Abstract
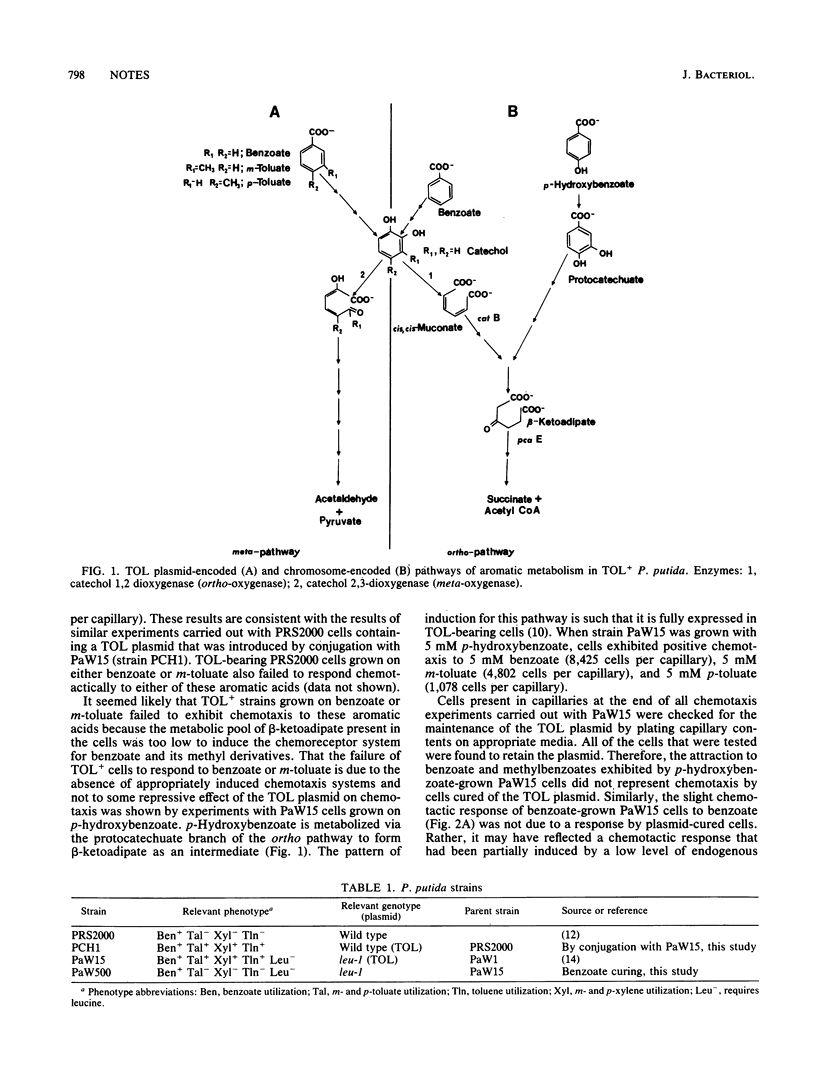
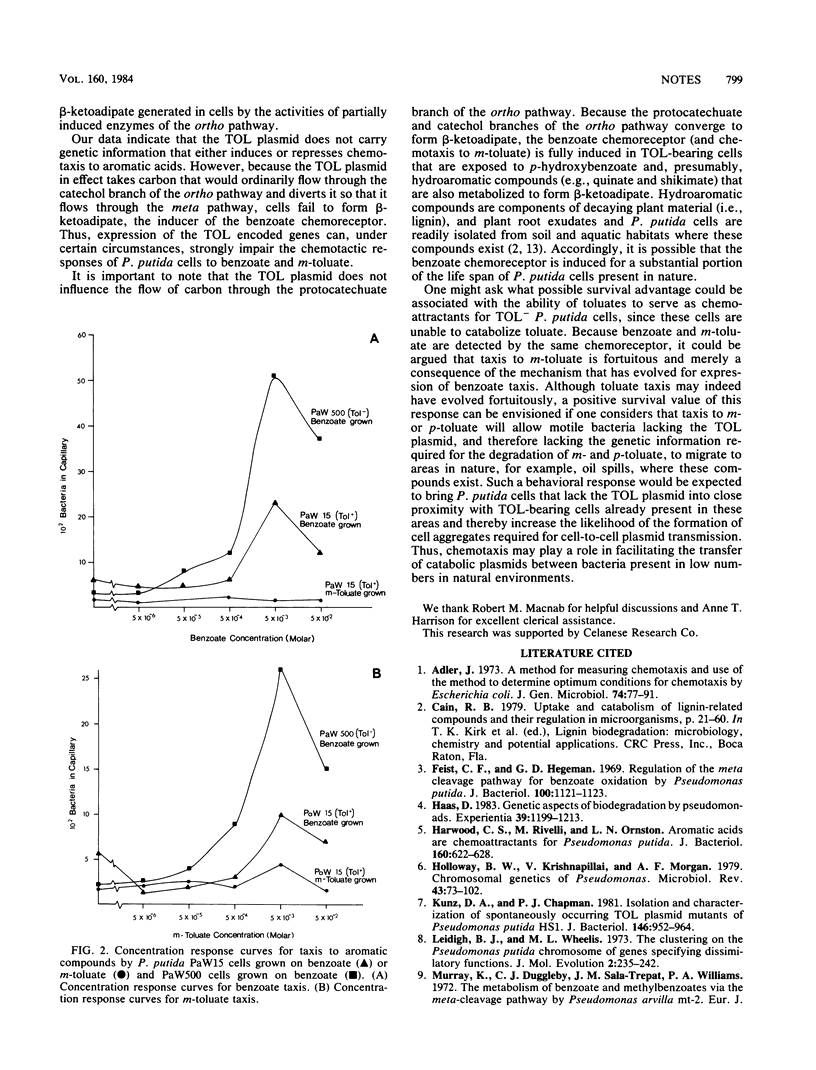
Growth conditions that elicited positive chemotaxis to benzoate and m-toluate in TOL- Pseudomonas putida cells failed to elicit taxis to these compounds in TOL+ cells. The inability of TOL+ cells to respond to these aromatic acids appears to be due to the preferential expression of TOL-encoded genes for aromatic degradation over chromosomally encoded genes. Expression of chromosomal genes for aromatic degradation is required for cells to form beta-ketoadipate, the inducer of benzoate and m-toluate taxis.
Full text
PDF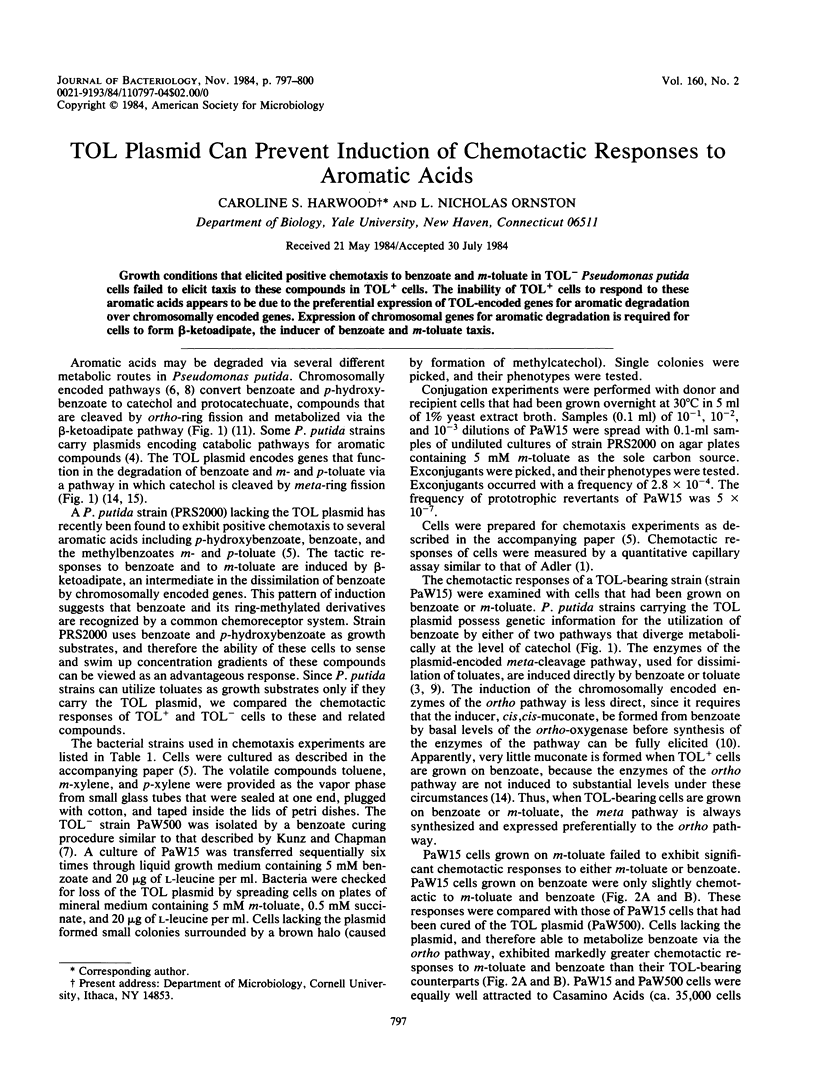

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Feist C. F., Hegeman G. D. Regulation of the meta cleavage pathway for benzoate oxidation by Pseudomonas putida. J Bacteriol. 1969 Nov;100(2):1121–1123. doi: 10.1128/jb.100.2.1121-1123.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D. Genetic aspects of biodegradation by pseudomonads. Experientia. 1983 Nov 15;39(11):1199–1213. doi: 10.1007/BF01990357. [DOI] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Morgan A. F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979 Mar;43(1):73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz D. A., Chapman P. J. Isolation and characterization of spontaneously occurring TOL plasmid mutants of Pseudomonas putida HS1. J Bacteriol. 1981 Jun;146(3):952–964. doi: 10.1128/jb.146.3.952-964.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidigh B. J., Wheelis M. L. The clustering on the Pseudomonas putida chromosome of genes specifying dissimilatory functions. J Mol Evol. 1973 Nov 27;2(4):235–242. doi: 10.1007/BF01654092. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Parke D. Properties of an inducible uptake system for beta-ketoadipate in Pseudomonas putida. J Bacteriol. 1976 Feb;125(2):475–488. doi: 10.1128/jb.125.2.475-488.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N. Regulation of catabolic pathways in Pseudomonas. Bacteriol Rev. 1971 Jun;35(2):87–116. doi: 10.1128/br.35.2.87-116.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- Schroth M. N., Hancock J. G. Disease-suppressive soil and root-colonizing bacteria. Science. 1982 Jun 25;216(4553):1376–1381. doi: 10.1126/science.216.4553.1376. [DOI] [PubMed] [Google Scholar]
- Williams P. A., Murray K. Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J Bacteriol. 1974 Oct;120(1):416–423. doi: 10.1128/jb.120.1.416-423.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. L., Dunn N. W. Transmissible plasmid coding for the degradation of benzoate and m-toluate in Pseudomonas arvilla mt-2. Genet Res. 1974 Apr;23(2):227–232. doi: 10.1017/s0016672300014853. [DOI] [PubMed] [Google Scholar]



