nuti M. P. Large plasmids of fast-growing rhizobia: homology studies and location of structural nitrogen fixation (nif) genes. J Bacteriol. 1981 Mar;145(3):1129–1136. doi: 10.1128/jb.145.3.1129-1136.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Sundaresan V., Ausubel F. M. Directed transposon Tn5 mutagenesis and complementation analysis of Rhizobium meliloti symbiotic nitrogen fixation genes. Cell. 1982 Jun;29(2):551–559. doi: 10.1016/0092-8674(82)90171-4. [DOI] [PubMed] [Google Scholar]
- Scolnik P. A., Haselkorn R. Activation of extra copies of genes coding for nitrogenase in Rhodopseudomonas capsulata. Nature. 1984 Jan 19;307(5948):289–292. doi: 10.1038/307289a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]