Abstract
Bone morphogenetic proteins (BMPs) are derived from inactive precursor proteins by endoproteolytic cleavage. Here we show that processing of Nodal and Myc-tagged BMP4 is significantly enhanced by SPC1/Furin or SPC4/PACE4, providing direct evidence that regulation of BMP signaling is likely to be controlled by subtilisin-like proprotein convertase (SPC) activities. Nodal processing is dramatically enhanced if two residues adjacent to the precursor cleavage site are substituted with amino acids found at the equivalent positions of Activin, demonstrating that structural constraints at the precursor cleavage site limit the processing efficiency. However, in transfection assays, mature Nodal is undetectable either in culture supernatants or in cell lysates, despite efficient cleavage of the precursor protein, suggesting that mature Nodal is highly unstable. Domain swap experiments support this conclusion since mature BMP4 or Dorsalin are also destabilized when expressed in conjunction with the Nodal pro domain. By contrast, mature Nodal is stabilized by the Dorsalin pro domain, which mediates the formation of stable complexes. Collectively, these data show that the half-life of mature BMPs is greatly influenced by the identity of their pro regions.
Keywords: transforming growth factor-β, Dorsalin, Nodal processing, pro domain, complex formation
TGF-β–related growth factors elicit different biological responses depending on their local concentration. In the Drosophila blastoderm for example, distinct cell fates are specified by a dorsal-ventral gradient of DPP activity (Ferguson and Anderson, 1992). Cells exposed to high concentrations of DPP acquire dorsal-most character (amnioserosa), whereas lower levels or the absence of DPP specify dorsal ectoderm or ventral cell types, respectively. At later stages, cells within the wing disc in proximity to the antero-posterior compartment boundary, a source of DPP, express both spalt and optomotorblind. In contrast, only optomotorblind is induced in cells situated more distantly where DPP levels are lower (Nellen et al., 1996). Studies in Xenopus support the idea that TGF-β family members can act in a concentration-dependent manner. Thus, as little as 1.5-fold changes in the concentration of Activin alter the dorsal-ventral character of the mesoderm which is induced in animal cap explants (Green et al., 1992; Symes et al., 1994). Similarly, graded activities of Activin and other TGF-β–related growth factors may also specify endodermal and ectodermal cell fates in Xenopus embryos (Hemmati-Brivanlou et al., 1994; Hemmati-Brivanlou and Melton, 1994; Schulte-Merker et al., 1994). In chick, a gradient of bone morphogenetic protein (BMP)1 activity controls the fate of mediolateral mesoderm cells. Thus, at low levels of BMP4, medial presomitic mesoderm cells are specified to form the lateral aspect of somites (Pourquie et al., 1996), whereas exposure to higher concentrations causes the same cells to become lateral plate mesoderm (Tonegawa et al., 1997).
Morphogenic gradients of BMP activities are established at least in part by the activities of diffusible antagonists like Follistatin, Chordin, Noggin, Cerberus, Gremlin, and DAN which bind and sequester TGF-β–related molecules (Nakamura et al., 1990; Bouwmeester et al., 1996; Piccolo et al., 1996; Zimmerman et al., 1996; Hsu et al., 1998). In Drosophila, the Chordin homologue SOG diffuses from ventral blastoderm cells into the adjacent dorsal DPP expression domain where it has been suggested to sequester DPP, thus contributing to the formation of a DPP gradient and permitting cells in the dorsolateral blastoderm to acquire an ectodermal fate (Francois et al., 1994). Moreover, genetic screens led to the identification of Tolloid, a metalloprotease enhancing DPP activity in the dorsal-most region of the blastoderm (Shimell et al., 1991). While initially thought to be involved in the proteolytic activation of DPP precursor molecules, Tolloid and its Xenopus homologue Xolloid have been shown recently to cleave SOG or Chordin, respectively (Marqués et al., 1997; Piccolo et al., 1997). Accordingly, Tolloid in the dorsal blastoderm increases DPP activity by antagonizing SOG. Similarly in Xenopus, Xolloid opposes Chordin to enhance the ventralizing activity of BMP4.
In addition, studies in Xenopus suggest that TGF-β–related activities are also regulated at the level of their proteolytic maturation. Thus, Vg-1, a critical determinant of dorsal-ventral axis specification, potently induces axial mesoderm in animal cap explants only if fused to a foreign BMP pro domain that allows efficient precursor processing (Thomsen and Melton, 1993). In contrast, mRNA encoding native Vg-1 yields little if any mature Vg-1 protein and has no detectable activity in explant assays, consistent with the idea that in the intact embryo localized production of Vg-1 may ensure correct placement of the primary body axis (Tannahill and Melton, 1989; Thomsen and Melton, 1993). These findings imply that the pro region is a key determinant in regulating the secretion, processing efficiency, and/or turnover of mature Vg-1. Interestingly, the activity of TGF-β is also controlled in part by its pro region, although presumably via a distinct mechanism. Thus, unlike more distant family members, mature TGF-β is well known to be secreted by most cell types in noncovalent association with a disulfide-linked homodimer of its pro domain masking the activity of TGF-β and prolonging its in vivo half-life (Gentry et al., 1988; Miyazono et al., 1988; Wakefield et al., 1988, 1990).
Candidate proteases responsible for TGF-β processing comprise members of the subtilisin-like proprotein convertase (SPC) family (regarding the SPC nomenclature used in this report see Steiner et al., 1992). Several of these proteases have been shown to specifically hydrolyze peptide bonds preceded by the sequence R-X-K/R-R or R-X-X-R (Molloy et al., 1992; Creemers et al., 1993; Hosaka et al., 1994), such as those found at the cleavage sites of TGF-β–related precursors (Table I). Indeed, SPC1, also known as Furin, enhances the processing of TGF-β1 (Dubois et al., 1995). This observation, together with our previous finding that SPC and BMP expression patterns partially overlap during mouse embryogenesis, led us to propose that BMPs may be cleaved by related proteases (Constam et al., 1996). For example, BMP2, -4, and -7 are coexpressed with SPC4 in the primitive heart, in the apical ectodermal ridge of developing limb buds, and in the interdigital mesenchyme of embryonic limbs. During neural tube patterning, high levels of SPC6 colocalize with BMP4 and BMP7 in the dorsal surface ectoderm, whereas SPC4 is coexpressed with BMP6 in the floorplate. In addition, SPC1 and SPC7 mRNAs are widely expressed, suggesting that the processing of individual BMPs may be controlled by the overlapping activities of several SPCs. In support of this, several SPCs, including SPC1, -4, -6, and -7, have been shown recently to process recombinant BMP4 in vitro (Cui et al., 1998). Although, in the context of a developing frog embryo, an SPC1- and SPC6-specific inhibitor is sufficient to block the ventralizing activities of BMP4, suggesting that SPC1/6-like activities are solely responsible for BMP4 cleavage at least during the stage examined (Cui et al., 1998).
Table I.
Comparison of BMP Precursor Cleavage Sites with the Corresponding Sequences Found within TGF-βs or Activin
| ↓ | |||
| Position (P)* | 8 7 6 5 4 3 2 1 | 1′2′3′4′5′ | |
| Nodal | . . . G W G R R Q R R | H H L P D . . | |
| Dorsalin | . . . Q H S S R S K R | S I G A N . . | |
| BMP2 | . . . P L H K R E K R | Q A K H K . . | |
| BMP3 | . . . S I E R R K K R | S T G V L . . | |
| BMP4 | . . . L T R R R A K R | S P K H H . . | |
| BMP5 | . . . E V L L R S V R | A A N K R . . | |
| BMP6 | . . . E V H V R T T R | S A S S R . . | |
| BMP7 | . . . E V H F R S I R | S T G S K . . | |
| DPP | . . . I R R R R A K R | S P K H H . . | |
| TGF-β1 | . . . L Q S S R H R R | A L D T N . . | |
| TGF-β2 | . . . Q T N R R K K R | A L D A A . . | |
| TGF-β3 | . . . Q G G Q R K K R | A L D T N . . | |
| Activin A | . . . H P H R R R R R | G L E C D . . |
The positions flanking the predicted cleavage sites (arrow) are numbered. The cleavage sites have been confirmed by NH2-terminal sequencing of purified Activin A, TGF-β1–3, BMP2-4, DPP, and Dorsalin (Derynck et al., 1985; Ling et al., 1986; de Martin et al., 1987; Graycar et al., 1989; Celeste et al., 1990; Panganiban et al., 1990; Hammonds et al., 1991; Basler et al., 1993). In the case of BMP3, multiple forms of processed proteins have been detected (Celeste et al., 1990), which may reflect further processing by exopeptidases. The residues shown in bold (positions P1 to P4) fit the consensus sequence R-X-X-R of SPC cleavage motifs.
Here we compare the abilities of SPC1, SPC4, and SPC7 to proteolytically cleave BMP precursors, including BMP4 and Nodal in cell transfection assays. We find that processing of these precursors is dramatically increased by coexpression of either SPC1 or SPC4, whereas SPC7 is significantly less active. Furthermore, domain swap experiments demonstrate that individual BMP pro regions greatly influence the stability of processed, mature ligands. Overall, these experiments suggest that the availability of biologically active BMPs may be controlled by their pro domains and by the limited actions of proprotein convertases.
Materials and Methods
Expression Vectors
A cDNA comprising the coding region of mouse SPC1 cloned into the pSVL expression vector (Pharmacia) was provided by W. Van de Ven (University of Leuven, Leuven, Belgium). The mouse SPC7 cDNA (Constam et al., 1996) lacking untranslated regions and provided with a Kozak consensus sequence was cloned into the same expression vector. A partial mouse SPC4 cDNA (gift of K. Nakayama) was modified at the 5′ end with a linker to provide a start codon and the first 14 amino acids present at the corresponding position of human SPC4 (Kiefer et al., 1991; Hosaka et al., 1994; Johnson et al., 1994). Thus a linker reconstituted with the oligonucleotides 5′-CGCCATGCCTCCGCGCGCGCCGCCTGCGCCCGGGCCCCGGCCGCCGCCCC-3′ (sense), and 5′-GGGGCGGCGGCCGGGGCCCGGGCGCAGGCGGCGCGCGCGGAGGCATGG-3′ (antisense) was ligated to SPC4 cDNA treated with SmaI. The resulting full-length cDNA was subcloned into pSVL.
The pMT21 expression vector containing Myc-tagged chick Dorsalin (Basler et al., 1993) was a gift from T. Jessell. A similar vector containing mouse Nodal (Zhou et al., 1993) was provided by J. Collignon. A related construct, also provided by J. Collignon, contained the Nodal cDNA modified by PCR mutagenesis to encode the sequence HHLPDRSEQKLI SEEDLQLCR... COOH-terminal to the precursor cleavage site, where the underlined residues correspond to a Myc epitope. Myc-tagged BMP4 cDNA (gift of S. Michael) encoding the sequence SPKHEQKLISEEDLHPQR... COOH-terminal to the cleavage site was subcloned into pMT21. Constructs encoding for chimeric BMPs were generated using an EcoRV site present within the Myc coding sequence. To generate a Nodal-MycBMP4 chimeric precursor, the Nodal pro domain encompassing the multibasic cleavage site and the NH2-terminal portion of the Myc epitope thus was fused to the mature domain of MycBMP4 providing the COOH-terminal portion of the Myc epitope. Conversely, a BMP4-MycNodal construct was obtained by ligating an EcoRI-EcoRV cDNA fragment of pro BMP4 to the EcoRV-XhoI fragment of the MycNodal cDNA. Dorsalin-MycNodal (gift of J. Collignon), Nodal-MycDorsalin, and Dorsalin-MycBMP4 constructs were generated analogously.
Modified versions of the above constructs were generated to insert Flag epitopes in the pro domains. In the case of Nodal, the cDNA was partially digested with AflII, and a linker composed of 5′-CATGGACTACAAGGACGACGATGACAAGTA-3′ (sense) and 5′-CATGTACTTGTCATCGTCGTCCTTGTAGTC-3′ (antisense) was inserted after nucleotide 445. The BMP4 pro domain was Flag-tagged by ligating a linker into the AhaII site at position 487 of the mouse BMP4 cDNA (GenBank accession number X56848). The linker was composed of 5′-CGCGACTACAAGGACGACGATGACAAG-3′ (sense) and 5′-CGCTTGTCATCGTCGTCCTTGTAGTCG-3′ (antisense). Overlap extension PCR (Ho et al., 1989) was performed to insert the sequence TACAAGGATGACGATGACAAACCC into the Dorsalin cDNA between nucleotides 219 and 223. The resulting construct encoded the sequence YKDDDDK between D43 and P44 of Dorsalin, reconstituting a Flag epitope. To substitute His246 and Leu247 within Nodal, two fragments were amplified by PCR using primers which at the corresponding positions coded for Leu and Glu, respectively. The first fragment comprising nucleotides 1–1071 of the Nodal cDNA was amplified using the primers 5′-AATACGACTCACTATAGCGC-3′ and 5′-TGGCTCTAGATGTCGGCGTTGCCT-3′, the latter providing an XbaI site. The second fragment corresponding to the mature domain of Nodal was amplified using the primers 5′-CATCTAGAGCCAGACAGAAGC-3′ and 5′-TGTCTCGAGAGAGGCACCCACACTCC-3′. After verifying the sequences of both PCR fragments, they were fused to each other via an XbaI site in a three part ligation with pMT21 to generate pNodal-H246L. To tag this construct with a Flag coding sequence, the EcoRI-NarI fragment (964 bp) encoding for the NH2-terminal portion of Nodal was replaced by the corresponding region of the FlagNodal construct described above.
The FlagNodal-H246L precursor was further modified by abolishing the SPC cleavage site represented by the amino acid sequence R-R-Q-R-R. A 400-bp fragment was amplified by PCR from the Nodal cDNA using the primers 5′-CATTGACATTTTCCACCAGGCCA-3′ (upper), and 5′ - TGGCTCTAGATGGCCGGCTTGAGATCTGCCCCATCCGCCCCTCT-3′ (lower). The resulting PCR product was digested with NarI and XbaI to isolate a 95-bp fragment which had incorporated the lower primer, encoding therefore at its 3′ end the sequence RSQAG and an XbaI restriction site. This PCR-derived 95-bp fragment was used to replace the corresponding sequence in pFlagNodal-H246L, generating the construct cmFlagNodal-H246L. Flag epitopes were also engineered into SPC4 and SPC7 using overlap extension PCR. The sequence flanking the precursor cleavage site in the resulting FlagSPC7 was RAKR′ DYKDDDDKSIH..., whereas in FlagSPC4 it was RAKR′DYKDDDDK QAR..., the Flag epitope being underlined.
COS Transfection and Immunoblot Analysis
COS cells were transfected in 6-cm dishes as described (Gonzalez and Joly, 1995) using DEAE dextran. After incubating transfected cells for 20 h in DMEM containing 10% fetal bovine serum, the medium was changed to serum-free OptiMEM (GIBCO BRL) and conditioned for 48 h. Supernatants (50–400 μl) precleared by centrifugation were precipitated by adding five volumes of cold acetone. The precipitates were collected and dissolved in 15 μl Laemmli buffer. Cell monolayers were lysed by adding 0.5 ml/dish of ice-cold Laemmli buffer to the culture dishes, followed by sonication on ice for 30 s. Proteins collected from supernatants (15 μl/ lane) and whole cell lysates (10 μl/lane) were fractionated by SDS-PAGE together with prestained low molecular weight markers (BioRad) using 12 or 7.5% separating gels. For Western blot analysis, proteins were transferred on nitrocellulose membranes (Schleicher & Schuell) in a Mini-Transblot apparatus (BioRad), followed by incubation with anti-Myc hybridoma supernatant 1-9E10.2 (American Type Culture Collection CRL-1729), or with anti-Nodal antiserum. The Nodal-specific antiserum was obtained from rabbits immunized with the peptide KRYQPHRVPSTC, and it was used at 1,500-fold dilution. The secondary antibodies used were goat anti–mouse Fcγ and goat anti–rabbit IgG, coupled to horseradish peroxidase (Jackson ImmunoResearch). Chemiluminescence was detected with an ECL kit (Amersham), with exposure times of 1–2 min (lysates) or 30–45 min (supernatants). Results shown were all reproduced in three to six independent transfection experiments. Immunoprecipitations were carried out as described (Harlow and Lane, 1988). For preclearing, 1 ml of COS cell conditioned medium was incubated for 1 h with 1 μg/ml of an unrelated mouse IgG1, followed by precipitation using protein G–Sepharose beads (GIBCO BRL). 1 μg/ml monoclonal anti-Flag M2 antibody (Eastman Kodak) was added to each supernatant for 1 h at 4°C. Immune complexes were precipitated with protein G–Sepharose beads, washed four times with ice-cold RIPA buffer, resuspended in Laemmli buffer containing 5% β-mercaptoethanol, and boiled for 3 min before anti-Myc immunoblotting.
Bioassay
C3H10T1/2 osteoblast cells (ATCC CCL-226) were plated in 96-well microtiter dishes at a density of 1.2 × 104 cells per well in DMEM containing 10% heat-inactivated fetal bovine serum. After 15 h, the medium was replaced by DMEM containing 0.25% heat-inactivated fetal bovine serum and 1 μM all-trans retinoic acid (Sigma Chemical Co.). Appropriately diluted COS cell supernatants were added in triplicates, and the plates were cultured for 48 h. Subsequently, cells were lysed in 25 mM Tris-HCl, pH 7.5 (100 μl/well), for 1 h at 4°C, and 25 μl lysate was added to 150 μl 1.5 M 2-amino-propanol, pH 10.3, containing 1 mM MgCl2 and 10 mM 4-nitrophenylphosphate to monitor alkaline phosphatase activity. After incubation for 45 min at 37°C, the reaction was stopped by adding 1 M sodium hydroxide (75 μl/well), and optical density was measured at 405 nm with an ELISA reader. Results shown correspond to mean values obtained in one out of three identical experiments.
Results
Proteolytic Processing of BMP4 Is Enhanced by Coexpression of SPCs
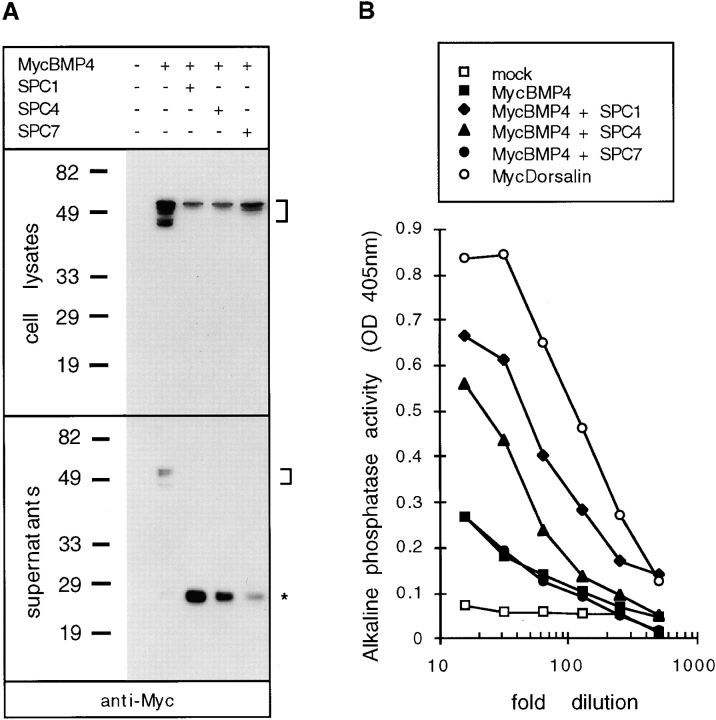
Implants of transfected COS-1 cells have been used previously as a source of biologically active BMP4, suggesting that BMP4 is at least partially processed in COS cells (Liem et al., 1995; Pourquie et al., 1996). In this study, we monitored BMP4 cleavage using a Myc-tagged construct. The Myc epitope was inserted in the COOH-terminal domain of BMP4, allowing detection of both mature and unprocessed forms. Thus, several bands corresponding to unprocessed MycBMP4 precursor were detected at 48–55 kD in lysates prepared from transfected COS-1 cells (Fig. 1 A). Also in conditioned medium, small quantities of precursor protein were observed in three out of six experiments. In addition, conditioned medium was found to contain small amounts of a 23-kD product (Fig. 1 A), corresponding in size to mature BMP4 (Hammonds et al., 1991). The low abundance of this 23-kD form suggests that BMP4 processing may be inefficient. COS cells constitutively express low levels of several SPCs, including SPC1, -4, -6, and -7 (Liu et al., 1993; Nakagawa et al., 1993; Yanagita et al., 1993; Seidah et al., 1996). To test whether overexpression of specific SPCs results in enhanced processing, MycBMP4 was cotransfected with individual SPC expression vectors. As shown in Fig. 1 A, cotransfection with SPC1 or SPC4 led to a substantial increase in the amount of mature MycBMP4 protein in conditioned medium. SPC7 was significantly less active in this assay, although high levels of expression of its precursor form were detected in Western blots (data not shown) using a Flag-tagged SPC7 construct (see Materials and Methods), ruling out the possibility that the effect of SPC1 and SPC4 is a protein overexpression artifact unrelated to the proteolytic activities of these enzymes. By contrast, we have been unable to detect any mature FlagSPC7 (data not shown), hence it cannot be ruled out that the failure of SPC7 to efficiently cleave BMP4 in COS cells reflects inefficient SPC7 maturation.
Figure 1.
MycBMP4 precursor cleavage in COS-1 cells cotransfected with SPC1/furin, SPC4/PACE4, or SPC7. (A) Western blot analysis showing the presence of MycBMP4 precursor (bracket) in lysates (top) or supernatants (bottom) of cells transfected with 2 μg of MycBMP4 expression vector. In cell supernatants, mature MycBMP4 is detected at 23 kD (asterisk). Molecular masses of standard proteins are indicated to the left in kilodaltons. (B) Induction of alkaline phosphatase activity in C3H10T1/2 cells treated with serially diluted supernatants of transfected COS cells. The legend to the right shows the cDNA expression vectors used. SPC1 expression vector alone was used as a mock control, whereas MycDorsalin served as a positive control (Basler et al., 1993).
Processing of Wild-Type and Mutant Forms of Nodal Precursor
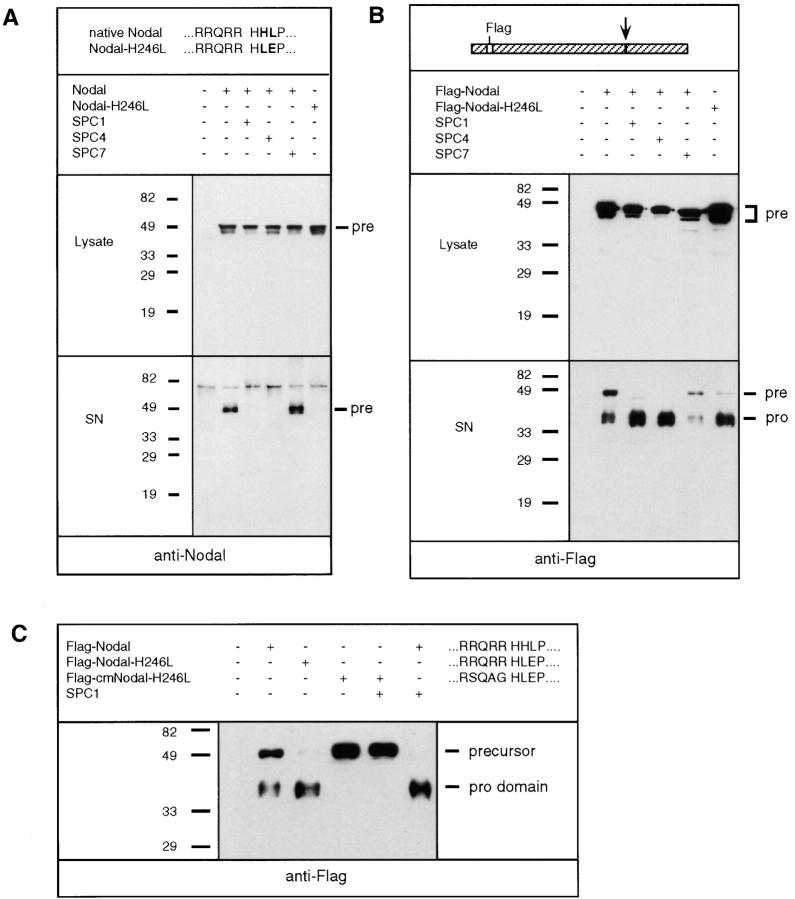
In addition to MycBMP4, we examined the processing of Nodal, a more distantly related BMP subfamily member. Initial attempts using various transfected cell lines including COS cells, Hi5 insect cells, embryonic stem cells, or an embryonic fibroblast cell line consistently failed to yield detectable amounts of the mature form of recombinant Nodal (data not shown). This suggests that the processing of this BMP-related precursor may be tightly regulated. Therefore, we explored the ability of SPCs to enhance Nodal processing in coexpression experiments. Using a Nodal antiserum raised against a peptide within the mature domain, the precursor form of Nodal migrating at 50 kD was readily detected in COS cell lysates and in conditioned medium (Fig. 2 A). Interestingly, coexpression of either SPC1 or SPC4 resulted in virtually complete disappearance of Nodal precursor in conditioned medium, whereas cotransfection with SPC7 had no effect. However, the decrease in the level of precursor was not accompanied by a corresponding increase in the amount of mature Nodal. Similar observations were made in cotransfection experiments using Myc-tagged Nodal precursor (data not shown), raising the possibility that mature Nodal is highly unstable (see below). Alternatively, Nodal processing may be exceptionally inefficient. Comparing cleavage site sequences of TGF-β family members (Table I), we noticed that 11 out of 12 other than Nodal have an aliphatic residue at position P2′, and in 12 out of 12, the amino acid at position P3′ is either glycine or a polar residue. By contrast in Nodal, histidine (H246) and leucine (L247) are present at P2′ and P3′, respectively. To test whether structural constraints imposed by these residues inhibit Nodal cleavage, H246 and L247 were substituted with leucine and glutamate, respectively, resulting in a sequence similar to that of Activin and of TGF-β isoforms. This mutant precursor, termed Nodal-H246L, was efficiently expressed in cell lysates, but failed to accumulate in culture supernatants (Fig. 2 A). Likewise, Nodal-H246L failed to yield any detectable mature Nodal.
Figure 2.
Nodal precursor cleavage is enhanced by SPC1 and SPC4, or by structural modification of the cleavage site. (A) Native Nodal or a mutant form, termed Nodal-H246L, was analyzed by Western blotting in cell lysates (top) or supernatants (bottom) using anti-Nodal antiserum directed against a peptide in the middle of the COOH-terminal domain. The mutant sequence in Nodal-H246L near the multibasic cleavage site is aligned with that of native Nodal (top). Where indicated, COS cells were cotransfected with 0.5 μg of either SPC1, SPC4, or SPC7 expression vector. Bands corresponding to unprocessed precursor are marked pre. Note the absence of any detectable mature Nodal. An additional, nonspecific band is present in all supernatants, suggesting that comparable amounts of protein were loaded. (B) Cleavage of Flag-tagged Nodal precursor and of FlagNodal-H246L visualized by anti-FLAG immunoblotting. The position of the Flag epitope (open box) relative to the cleavage site (arrow) within Nodal and Nodal-H246L is shown schematically. The Nodal pro domain (pro) accumulates in cell supernatants (bottom), but not in lysates (top). Cotransfection with SPC1 or SPC4, but not SPC7, resulted in enhanced precursor cleavage. (C) The multibasic motif R-Q-R-R present in Nodal-H246L is replaced by SQAG in the Flag-cmNodal-H246L construct, resulting in complete inhibition of processing by either resident COS cell proteases or by SPC1 transfection.
To address the stability of mature Nodal and to assess precursor cleavage, a Flag epitope was engineered into the Nodal pro region, allowing detection of the NH2-terminal cleavage product. As shown in Fig. 2 B, only the unprocessed form of FlagNodal was detectable in cell lysates. However, culture supernatants contained both precursor and a 37-kD product corresponding to the Flag-tagged Nodal pro domain, suggesting that Nodal is partially cleaved by resident proteolytic activities. Cleavage of Nodal was markedly enhanced by cotransfection with SPC1 or SPC4, but not SPC7 (Fig. 2 B). Interestingly, cleavage of Flag-tagged Nodal-H246L was enhanced even in the absence of cotransfected SPCs, confirming that the H246L modification of the Nodal cleavage site facilitates processing. Moreover, ablation of the canonical SPC cleavage site abolished cleavage of the 50-kD Nodal precursor, ruling out the possibility that the 37-kD product arises due to nonspecific degradation (Fig. 2 C).
Distinct Pro Regions Affect the Stability of Mature BMPs
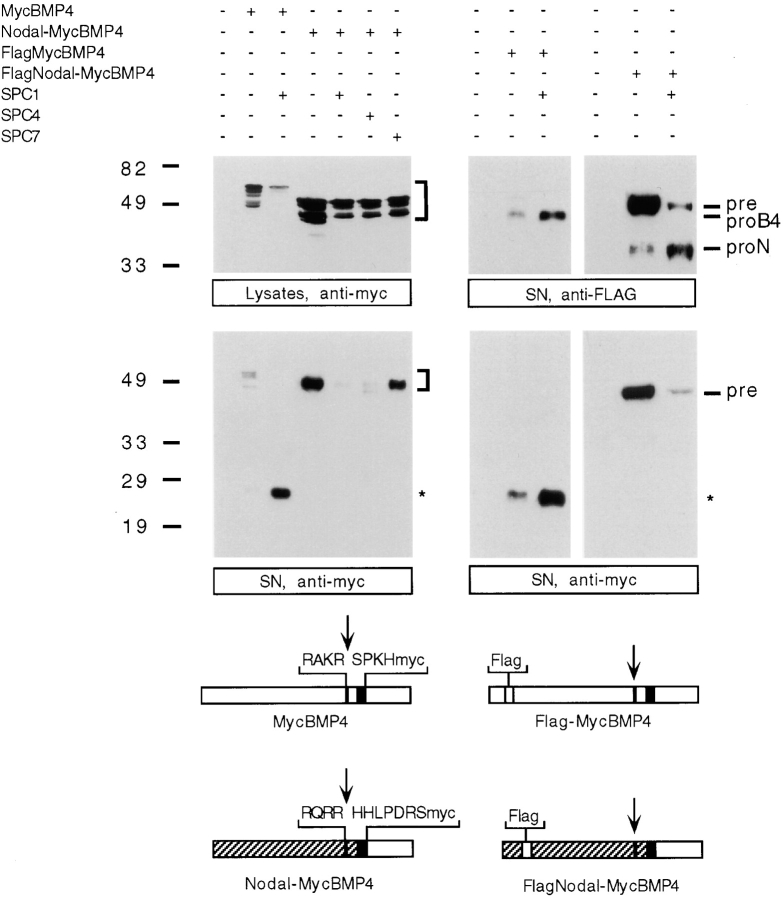
Our results demonstrate that mature Nodal, unlike MycBMP4, fails to accumulate in culture supernatants even under conditions where precursor cleavage is highly efficient. To test whether the pro domains may influence the stability of mature BMPs, we next examined the behavior of a chimeric Nodal-MycBMP4 where the pro domain and seven amino acids COOH-terminal to the cleavage site are derived from Nodal sequence (Fig. 3). We also designed control constructs where mature MycBMP4 is fused to a Flag-tagged pro region derived either from Nodal (giving rise to FlagNodal-MycBMP4) or BMP4 (giving rise to Flag-MycBMP4). Interestingly, Nodal-MycBMP4 fusion protein is efficiently secreted, but no mature MycBMP4 is found in either cell lysates or culture supernatants (Fig. 3). Cotransfection with SPC1 leads to a marked reduction in the amount of precursor and to a corresponding increase in the amount of the Flag-tagged NH2-terminal cleavage product. However, no mature BMP4 protein is detectable. These findings demonstrate that Nodal-MycBMP4 is cleaved and secreted, but fails to yield a stable mature product. Similar results were obtained with chimeric constructs consisting of mature MycDorsalin, another BMP family member, fused to the Nodal pro domain (data not shown), reinforcing the idea that the Nodal pro region confers a destabilizing influence on both its own mature domain or that of BMP4 or Dorsalin.
Figure 3.
The Nodal pro region destabilizes mature MycBMP4. Expression of the chimeric protein Nodal-MycBMP4 in comparison with MycBMP4 analyzed in cell lysates (top left) or supernatants (SN, bottom left). The positions of precursor (bracket) and mature (asterisk) forms are indicated. Processed MycBMP4 accumulates in cell supernatants only if expressed in the context of its own pro domain, but not in association with the Nodal pro region. Flag-tagged pro BMP4 (proB4) and pro Nodal (proN) were detected in cell supernatants (top right), showing that Nodal-MycBMP4 is processed. The structures of the various precursors and the position of Flag (open boxes) and Myc epitopes (black boxes) relative to the precursor cleavage sites (arrow) are shown schematically.
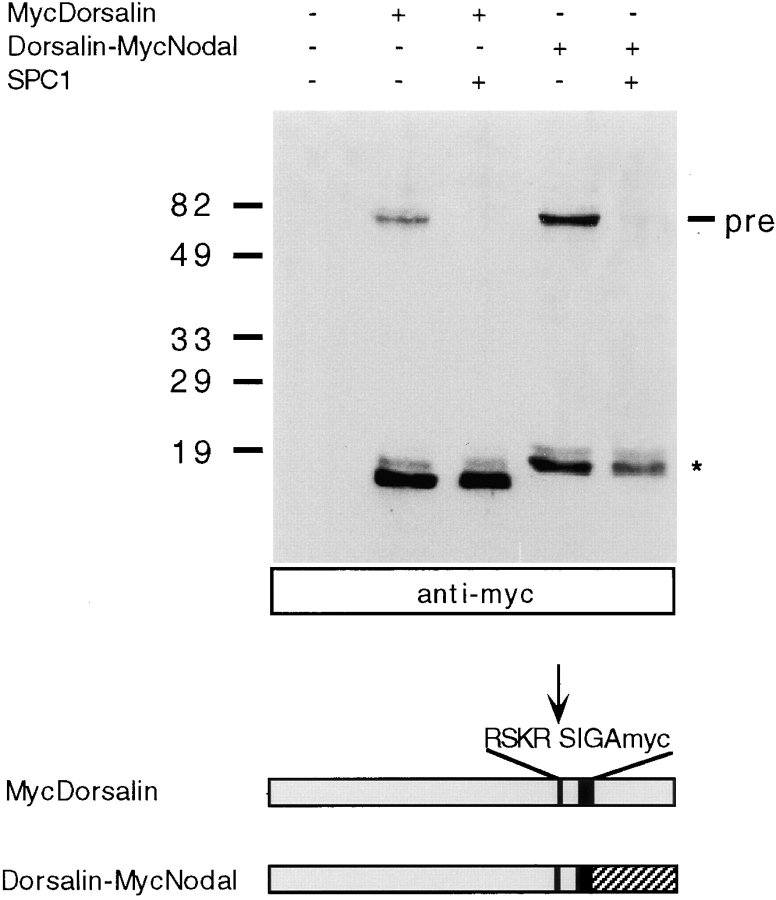
We next examined whether the pro domain of Dorsalin may stabilize mature Nodal, since Dorsalin is known to be efficiently processed and secreted by COS-1 cells (Basler et al., 1993). As expected, mature Myc-tagged Dorsalin migrating at 16–18 kD was readily detected in culture supernatants (Fig. 4). Transfection of the chimeric Dorsalin-MycNodal construct yielded both a 65-kD precursor, and mature MycNodal protein migrating at ∼18 kD. Moreover, after cotransfection with SPC1, only the mature 18-kD form is detected. These data establish that the Dorsalin pro region confers enhanced stability to MycNodal.
Figure 4.
Mature Nodal is stabilized when expressed as a fusion protein with the Dorsalin pro region. MycDorsalin and Dorsalin-MycNodal fusion protein in COS cell supernatants analyzed by anti-Myc immunoblotting. Note that mature MycNodal (asterisk) is stable when expressed in conjunction with the Dorsalin pro region.
The Dorsalin Pro Region Stably Associates with Mature Protein
TGF-β precursors are known to form homodimers covalently linked in their NH2- and COOH-terminal domains via disulfide bonds (Gentry et al., 1988; Miyazono et al., 1988; Wakefield et al., 1988). After precursor cleavage, the TGF-β pro region remains noncovalently associated with the mature domain to form inactive latent complexes (Miyazono et al., 1988; Wakefield et al., 1988). In association with its pro region, mature TGF-β is stabilized, exhibiting a 30–50-fold increase in its in vivo half-life (Wakefield et al., 1990). However, unlike TGF-β isoforms, the activities of other family members such as Dorsalin have not been reported to be stabilized through an association with their pro domains. To test whether mature Nodal may stably interact with the Dorsalin pro region, immunoprecipitation experiments were carried out using a Flag-tagged Dorsalin pro region. As shown in Fig. 5 A, the Flag epitope did not interfere with the expression or proteolytic processing of either FlagDorsalin or a FlagDorsalin-MycNodal fusion protein. When these same supernatants were immunoprecipitated using the M2 anti-Flag antibody, followed by anti-Myc immunoblotting, mature MycDorsalin was found to coprecipitate with its cognate Flag-tagged pro region, indicating that these two moieties form stable complexes (Fig. 5 B). Similarly, processed MycNodal was recovered in immunoprecipitates of FlagDorsalin-MycNodal fusion protein. Thus, we conclude that mature Nodal similar to mature Dorsalin can form stable complexes with the Dorsalin pro region.
Figure 5.
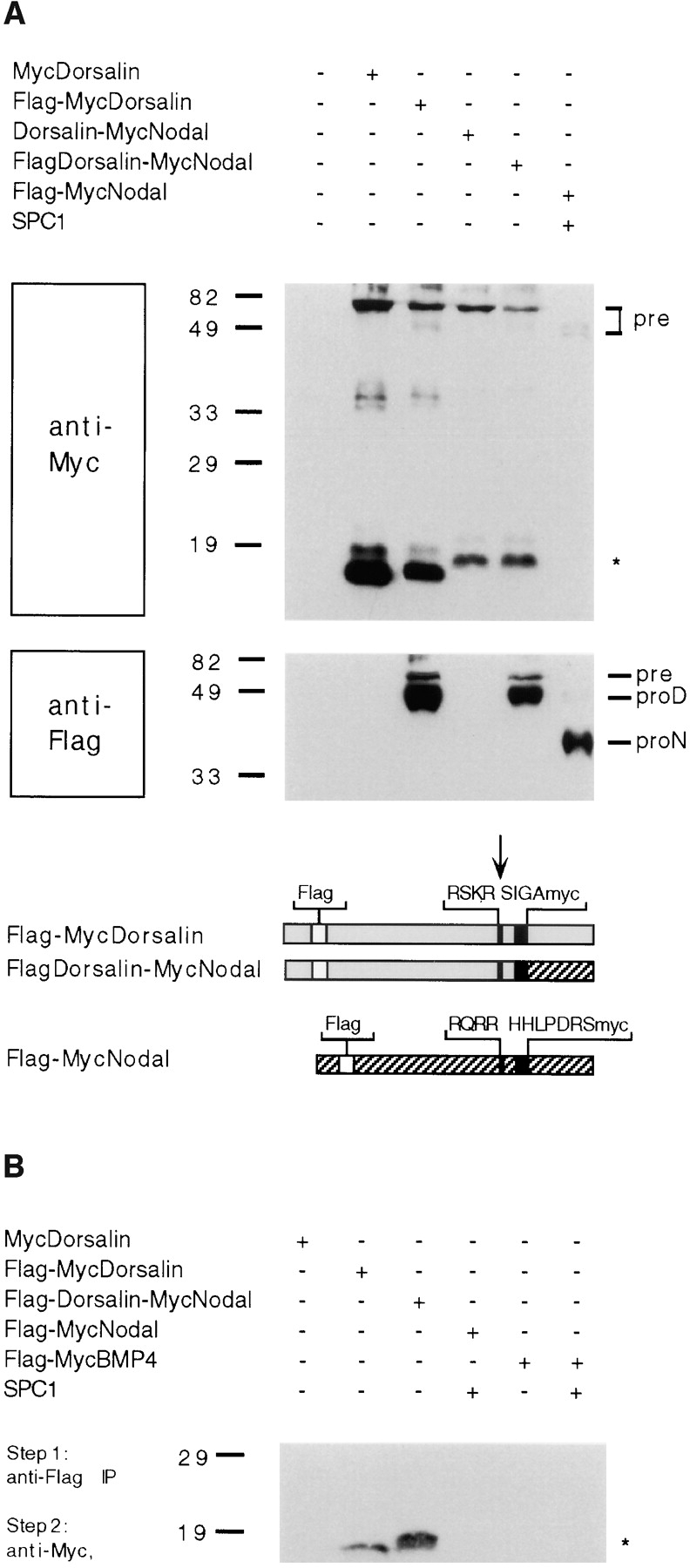
Mature MycNodal and mature MycDorsalin stably associate with the Dorsalin pro domain. (A) Insertion of a Flag epitope (open boxes) in their pro domains does not interfere with the processing of MycDorsalin, Dorsalin-MycNodal, or MycNodal (top), but allows detection of pro Dorsalin (proD) and pro Nodal (proN) fragments and unprocessed precursor (pre) proteins (bottom). (B) The supernatants shown in A were immunoprecipitated using anti-Flag antibodies and analyzed by anti-Myc immunoblotting. Mature MycDorsalin or MycNodal, respectively, coprecipitate with the Dorsalin pro region.
Formation of Disulfide Bonds and High Molecular Weight Aggregates
Similar to other BMPs, Nodal is predicted to form disulfide-linked homodimers, but its failure to be stabilized when derived from the Nodal pro region may reflect inefficient dimerization. To test this possibility, we analyzed disulfide bond formation of Nodal in nonreducing SDS gels. Under nonreducing conditions, Flag-tagged Nodal precursor was found at the position expected for a dimer comprised of two 50-kD subunits (Fig. 6 A). Likewise, Flag-cmNodalH246L lacking a functional SPC cleavage site also migrated at ∼100 kD as predicted for the intact precursor, although dimerization of this mutant construct may be slightly inhibited as indicated by the presence of some residual 50-kD form. In contrast, the FlagNodal pro domain displayed a molecular mass of 37 kD both under reducing and nonreducing conditions, suggesting that it is monomeric. Thus, we conclude that disulfide bonds in the COOH-terminal domain of Nodal mediate efficient homodimerization.
Figure 6.
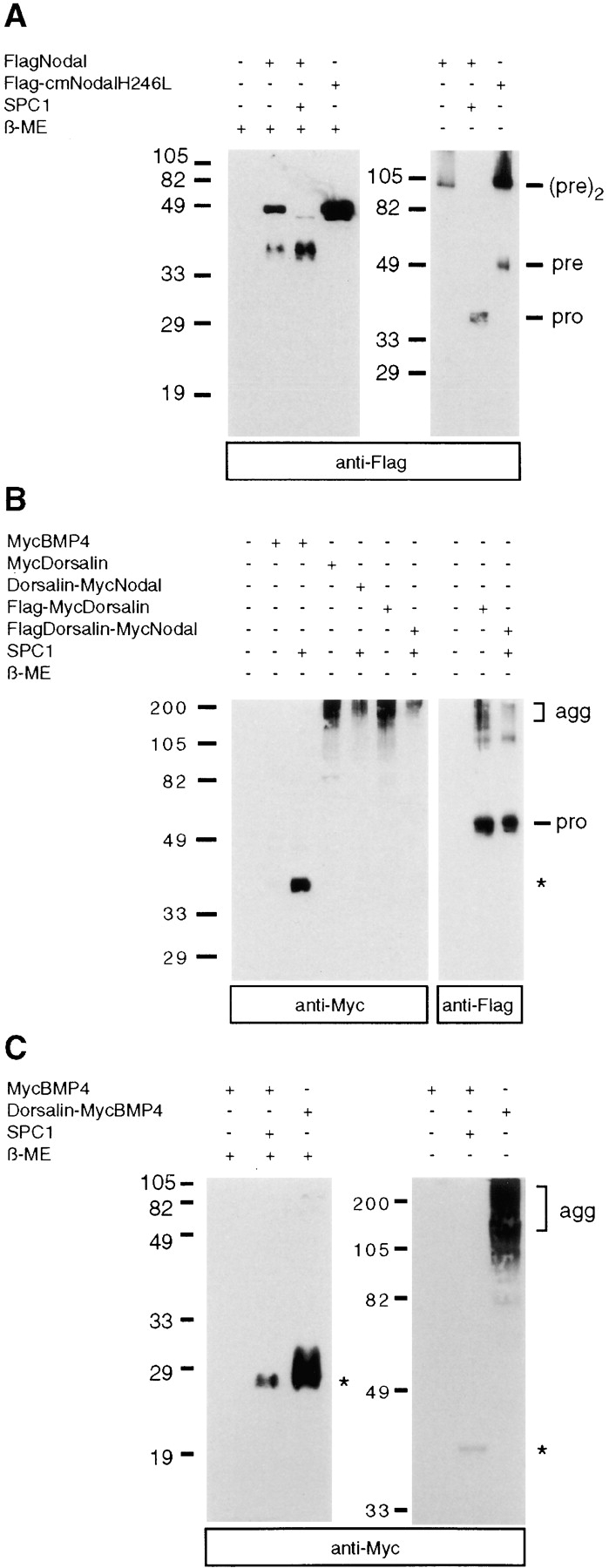
Formation of higher order BMP complexes. (A) Nodal proteins analyzed under reducing (left) or nonreducing conditions (right) by anti-Flag immunoblotting. Note the presence of a 100-kD protein corresponding in size to a dimer of unprocessed Nodal precursor, (pre)2, and of monomeric pro fragments (pro) in A and B. (B) MycBMP4, MycDorsalin, and Dorsalin-MycNodal analyzed in nonreducing SDS gels, followed by anti-Myc (left) or anti-Flag (right) immunoblotting. (C) Dorsalin pro region–mediated aggregation of mature Myc BMP4. agg, aggregates; asterisk, position of mature MycBMP4; β-ME, β-mercaptoethanol.
Next, we compared disulfide bond formation of MycBMP4, MycDorsalin, and Dorsalin-MycNodal in nonreducing SDS gels. Processed MycBMP4 was detected at 40 kD (Fig. 6 B), confirming earlier reports that the 23-kD subunit forms disulfide-linked homodimers (Hammonds et al., 1991). Unexpectedly, the mature form of MycDorsalin, which migrates at 16–18 kD when it is reduced (Fig. 5 A), was detected exclusively in high molecular mass aggregates under nonreducing conditions (Fig. 6 B). These aggregates specifically contain the COOH-terminal cleavage product and probably some residual uncleaved precursor form of Dorsalin, but not the Dorsalin pro domain fragment which is monomeric and migrates at 52 kD under nonreducing conditions (Fig. 6 B). Similar results were obtained in the case of chimeric Dorsalin-MycNodal (Fig. 6 B) and Dorsalin-MycBMP4 constructs (Fig. 6 C), strongly suggesting that the Dorsalin pro region mediates aggregation of both mature Dorsalin, mature Nodal, and mature BMP4.
Discussion
Here we provide the first direct evidence that members of the SPC family enhance cleavage of BMP precursors. Thus, in COS cells transfected with a BMP4 expression vector, we find that the amount of mature BMP4 secreted into culture medium is dramatically increased by cotransfection of SPC1 or SPC4. Consistent with earlier reports (Hammonds et al., 1991), the processed form of BMP4 migrated at 23 kD in SDS gels, and it forms disulfide-linked homodimers that are biologically active in an alkaline phosphatase induction assay, demonstrating that cleavage gives rise to functional protein. In addition, we analyzed Nodal precursor processing using a peptide-specific anti-Nodal antiserum. Whereas mature Nodal is 42% identical to BMP4, its pro domain and sequences flanking the precursor cleavage site are significantly less conserved, implying that Nodal potentially is cleaved by distinct proteases. However, we found that similar to BMP4, Nodal precursor protein is efficiently processed in the presence of SPC1 or SPC4, and that this cleavage requires an intact R-Q-R-R sequence within the precursor, confirming the prediction that this motif is a functional cleavage site. A similar motif (R-A-K-R) identified by NH2-terminal sequencing of the mature protein precedes the BMP4 cleavage site (Hammonds et al., 1991) and is required for precursor processing (Hawley et al., 1995). Therefore, endogenous SPCs including SPC1 and SPC4, and perhaps SPC6, seem most likely to account for the constitutive processing of BMP4 and Nodal in COS cells.
The processing of other TGF-β family members including TGF-β1 (Dubois et al., 1995), Activin A (Roebroek et al., 1993), and Muellerian inhibitory substance (MIS) (Nachtigal and Ingraham, 1996) has been shown previously to be enhanced in cells overexpressing SPC1. MIS cleavage is also enhanced by SPC6, which appears to be coexpressed with MIS during gonadal development. Thus, SPC6 is a likely candidate for mediating MIS cleavage in vivo (Nachtigal and Ingraham, 1996). Support for a role of SPCs in processing of BMPs has come from analysis of their expression patterns during embryogenesis (Constam et al., 1996). Thus, we previously proposed that BMP processing may be controlled by the overlapping activities of SPC1, -4, -6, and -7. Indeed, cleavage of BMP4 and Nodal precursors is enhanced by cotransfection with either SPC1 or SPC4. Proteolytic activation most likely is mediated directly, considering that a purified soluble form of SPC1 can specifically cleave recombinant TGF-β1 precursor in vitro (Dubois et al., 1995). Moreover, direct cleavage of BMP4 by recombinant SPC1, -4, -6, and -7 has been reported recently by Cui et al. (1998). Interestingly, while our results on BMP4 cleavage by SPC1 and SPC4 agree with those of Cui et al., the same is not true for SPC7 which appeared to be significantly less active in COS cells, possibly owing to inefficient maturation. Alternatively, the soluble SPC7 used by Cui et al. (1998) may differ in its activity due to species differences or engineered deletion of its COOH-terminal 118 amino acids.
Recent genetic studies revealed unique as well as partially redundant functions for individual SPCs. Thus, mice lacking functional SPC2 eventually become hypoglycemic due to the failure of pancreatic α cells to generate mature glucagon (Furuta et al., 1997). An additional fourfold reduction in the level of mature insulin in pancreatic extracts suggests that SPC2 is also important for the conversion of proinsulin, although some proinsulin seems to be processed due to overlapping proteolytic activities (Furuta et al., 1997, 1998). A likely candidate is SPC3 which is coexpressed with SPC2 in β cells, and can cleave both sites flanking the C-peptide in proinsulin when expressed at high levels (Irminger et al., 1996). On the other hand, an obese patient with elevated levels of plasma proinsulin and pro-opiomelanocortin, but very low levels of insulin, was found to be genetically deficient for SPC3/PC1(3), confirming that SPC3 is very important for proinsulin processing, but that some residual insulin can be generated by other proteases. A unique role has been attributed to SPC5/PC4. Consistent with the exclusive expression of SPC5 in testicular germ cells, sperm produced by SPC5−/− males is reduced in its capacity to fertilize eggs. When fertilization occurs, the resulting embryos die before the blastocyst stage, presumably because they lack paternal factor(s) activated by SPC5 during normal sperm maturation (Mbikay et al., 1997). Additionally, a loss of function mutation has been generated at the SPC1 locus. Embryos lacking SPC1 die at around embryonic day 10.5, exhibiting severe ventral closure defects including cardia bifida, and fail to undergo axial rotation (Roebroek et al., 1998). Thus it appears that closely related proteases cannot fully compensate for the loss of SPC1. Interestingly, similar to embryos compromised in both Nodal and Smad2 signaling (Nomura and Li, 1998), embryos lacking SPC4 display varying degrees of holoprosencephaly (cyclopia; Constam, D.B., and E.J. Robertson, manuscript in preparation). In contrast, SPC7−/− embryos apparently develop normally (Constam, D.B., and E.J. Robertson, manuscript in preparation). These findings are consistent with the idea that SPC4, but not SPC7, plays a pivotal role in the maturation of BMP activities.
In comparison with mature Dorsalin, we detected far less mature BMP4 in COS cell supernatants, even though both precursors are highly abundant in cell lysates. These proteins thus potentially differ in their stability, maturation efficiency, or both. Our data suggest that the expression level of mature BMP4 is limited by the availability of convertase activities rather than protein stability, since mature BMP4 stably accumulates in supernatants upon cotransfection with SPC1 or SPC4. Relatively little is known about the regulatory mechanisms controlling the susceptibility of individual TGF-β family members and other proproteins to proteolytic cleavage. Several independent studies have shown that the R-X-X-R motif is required (Molloy et al., 1992), but additional structural constraints also influence the efficiency of processing. For example, synthetic peptides mimicking the dibasic proalbumin cleavage site are not hydrolyzed by SPC1 if the aspartic acid in position P1′ is replaced by a basic residue (Brennan and Nakayama, 1994). Furthermore, replacing aspartate at position P6 by arginine results in increased cleavage of prorenin (Watanabe et al., 1993). In contrast, prorenin processing is completely inhibited if the P1′ serine is substituted by leucine (Watanabe et al., 1992). These results suggest that the arginine and serine residues in positions P6 and P1′, respectively, are unlikely to inhibit BMP4 processing. Moreover, the best SPC1 substrates selected among random peptides using phage display were frequently found to contain an additional basic residue at position P5, and proline in position P7 or P2′ (Matthews et al., 1994). However, BMP4 contains both an arginine at P5 and a proline at position P2′. Thus, it remains unclear why BMP4 cleavage in COS cells is inefficient compared with MycDorsalin, and what additional parameters govern the efficiency of precursor processing.
To visualize Nodal processing, we monitored the accumulation of the NH2-terminal cleavage product. Thus, Nodal appeared to be inefficiently cleaved in COS cells. However, Nodal processing was significantly enhanced when we substituted the P2′ histidine and the P3′ leucine with leucine and glutamate, respectively. Most likely this is due to increased flexibility at the cleavage site because histidine is replaced by a more flexible residue. Therefore, it may be interesting to determine whether similar steric constraints inhibit Nodal processing in vivo, and whether in vivo Nodal-H246L acts as a gain-of-function mutation. Indeed, an active form of Vg-1 bypassing the tight regulation of Vg-1 processing was instrumental in identifying its role as an inducer of axial mesoderm in Xenopus embryos (Thomsen and Melton, 1993).
Unexpectedly, we were unable to detect mature Nodal either in whole cell lysates or conditioned medium, even under conditions where Nodal precursor protein is completely processed. This strongly argues that the mature form of Nodal is highly unstable. The instability of Nodal is unlikely to reflect lack of dimerization since under nonreducing conditions Nodal migrates at 100 kD, corresponding in size to dimerized precursor. Since the Nodal pro region is monomeric, precursor dimerization appears to be mediated via disulfide bonds in the COOH-terminal domain, as is predicted by analogy to other TGF-β family members. Domain swap experiments confirmed that the instability of Nodal is conferred at least in part by its pro region which can similarly destabilize mature BMP4 or Dorsalin. This effect cannot be explained by aberrant protein folding since misfolded proteins are usually retained within the endoplasmic reticulum (Hammond and Helenius, 1995), whereas the Nodal-BMP4 and Nodal-Dorsalin fusion proteins are very efficiently secreted. Thus, we found that the Nodal pro region dramatically increases the secretion of BMP4 precursor (Fig. 3). As a result, BMP4 may be underglycosylated, possibly accounting for decreased stability as observed for other glycoproteins (Hori et al., 1988; McCracken and Brodsky, 1996; reviewed in Wang et al., 1996). Alternatively, BMP4 may require a stabilizing interaction with its cognate pro domain or a fragment thereof, or with a hypothetical BMP4 pro domain– associated factor. However, an association of BMP4 with its pro domain has not been observed in our experiments (Fig. 5 B), nor by other investigators (Wozney et al., 1988; Hammonds et al., 1991). In marked contrast, we found that the Dorsalin pro region forms stable complexes with its mature domain, and with mature Nodal. Thus, the Dorsalin pro region remains stably associated with the mature protein even after precursor cleavage. In the case of the chimeric Dorsalin-Nodal fusion protein, this association correlates with a marked stabilization of mature Nodal. It will be interesting to determine whether specific pro regions can be used to manipulate the stability of mature BMPs in vivo, and whether this interaction influences the range of BMP signaling.
The physiological significance of the complexes formed with the Dorsalin pro region remains unknown. Possibly, they serve to stabilize the mature protein, as is the case for TGF-β1 which is secreted in a noncovalent complex with its pro region (Lawrence et al., 1985; Miyazono et al., 1988; Wakefield et al., 1988, 1990). Alternatively, they may represent an artifact resulting from overexpression in COS cells. To evaluate this possibility, samples were analyzed in nonreducing gels. Thus, the Dorsalin pro fragment was found to be predominantly monomeric, suggesting that it does not form disulfide bonds. Unexpectedly, mature Dorsalin, Nodal, and BMP4 all were found exclusively in high molecular weight aggregates when derived from the Dorsalin pro domain. How these aggregates form remains unknown. Although we cannot fully rule out the possibility that the aggregation of mature protein is due to aberrant disulfide bond formation, this seems unlikely since we and others observed that COS cell–derived Dorsalin is a very potent inducer of alkaline phosphatase in C3H10T1/2 cells (Fig. 1 B; Basler et al., 1993). This strongly suggests Dorsalin is properly folded, and its activity is not masked by either its pro region or aggregate formation.
Collectively, our data show that processing of BMP4 and Nodal is limited in transfected mammalian cells. However, upregulation of either SPC1 or SPC4 leads to significantly enhanced processing, suggesting that BMP activities are likely to be controlled in vivo in part by the availability of SPCs. Additionally, the efficiency of Nodal processing appears to be restricted by structural constraints imposed by sequences adjacent to the cleavage site. Strikingly we find that mature Nodal is far less stable than mature BMP4 or Dorsalin, raising the interesting possibility that the short half life of mature Nodal may limit the range of Nodal signaling in vivo. In the embryo which is still very small at the time when Nodal is transiently expressed, a short half-life could represent an important mechanism to prevent widespread diffusion of this signaling molecule. Finally, we have shown that individual pro regions differ markedly in their ability to stabilize mature BMPs. While the physiological significance of this observation remains to be clarified, this finding suggests that experiments using chimeric BMP precursors should be interpreted with caution since enhanced or ectopic activities may in part reflect a change in the half-life of mature protein. Future experiments will test whether BMP activities can be modified in vivo by manipulating either their processing efficiency or stability.
Acknowledgments
We would like to thank E. Bikoff, S. Michael, and A. Dudley for valuable discussions and comments on the manuscript. We also thank E. Bikoff for providing anti-Nodal antibodies, and T. Jessell, J. Collignon, and S. Michael for providing expression vectors for MycDorsalin, Nodal, and MycBMP4, respectively. We are further indebted to W. Van de Ven and K. Nakayama for the gifts of SPC1/Furin and SPC4/PACE4 cDNAs, respectively.
This work was supported by a grant from the National Institutes of Health (E.J. Robertson). D.B. Constam was supported by fellowships from EMBO (ATLF 338-1994) and from the Swiss National Science Foundation (823A-46642).
Abbreviations used in this paper
- BMP
bone morphogenetic protein
- MIS
Muellerian inhibitory substance
- SPC
subtilisin-like proprotein convertase
References
- Basler K, Edlund T, Jessell TM, Yamada T. Control of cell pattern in the neural tube: regulation of cell differentiation by dorsalin-1, a novel TGF beta family member. Cell. 1993;73:687–702. doi: 10.1016/0092-8674(93)90249-p. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- Brennan SO, Nakayama K. Cleavage of proalbumin peptides by furin reveals unexpected restrictions at the P2 and P′1 sites. FEBS Lett. 1994;347:80–84. doi: 10.1016/0014-5793(94)00511-7. [DOI] [PubMed] [Google Scholar]
- Celeste AJ, Iannazzi JA, Taylor RC, Hewick RM, Rosen V, Wang EA, Wozney JM. Identification of transforming growth factor β family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci USA. 1990;87:9843–9847. doi: 10.1073/pnas.87.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constam DB, Calfon M, Robertson EJ. SPC4, SPC6, and the novel protease SPC7 are coexpressed with bone morphogenetic proteins at distinct sites during embryogenesis. J Cell Biol. 1996;134:181–191. doi: 10.1083/jcb.134.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers JWM, Kormelink PJ, Roebroek AJM, Nakayama K, Van de Ven WJM. Proprotein processing activity and cleavage site selectivity of the Kex2-like endoprotease PACE4. FEBS Lett. 1993;336:65–69. doi: 10.1016/0014-5793(93)81610-c. [DOI] [PubMed] [Google Scholar]
- Cui Y, Jean F, Thomas G, Christian JL. BMP-4 is proteolytically activated by furin and/or PC6 during vertebrate embryonic development. EMBO (Eur Mol Biol Organ) J. 1998;17:4735–4743. doi: 10.1093/emboj/17.16.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martin R, Haendler B, Hofer-Warbinek R, Gaugitsch H, Wrann M, Schlusener H, Seifert JM, Bodmer S, Fontana A, Hofer E. Complementary DNA for human glioblastoma-derived T cell suppressor factor, a novel member of the transforming growth factor-beta gene family. EMBO (Eur Mol Biol Organ) J. 1987;6:3673–3677. doi: 10.1002/j.1460-2075.1987.tb02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Jarrett JA, Chen EY, Eaton DH, Bell JR, Assoian RK, Roberts AB, Sporn MB, Goeddel DV. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985;316:701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R. Processing of transforming growth factor beta 1 precursor by human furin convertase. J Biol Chem. 1995;270:10618–10624. doi: 10.1074/jbc.270.18.10618. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Anderson KV. Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophilaembryo. Cell. 1992;71:451–461. doi: 10.1016/0092-8674(92)90514-d. [DOI] [PubMed] [Google Scholar]
- Francois V, Solloway M, O'Neill JW, Emery J, Bier E. Dorsal-ventral patterning of the Drosophilaembryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev. 1994;8:2602–2616. doi: 10.1101/gad.8.21.2602. [DOI] [PubMed] [Google Scholar]
- Furuta M, Yano H, Zhou A, Rouille Y, Holst JJ, Carroll R, Ravazzola M, Orci L, Furuta H, Steiner DF. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci USA. 1997;94:6646–6651. doi: 10.1073/pnas.94.13.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M, Carroll R, Martin S, Swift HH, Ravazzola M, Orci L, Steiner DF. Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2. J Biol Chem. 1998;273:3431–3437. doi: 10.1074/jbc.273.6.3431. [DOI] [PubMed] [Google Scholar]
- Gentry LE, Lioubin MN, Purchio AF, Marquardt H. Molecular events in the processing of recombinant type 1 pre-pro-transforming growth factor beta to the mature polypeptide. Mol Cell Biol. 1988;8:4162–4168. doi: 10.1128/mcb.8.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AL, Joly E. A simple procedure to increase efficiency of DEAE-dextran transfection of COS cells. Trends Genet. 1995;11:216–217. doi: 10.1016/s0168-9525(00)89051-4. [DOI] [PubMed] [Google Scholar]
- Graycar JL, Miller DA, Arrick BA, Lyons RM, Moses H, Derynck R. Human transforming growth factor-beta 3: recombinant expression, purification, and biological activities in comparison with transforming growth factors-beta 1 and -beta 2. Mol Endocrinol. 1989;3:1977–1986. doi: 10.1210/mend-3-12-1977. [DOI] [PubMed] [Google Scholar]
- Green JB, New HV, Smith JC. Responses of embryonic Xenopuscells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell. 1992;71:731–739. doi: 10.1016/0092-8674(92)90550-v. [DOI] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Quality control in the secretory pathway. Curr Opin Cell Biol. 1995;7:523–529. doi: 10.1016/0955-0674(95)80009-3. [DOI] [PubMed] [Google Scholar]
- Hammonds RJ, Schwall R, Dudley A, Berkemeier L, Lai C, Lee J, Cunningham N, Reddi AH, Wood WI, Mason AJ. Bone-inducing activity of mature BMP-2b produced from a hybrid BMP-2a/2b precursor. Mol Endocrinol. 1991;5:149–155. doi: 10.1210/mend-5-1-149. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Hawley SHB, Wünnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, Cho KWY. Disruption of BMP signals in embryonic Xenopusectoderm leads to direct neural induction. Genes Dev. 1995;9:2923–2935. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton DA. Inhibition of activin receptor signaling promotes neuralization in Xenopus. . Cell. 1994;77:273–281. doi: 10.1016/0092-8674(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Kelly OG, Melton DA. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994;77:283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hori H, Yoshino T, Ishizuka Y, Yamauchi T, Murakami K. Role of N-linked oligosaccharides attached to human renin expressed in COS cells. FEBS Lett. 1988;232:391–394. doi: 10.1016/0014-5793(88)80777-4. [DOI] [PubMed] [Google Scholar]
- Hosaka M, Murakami K, Nakayama K. PACE4 is a ubiquitous endoprotease that has similar but not identical substrate specificity to other KEX2-like processing endoproteases. Biomed Res. 1994;16:383–390. [Google Scholar]
- Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopusdorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell. 1998;1:673–683. doi: 10.1016/s1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
- Irminger JC, Meyer K, Halban P. Proinsulin processing in the rat insulinoma cell line INS after overexpression of the endoproteases PC2 or PC3 by recombinant adenovirus. Biochem J. 1996;320:11–15. doi: 10.1042/bj3200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RC, Darlington DN, Hand TA, Bloomquist BT, Mains RE. PACE4: a subtilisin-like endoprotease prevalent in the anterior pituitary and regulated by thyroid status. Endocrinology. 1994;135:1178–1185. doi: 10.1210/endo.135.3.8070361. [DOI] [PubMed] [Google Scholar]
- Kiefer MC, Tucker JE, Joh R, Landsberg KE, Saltman D, Barr PJ. Identification of a second human subtilisin-like protease gene in the fes/fps region of chromosome 15. DNA Cell Biol. 1991;10:757–769. doi: 10.1089/dna.1991.10.757. [DOI] [PubMed] [Google Scholar]
- Lawrence DA, Pircher R, Jullien P. Conversion of a high molecular weight latent beta-TGF from chicken embryo fibroblasts into a low molecular weight active beta-TGF under acidic conditions. Biochem Biophys Res Commun. 1985;133:1026–1034. doi: 10.1016/0006-291x(85)91239-2. [DOI] [PubMed] [Google Scholar]
- Liem KFJ, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Ling N, Ying S, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R. Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature. 1986;321:779–782. doi: 10.1038/321779a0. [DOI] [PubMed] [Google Scholar]
- Liu YC, Kawagishi M, Mikayama T, Inagaki Y, Takeuchi T, Ohashi H. Processing of a fusion protein by endoprotease in COS-1 cells for secretion of mature peptide by using a chimeric expression vector. Proc Natl Acad Sci USA. 1993;90:8957–8961. doi: 10.1073/pnas.90.19.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués G, Musacchio M, Shimell MJ, Wünnenberg-Stapleton K, Cho KWY, O'Connor MB. Production of a DPP activity gradient in the early Drosophilaembryo through the opposing action of the SOG and TLD proteins. Cell. 1997;91:417–426. doi: 10.1016/s0092-8674(00)80425-0. [DOI] [PubMed] [Google Scholar]
- Matthews DJ, Goodman LJ, Gorman CM, Wells JA. A survey of furin substrate specificity using substrate phage display. Protein Sci. 1994;3:1197–1205. doi: 10.1002/pro.5560030805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbikay M, Tadros H, Ishida N, Lerner CP, De Lamirande E, Chen A, El-Alfy M, Clermont Y, Seidah NG, Chretien M, Gagnon C, Simpson EM. Impaired fertility in mice deficient for the testicular germ-cell protease PC4. Proc Natl Acad Sci USA. 1997;94:6842–6846. doi: 10.1073/pnas.94.13.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken AA, Brodsky JL. Assembly of ER-associated protein degradation in vitro: dependence on cytosol, calnexin, and ATP. J Cell Biol. 1996;132:291–298. doi: 10.1083/jcb.132.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Hellman U, Wernstedt C, Heldin CH. Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J Biol Chem. 1988;263:6407–6415. [PubMed] [Google Scholar]
- Molloy SS, Bresnahan PA, Leppla SH, Klimpel KR, Thomas G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem. 1992;267:16396–16402. [PubMed] [Google Scholar]
- Nachtigal MW, Ingraham HA. Bioactivation of Mullerian inhibiting substance during gonadal development by a kex2/subtilisin-like endoprotease. Proc Natl Acad Sci USA. 1996;93:7711–7716. doi: 10.1073/pnas.93.15.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Hosaka M, Torii S, Watanabe T, Murakami K, Nakayama K. Identification and functional expression of a new member of the mammalian Kex2-like processing endoprotease family: its striking structural similarity to PACE4. J Biochem. 1993;113:132–135. doi: 10.1093/oxfordjournals.jbchem.a124015. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Takio K, Eto Y, Shibai H, Titani K, Sugino H. Activin-binding protein from rat ovary is follistatin. Science. 1990;247:836–838. doi: 10.1126/science.2106159. [DOI] [PubMed] [Google Scholar]
- Nellen D, Burke R, Struhl G, Basler K. Direct and long-range action of a DPP morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- Panganiban GE, Rashka KE, Neitzel MD, Hoffmann FM. Biochemical characterization of the Drosophiladpp protein, a member of the transforming growth factor beta family of growth factors. Mol Cell Biol. 1990;10:2669–2677. doi: 10.1128/mcb.10.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourquie O, Fan CM, Coltey M, Hirsinger E, Watanabe Y, Breant C, Francis-West P, Brickell P, Tessier-Lavigne M, Le Douarin NM. Lateral and axial signals involved in avian somite patterning: a role for BMP4. Cell. 1996;84:461–471. doi: 10.1016/s0092-8674(00)81291-x. [DOI] [PubMed] [Google Scholar]
- Roebroek AJM, Creemers JWM, Pauli IGL, Bogaert T, Van de Ven WJM. Generation of structural and functional diversity in furin-like proteins in Drosophila melanogasterby alternative splicing of the Dfur1 gene. EMBO (Eur Mol Biol Organ) J. 1993;12:1853–1870. doi: 10.1002/j.1460-2075.1993.tb05834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroek AJM, Umans L, Pauli IGL, Robertson EJ, van Leuven F, Van de Ven WJM, Constam DB. Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase Furin. Development. 1998;125:4863–4876. doi: 10.1242/dev.125.24.4863. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Smith JC, Dale L. Effects of truncated activin and FGF receptors and of follistatin on the inducing activities of BVg1 and activin: does activin play a role in mesoderm induction? . EMBO (Eur Mol Biol Organ) J. 1994;13:3533–3541. doi: 10.1002/j.1460-2075.1994.tb06660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, Hamelin J, Mamarbachi M, Dong W, Tardos H, Mbikay M, Chretien M, Day R. cDNA structure, tissue distribution, and chromosomal localization of rat PC7, a novel mammalian proprotein convertase closest to yeast kexin-like proteinases. Proc Natl Acad Sci USA. 1996;93:3388–3393. doi: 10.1073/pnas.93.8.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimell MJ, Ferguson EL, Childs SR, O'Connor MB. The Drosophiladorsal-ventral patterning gene tolloid is related to human bone morphogenetic protein 1. Cell. 1991;67:469–481. doi: 10.1016/0092-8674(91)90522-z. [DOI] [PubMed] [Google Scholar]
- Steiner DF, Smeekens SP, Ohagi S, Chan SJ. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992;267:23435–23438. [PubMed] [Google Scholar]
- Symes K, Yordan C, Mercola M. Morphological differences in Xenopusembryonic mesodermal cells are specified as an early response to distinct threshold concentrations of activin. Development. 1994;120:2339–2346. doi: 10.1242/dev.120.8.2339. [DOI] [PubMed] [Google Scholar]
- Tannahill D, Melton DA. Localized synthesis of the Vg1 protein during early Xenopusdevelopment. Development. 1989;106:775–785. doi: 10.1242/dev.106.4.775. [DOI] [PubMed] [Google Scholar]
- Thomsen GH, Melton DA. Processed Vg1 protein is an axial mesoderm inducer in Xenopus. . Cell. 1993;74:433–441. doi: 10.1016/0092-8674(93)80045-g. [DOI] [PubMed] [Google Scholar]
- Tonegawa A, Funayama N, Ueno N, Takahashi Y. Mesodermal subdivision along the mediolateral axis in chicken controlled by different concentrations of BMP-4. Development. 1997;124:1975–1984. doi: 10.1242/dev.124.10.1975. [DOI] [PubMed] [Google Scholar]
- Wakefield LM, Smith DM, Flanders KC, Sporn MB. Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem. 1988;263:7646–7654. [PubMed] [Google Scholar]
- Wakefield LM, Winokur TS, Hollands RS, Christopherson K, Levinson AD, Sporn MB. Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J Clin Invest. 1990;86:1976–1984. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Eufemi M, Turano C, Giartosio A. Influence of the carbohydrate moiety on the stability of glycoproteins. Biochemistry. 1996;35:7299–7307. doi: 10.1021/bi9517704. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa T, Ikemizu J, Nagahama M, Murakami K, Nakayama K. Sequence requirements for precursor cleavage within the constitutive secretory pathway. J Biol Chem. 1992;267:8270–8274. [PubMed] [Google Scholar]
- Watanabe T, Murakami K, Nakayama K. Positional and additive effects of basic amino acids on processing of precursor proteins within the constitutive secretory pathway. FEBS Lett. 1993;320:215–218. doi: 10.1016/0014-5793(93)80589-m. [DOI] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsok LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Yanagita M, Hoshino H, Nakayama K, Takeuchi T. Processing of mutated proinsulin with tetrabasic cleavage sites to mature insulin reflects the expression of furin in nonendocrine cell lines. Endocrinology. 1993;133:639–644. doi: 10.1210/endo.133.2.8344203. [DOI] [PubMed] [Google Scholar]
- Zhou X, Sasaki H, Lowe L, Hogan BLM, Kuehn MR. Nodal is a novel TGF-beta-like gene expressed in the mouse node during gastrulation. Nature. 1993;361:543–547. doi: 10.1038/361543a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]