Abstract
Integrins are widely expressed plasma membrane adhesion molecules that tether cells to matrix proteins and to one another in cell–cell interactions. Integrins also transmit outside-in signals that regulate functional responses of cells, and are known to influence gene expression by regulating transcription. In previous studies we found that platelets, which are naturally occurring anucleate cytoplasts, translate preformed mRNA transcripts when they are activated by outside-in signals. Using strategies that interrupt engagement of integrin αIIbβ3 by fibrinogen and platelets deficient in this integrin, we found that αIIbβ3 regulates the synthesis of B cell lymphoma 3 (Bcl-3) when platelet aggregation is induced by thrombin. We also found that synthesis of Bcl-3, which occurs via a specialized translation control pathway regulated by mammalian target of rapamycin (mTOR), is induced when platelets adhere to immobilized fibrinogen in the absence of thrombin and when integrin αIIbβ3 is engaged by a conformation-altering antibody against integrin αIIbβ3. Thus, outside-in signals delivered by integrin αIIbβ3 are required for translation of Bcl-3 in thrombin-stimulated aggregated platelets and are sufficient to induce translation of this marker protein in the absence of thrombin. Engagement of integrin α2β1 by collagen also triggered synthesis of Bcl-3. Thus, control of translation may be a general mechanism by which surface adhesion molecules regulate gene expression.
Keywords: adhesion, integrins, platelets, translation, gene regulation
Integrins are plasma membrane proteins that mediate adhesion of cells to other cells and to matrix structures (Hynes, 1992). Individual α and β subunits pair to form heterodimers of characteristic ligand specificity that interact via their cytoplasmic domains with cytoskeletal and other intracellular proteins (Hynes, 1992; Shattil and Ginsberg, 1997). Interaction of integrin intracellular domains with cytoplasmic proteins confers the ability to transmit outside-in signals when the extracellular domains are engaged by specific ligands, in addition to providing a mechanism by which affinity and avidity of integrins for their ligands can be regulated (Hynes, 1992; Clark and Brugge, 1995; Schwartz et al., 1995; Shattil and Ginsberg, 1997). Outside-in signaling resulting from integrin engagement triggers a variety of responses in cells, including fluxes in intracellular calcium, sodium–proton exchange and alterations in intracellular pH, phosphatidylinositol metabolism, activation of calpain, focal adhesion kinase, mitogen-activated protein kinases and other enzymes, and induction of nuclear signaling pathways leading to expression of new gene products (Clark and Brugge, 1995; Schwartz et al., 1995; Howe et al., 1998). These biochemical events lead to both rapid and delayed changes in cellular function and phenotype, including motility, growth, and differentiation. Outside-in signaling by integrins is both heterodimer- and cell-specific (Shattil and Ginsberg, 1997) and has been studied frequently in the context of adhesion of cells to matrix ligands. Although integrins are also prototypic tethering factors in cell–cell interactions (Zimmerman et al., 1996), less is known of their outside-in signaling roles in this context compared with cell–matrix interactions.
The mechanisms by which gene expression is regulated by cellular adhesion are of considerable interest because this imposes both spatial and biochemical control on the process. Studies of fibroblasts and human monocytic cells demonstrate that engagement of integrins by antibodies against their extracellular domains or by matrix ligands induces activation and/or nuclear translocation of Rel (NF-κB) and other transcription factors, and consequent transcription of specific mRNAs (Schwartz et al., 1995; Juliano, 1996). In some cases, only mRNA transcripts are induced by integrin engagement alone, and a second signal is required for their translation and expression of the corresponding protein (Juliano, 1996). Whether integrins directly or indirectly regulate posttranscriptional pathways is largely unknown, and is a challenging issue to study because of the clear and potent influence of integrin engagement on transcriptional events (Juliano and Haskill, 1993; Schwartz et al., 1995).
Recently, we found that stimulated human platelets synthesize proteins from preformed mRNA in an activation-dependent fashion (Weyrich et al., 1998). Because they are primary anucleate cytoplasts that bear receptors and surface adhesion molecules capable of mediating outside-in signaling (Shattil et al., 1994, 1998), platelets are a unique system in which to study activation-dependent translational events independent of nuclear influences. In activated platelets, the synthesis of several induced proteins is inhibited by the immunosuppressant rapamycin in addition to general inhibitors of translation (Weyrich et al., 1998), indicating the presence of a specialized pathway of translational control regulated by mammalian target of rapamycin (mTOR)1 (Brown and Schreiber, 1996; Thomas and Hall, 1997). The transcript for B cell lymphoma protein 3 (Bcl-3), an intracellular regulatory factor, is present in platelets and is translated via this pathway when platelets are stimulated with thrombin (Weyrich et al., 1998), making it a useful marker for studies of regulated protein synthesis in this cell type. Here we show that translation of Bcl-3 is an adhesion-dependent event that requires engagement of integrin αIIbβ3 in platelets activated by thrombin. Integrin αIIbβ3 is expressed only by platelets and megakaryocytes, and transmits outside-in signals in addition to mediating cellular aggregation and adhesion (Phillips et al., 1991; Hynes, 1992; Shattil et al., 1998). We also show that direct engagement of integrin αIIbβ3 induces expression of Bcl-3 in the absence of thrombin or other exogenous agonists, and that synthesis of Bcl-3 is induced when platelets adhere to collagen via integrin α2β1. These experiments demonstrate for the first time that integrins can directly control expression of gene products at translational checkpoints, and that their influence on the flow of genetic information is not limited to regulation of transcription.
Materials and Methods
Cell Isolation
Platelets were isolated using the methods of Hamburger and McEver (1990). In brief, human blood was drawn into acid-citrate-dextrose (ACD; 7 ml ACD/42 ml of blood) and was centrifuged (200 g for 20 min) to obtain platelet-rich plasma. Platelet-rich plasma was recentrifuged (500 g for 20 min) in the presence of 100 nM prostaglandin E-1. The supernatant was discarded and platelet pellet was resuspended in 50 ml of Pipes/saline/glucose (5 mM Pipes, 145 mM NaCl, 4 mM KCl, 50 μM Na2HPO4, 1 mM MgCl2-6 H2O, and 5.5 mM glucose), containing 100 nM of prostaglandin E-1 (Sigma Chemical Co.). The platelet suspension was centrifuged (500 g for 20 min), the supernatant was discarded, and the platelet pellet was resuspended in M199 (phenol red free; Whittaker M.A. Bioproducts). In selected studies, the platelets were suspended in Ca2+ and Mg2+-free HBSS containing 5 mM EGTA to chelate Ca2+. 2.5 × 108 platelets were used for each experimental point. Platelets were stimulated with thrombin (Sigma Chemical Co.), collagen (from human placenta; Sigma Chemical Co.), fibrinogen (Sigma Chemical Co.), or an activating antibody, D3GP3 (provided by Dr. L.K. Jennings, University of Tennessee, Memphis, TN) for 1 h at 37°C while gently rocking in small volume conical tubes. In selected studies, platelets were preincubated with αIIbβ3 blocking antibodies, 7E3 (obtained commercially and provided by Dr. S. Tam, Centocor, Malvern, PA), 10E5 (provided by Dr. B.S. Coller, Mount Sinai School of Medicine, New York), G4120, G4709 (provided by Dr. T. Gadek, Genentech, San Francisco, CA), or integrilin (eptifibatide) (provided by Dr. S. Hollenbach, COR Therapeutics, South San Francisco, CA) before stimulation with thrombin. Isotype-matched antibodies were used as controls as indicated in the text and figure legends (α-LFA-1 and CD31 from R&D Systems). After 1 h, the platelet pellets were collected and prepared for Western analysis as described below.
Human tissues for examination of platelets in situ were collected at the time of surgical intervention. Surgical tissues were collected according to protocols approved by the University of Utah internal review board after informed consent.
Platelet Adhesion Assay
Platelet adhesion to fibrinogen was studied in 4-well polystyrene chambers (Nunc Inc.) precoated overnight at 4°C with HBSS-human serum albumin (2%), which served as the control, fibrinogen (1 mg/ml; Sigma Chemical Co.), or collagen (50 μg/ml; Sigma Chemical Co.). Plates were washed three times and blocked for 2 h with 1 ml of HBSS-human serum albumin (2%), and washed three times with HBSS followed by three more washes with HBSS containing 0.01% Tween 20. Residual buffer was removed by aspiration and 2.5 × 108 platelets/ml were added to the matrix-coated wells for 1 h at 37°C. After this time, adherent platelets were scraped into Eppendorf tubes and the suspensions were centrifuged for 2 min, 1,000 g at room temperature, and the supernatants were removed. The cell pellets were placed in SDS-PAGE reducing buffer for Western analysis as described below.
Immunoblotting Procedure
Platelet pellets, collected from activated cells in suspension or those adherent to fibrinogen, were placed in SDS-PAGE reducing buffer, electrophoresed on a 9% SDS-polyacrylamide gel, and transferred to a nitrocellulose membrane. Western analysis was conducted using affinity-purified, rabbit polyclonal anti–Bcl-3 antibody (Santa Cruz Technology). Immunoreactive protein was detected by affinity-isolated goat anti–rabbit antibody conjugated to peroxidase (Biosource Int.) and an enhanced chemiluminescence detection reagent (Amersham Life Science).
Immunocytochemical and Immunohistochemical Procedures
Immunocytochemical procedures were performed as described previously, with minor modifications (Weyrich et al., 1996, 1998). In brief, platelets were spun onto glass slides and immediately fixed with 1% paraformaldehyde. After a methanol permeabilization step, the cells were blocked and probed with anti–Bcl-3 (Santa Cruz Technology). Immunoreactive protein for Bcl-3 was detected using an ABC kit from Vectastain (Vector Laboratories, Inc.) for horseradish peroxidase detection that yields a brown immunostain product. Control slides included omission of the primary antibody, omission of the secondary antibody, and/or substitution of nonimmune rabbit IgG. Tissue specimens from abdominal aortic aneurysms were collected and placed in Histochoice MB fixative (Amresco Inc.). After fixation, the specimens were embedded in paraffin, sectioned into 5-μm slices, and immunoreactivity for Bcl-3 was assayed as described previously (Weyrich et al., 1993). Sections were viewed and photographed by Nomarski interference contrast optics using a Zeiss Axioplan light microscope. Tissue collection procedures were approved by the University of Utah Institutional Review Board.
Aggregometry
0.5-ml aliquots of platelets (2.5 × 108/ml) were preincubated for 5 min at 37°C in the presence of buffer or antibodies before aggregation was initiated by thrombin. Platelets were placed in siliconized cuvettes and aggregation was monitored by a Sienco aggregometer (model DP-247-E) with constant stirring at 1,000 rpm at a constant temperature of 37°C as described previously (Kouns et al., 1990).
ELISA
Concentrations of RANTES were measured by ELISA as described previously (Weyrich et al., 1996).
Results
The Expression of Bcl-3 Is Enhanced in Aggregated Human Platelets
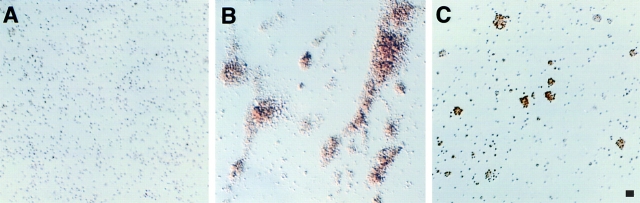
In previous experiments, we found that isolated human platelets translate constitutively present mRNA into proteins in an activation-dependent fashion, that this occurs in platelets stimulated with thrombin, and that Bcl-3 is an informative marker protein to examine in analyses of the synthetic response in this system (Weyrich et al., 1998). In addition, we found that when suspensions of thrombin-stimulated platelets were stained using an antibody against Bcl-3, expression of the protein appeared to be enhanced in aggregated cells compared with single cells. This suggested that signaling of protein synthesis in stimulated platelets is influenced by adhesion. To further explore this issue we performed additional immunocytochemical analyses and found that Bcl-3 protein is rapidly expressed in platelet aggregates after thrombin stimulation, with lesser amounts in thrombin-stimulated single cells and little or no protein detectable in platelets in the absence of thrombin (Fig. 1). When the anti–Bcl-3 antibody was deleted or replaced with a control rabbit immunoglobulin, there was no staining of Bcl-3 (Weyrich et al., 1998; data not shown). We also found that Bcl-3 is present in aggregated platelets in microvessels of inflamed tissue (Fig. 2), demonstrating that its synthesis in isolated platelets (Fig. 1) models in vivo events. The accumulation of Bcl-3 in thrombin-stimulated aggregated platelets examined in vitro was time- and concentration-dependent (Fig. 3 and data not shown), consistent with our earlier studies characterizing its synthesis in this cell type (Weyrich et al., 1998). Because aggregation of human platelets depends on engagement of integrin αIIbβ3 by fibrinogen or von Willebrand factor (Gawaz et al., 1991; Williams et al., 1995), these observations suggested that outside-in signaling via this integrin heterodimer regulates the synthetic pathway leading to expression of the Bcl-3 protein. Therefore, we characterized the role of integrin αIIbβ3 in detail.
Figure 1.
Bcl-3 is expressed in aggregated human platelets activated by thrombin. Platelets (2.5 × 108/ml) were isolated as described (Materials and Methods) and treated with control buffer or thrombin (0.1 U/ml) for 1 h at 37°C while being gently rocked in small volume conical tubes. Immunostaining using an antibody against Bcl-3 was done as described (Materials and Methods; Weyrich et al., 1998). (A) Platelets treated with buffer alone remained single with little or no staining by anti– Bcl-3 when viewed by low power or high power (not shown) microscopy. (B) Large multicellular aggregates formed in response to thrombin with smaller aggregates also visible and few single cells remaining in suspension. The aggregates stained intensely for Bcl-3, obscuring individual cellular detail at low power. (C) Pretreatment with the blocking anti-αIIbβ3, mAb 10E5 (see text), dramatically inhibited platelet aggregation and staining for Bcl-3. As shown, in some experiments inhibition of aggregation was not complete and Bcl-3 protein was present in the scattered small residual aggregates. The figures are representative of multiple experiments with thrombin-stimulated platelets and of six experiments examining the effects of antiintegrin antibodies. Bar, 10 μm.
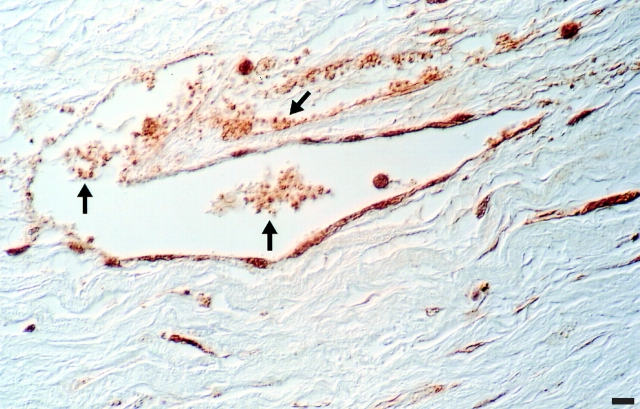
Figure 2.
Bcl-3 is expressed in aggregated human platelets in situ. Tissue sections from vessels resected at the time of surgical intervention in patients with abdominal aortic aneurysms were examined by immunohistochemical analysis. Bcl-3, indicated by the brown reaction product, is present in intravascular platelet aggregates and in platelets adherent to walls of adventitial microvessels (arrows). The adventitia of aneurysmal vessels is an area of inflammatory and thrombotic signaling and cell–cell interactions (Modur et al., 1997). Bcl-3 is also present in endothelial cells and leukocytes in this section, consistent with in vitro observations (Ohno et al., 1990; Pan and McEver, 1995). This figure is representative of surgical specimens from three different subjects. Bar, 10 μm.
Figure 3.

Removal of extracellular calcium prevents platelet aggregation and inhibits Bcl-3 synthesis. Platelets (2.5 × 108/ml) were isolated as described in Materials and Methods, pretreated for 30 min with vehicle or 5 mM EGTA, and then activated with thrombin for 1 h. (a) Chelation of extracellular calcium abolished platelet aggregation in response to thrombin. (b) In control platelets, thrombin induced Bcl-3 accumulation in a concentration-dependent fashion when assayed by Western analysis or immunostaining (not shown). The accumulation of Bcl-3 was inhibited in platelets stimulated with thrombin in Ca2+-free buffer in the presence of the chelator. (c) Thrombin triggered release of RANTES by platelets pretreated with EGTA as well as by control platelets. These results are representative of seven independent experiments.
Chelation of Extracellular Calcium Inhibits Platelet–Platelet Clustering and Abolishes Bcl-3 Synthesis
We determined if chelation of extracellular calcium interrupts Bcl-3 synthesis, since it is known to block platelet aggregation (Fitzgerald et al., 1985; Shattil et al., 1985). Platelets stimulated with thrombin in calcium-free buffer containing EGTA (5 mM) did not aggregate in response to thrombin (Fig. 3) and did not synthesize Bcl-3 (Fig. 3 b). As a control to test whether signaling via the thrombin receptor was still intact under these conditions, we measured the secretion of RANTES, a preformed chemokine that is stored in alpha granules (Kameyoshi et al., 1992; Weyrich et al., 1996), and found that it was released in response to stimulation (Fig. 3 c). These findings suggested that disruption of adhesive interactions between the aggregating platelets by removal of extracellular cations resulted in impaired synthesis of Bcl-3. Alterations in cation concentrations also have the potential to inhibit intracellular enzymes and other components required for protein synthesis, however, so we pursued this issue further using additional experimental strategies.
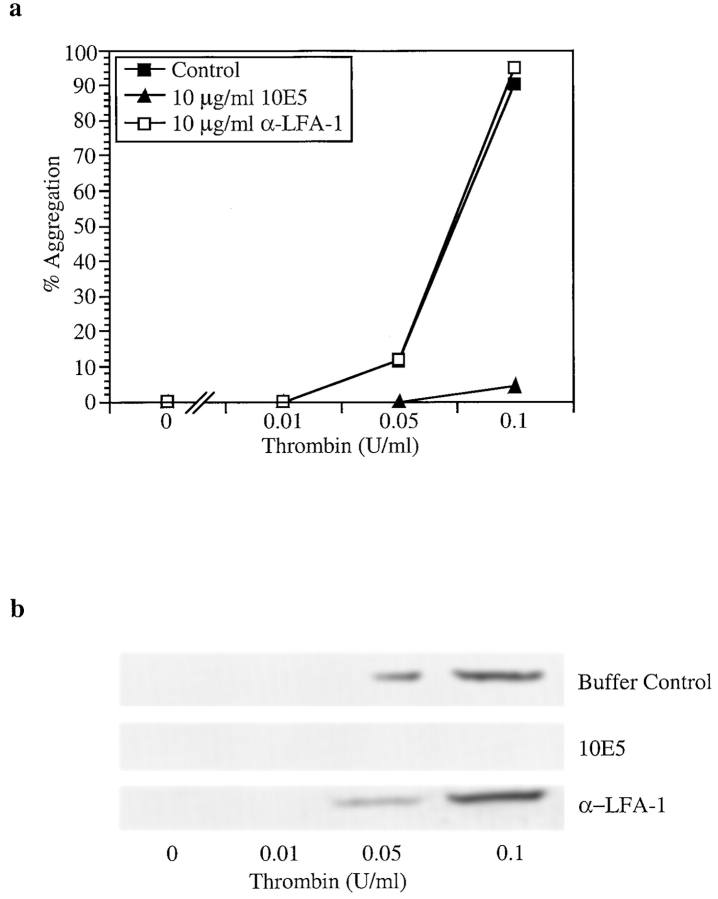
mAbs Directed Against Integrin αIIbβ3 Inhibit Platelet Aggregation and Bcl-3 Synthesis
We next determined if engagement of the αIIbβ3 integrin is required for Bcl-3 synthesis in thrombin-stimulated platelets. Platelet aggregation occurs when αIIbβ3 heterodimers on adjacent activated platelets are engaged by fibrinogen released from alpha granules or added exogenously (Gawaz et al., 1991; reviewed in Williams et al., 1995). mAb 10E5 blocks binding of fibrinogen to αIIbβ3 and prevents platelet aggregation (Coller et al., 1983; Coller, 1985). Therefore, we determined if mAb 10E5 inhibits Bcl-3 accumulation in stimulated human platelets. As expected, preincubation of platelets with mAb 10E5 markedly attenuated thrombin-induced platelet aggregation compared with control cells in the absence of antibody (Figs. 1 and 4). In addition, Bcl-3 accumulation was inhibited by pretreatment of platelets with mAb 10E5 when examined by immunocytochemistry (Fig. 1) and Western analysis (Fig. 4 b). In some incubations, there were scattered small residual aggregates that were visible by microscopy in suspensions pretreated with mAb 10E5 and then stimulated with thrombin, although the majority of cells did not aggregate; these residual aggregates contained Bcl-3 when examined by immunocytochemical analysis (Fig. 1 c). An isotype-matched mAb against αLβ2 integrin (α-LFA-1; IgG2a) did not inhibit Bcl-3 accumulation in thrombin-stimulated platelets or block platelet aggregation (Fig. 4).
Figure 4.
An antibody that blocks engagement of integrin αIIbβ3 inhibits stimulated synthesis of Bcl-3. Platelets were isolated, preincubated with control buffer, mAb 10E5 (IgG2a), or a control isotype-matched mAb against integrin αLβ2 (α-LFA-1) for 5 min at 37°C. They were then stimulated with thrombin or treated with control buffer, and platelet aggregation and accumulation of Bcl-3 were examined in parallel as described in Materials and Methods and Figs. 1 and 2. (a) mAb 10E5 inhibits aggregation of thrombin-stimulated platelets whereas anti-αLβ2 did not. (b) mAb 10E5 inhibited accumulation of Bcl-3 in platelets stimulated with thrombin in the concentrations shown for 1 h. The isotype-matched control antibody did not attenuate Bcl-3 accumulation compared with that in platelets treated with control buffer alone. The results shown are representative of six independent events.
We performed similar experiments with the Fab fragment of the chimeric human-murine mAb 7E3. This antibody recognizes integrins αIIbβ3 and αvβ3, inhibits fibrinogen binding to platelets, and has been used to block platelet aggregation as a clinical antithrombotic agent (Coller, 1985; Reverter et al., 1996; Coller, 1997). mAb 7E3 attenuated Bcl-3 accumulation in thrombin-stimulated platelets and also inhibited aggregation in parallel incubations (not shown). In contrast, a mAb against αvβ3 did not block Bcl-3 expression.
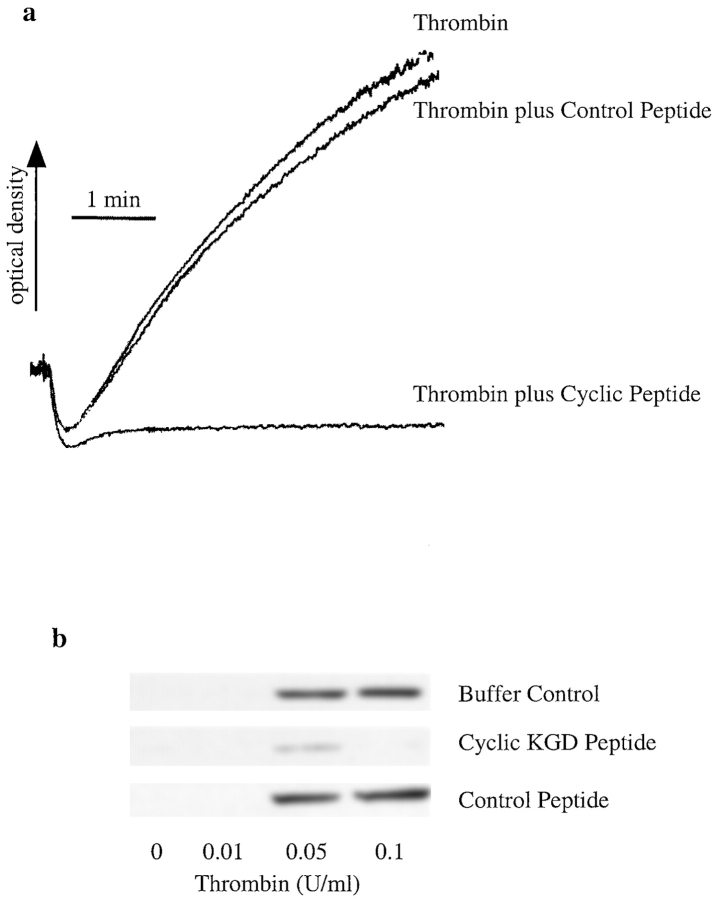
Peptides That Block Engagement of Integrin αIIbβ3 Inhibit Synthesis of Bcl-3 in Stimulated Human Platelets
Binding of fibrinogen to integrin αIIbβ3 on activated platelets requires engagement of a dodecapeptide sequence in the γ chain of fibrinogen by the integrin heterodimer, an event that can be blocked by peptides that contain arginine-glycine-asparagine (RGD) sequences (Du et al., 1991; Phillips et al., 1991). Substitution of lysine (K) for arginine (R) in the RGD sequence confers specificity for integrin αIIbβ3 compared with other integrins, and cyclic peptides containing the KGD sequence that are based on the snake venom disintegrin, barbourin, potently inhibit fibrinogen binding to αIIbβ3 and platelet aggregation (Scarborough et al., 1991; Scarborough et al., 1993). We first examined linear RGD peptides as antagonists of aggregation-dependent Bcl-3 synthesis in human platelets and found that under some conditions the peptides themselves had weak agonist effect (not shown), consistent with previous observations (Du et al., 1991). Then, we examined a cyclic KGD heptapeptide that specifically binds to integrin αIIbβ3 (Schulman et al., 1996; Pursuit Trial Investigators, 1998). The cyclic peptide antagonist inhibited both thrombin-induced platelet aggregation (Fig. 5 a) and Bcl-3 accumulation (Fig. 5 b). The inhibition was concentration-dependent (range 1–10 μg/ml) with maximal inhibition at 10 μg/ml (Fig. 5 b). A control peptide had no effect. A second blocking cyclic peptide, G4120 (Barker et al., 1992), also inhibited Bcl-3 synthesis in a concentration-dependent fashion, whereas a control peptide did not (not shown). Thus, inhibition of engagement of αIIbβ3 integrin with competitive peptides attenuates accumulation of Bcl-3 in platelets stimulated with thrombin (Fig. 5), as does treatment of the platelets with blocking antibodies (Fig. 4).
Figure 5.
A cyclic KGD peptide that specifically blocks engagement of integrin αIIbβ3 inhibits Bcl-3 synthesis in thrombin-stimulated human platelets. Platelets were isolated as described in Materials and Methods, pretreated with a cyclic KGD heptapeptide specific for integrin αIIbβ3 or a control peptide (5 min at 37°C), and then activated with thrombin. (a) Thrombin-induced (0.1 U/ml) platelet aggregation was inhibited when platelets were pretreated with the cyclic KGD peptide (10 μg/ml) but not by a control peptide in an equivalent concentration. (b) Pretreatment of platelets with the cyclic KGD peptide attenuated Bcl-3 synthesis in thrombin-activated platelets, whereas a control KGD peptide at the same concentration did not. These results are representative of four independent experiments.
Bcl-3 Synthesis Is Absent or Reduced When Platelets Deficient in Integrin αIIbβ3 Are Stimulated with Thrombin
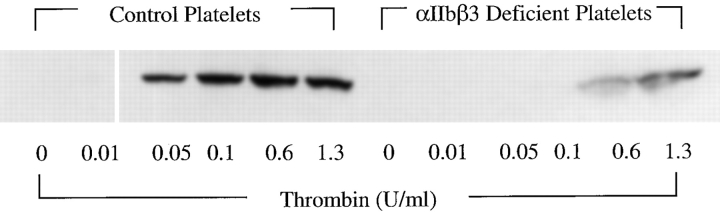
Platelets from patients with Glanzmann thrombasthenia have significant reductions or absence of αIIbβ3 on their surfaces (George et al., 1990; Newman and Poncz, 1995). The deficiency in integrin αIIbβ3 prevents normal binding of ligands and consequent platelet aggregation, accounting for the hemostatic defect that characterizes these subjects. Platelets from patients with Glanzmann thrombasthenia also have impaired outside-in signaling (Wang et al., 1997). We studied platelets from a patient with type I Glanzmann thrombasthenia that do not aggregate when stimulated by thrombin or a variety of other agonists (Jin et al., 1996). When compared with platelets from a normal control subject isolated in parallel, these mutant platelets exhibited a dramatic defect in accumulation of Bcl-3 in response to thrombin stimulation (Fig. 6). There was no accumulation of Bcl-3 in the integrin αIIbβ3-deficient platelets at concentrations of thrombin (0.05, 0.1 U/ml) that induced synthesis of the protein marker in the simultaneously assayed control platelets (Fig. 6) or in platelets from other control subjects (Figs. 1 and 3–5). At higher concentrations of thrombin, accumulation of Bcl-3 in the αIIbβ3-deficient platelets was attenuated but not absent (Fig. 6).
Figure 6.
Platelets deficient in integrin αIIbβ3 have absent or attenuated synthesis of Bcl-3 when stimulated with thrombin. Platelets (2.5 × 108/ml) were isolated from a subject with Glanzmann thrombasthenia and from a control subject in parallel and were stimulated with thrombin at the indicated concentrations for 2 h at 37°C. They were then assayed for Bcl-3 by Western analysis as described in Materials and Methods and Fig. 3. Additional measurements indicated equivalent loading of protein in samples of the control and αIIbβ3 integrin–deficient platelets (not shown).
Direct Activation of Integrin αIIbβ3 Induces Bcl-3 Synthesis
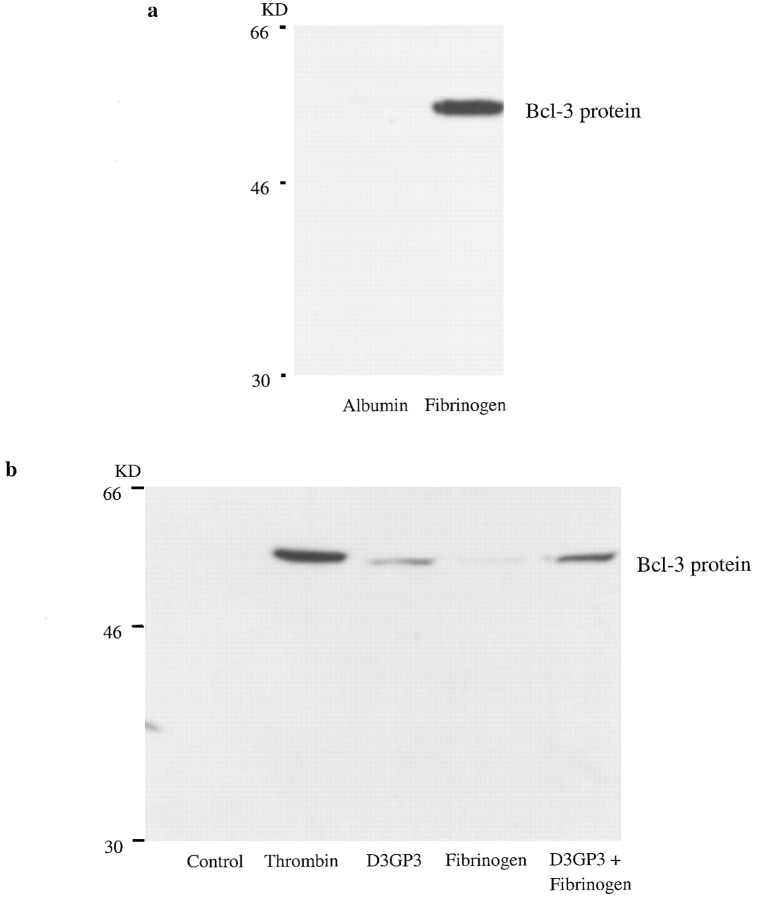
We showed previously that adhesion of platelets to purified immobilized fibrinogen induces the synthesis of multiple proteins in the absence of thrombin or another agonist (Weyrich et al., 1998). This is consistent with earlier studies indicating that integrin αIIbβ3 on the platelet surface can engage immobilized fibrinogen and transmit outside-in signals without requiring an exogenous agonist, whereas engagement by soluble fibrinogen requires agonist-stimulated cellular activation (Savage and Ruggeri, 1991; Haimovich et al., 1993; Shattil et al., 1994). To determine if engagement of integrin αIIbβ3 is sufficient to induce translation of the marker protein Bcl-3, we first used this immobilized fibrinogen system. We found that platelets adherent to a fibrinogen matrix accumulated Bcl-3 (Fig. 7 a). In contrast, there was little or no Bcl-3 in platelets incubated in suspension (not shown) or on immobilized albumin in parallel. mAbs 10E5 and 7E3 (see above) inhibited Bcl-3 accumulation in platelets adherent to immobilized fibrinogen. In addition, LY 294002 and Wortmannin, which inhibit phosphatidylinositol-3-kinase (PI3K), blocked Bcl-3 expression in platelets adherent to immobilized fibrinogen (not shown), as they do in thrombin-stimulated aggregated platelets (Weyrich et al., 1998).
Figure 7.
Engagement of integrin αIIbβ3 by immobilized ligand or an activating antibody induces synthesis of Bcl-3 in human platelets. (a) Adhesion of platelets to immobilized fibrinogen induces synthesis of Bcl-3. Isolated platelets were incubated on an immobilized fibrinogen matrix or on immobilized albumin for 1 h at 37°C and then examined for the accumulation of Bcl-3 by Western analysis as described in Materials and Methods and Fig. 3. The fibrinogen matrix and control surface were prepared (Materials and Methods) using a modification of a previously described method (Haimovich et al., 1993). This result is representative of four experiments. (b) An activating antibody against integrin αIIbβ3, D3GP3, triggers synthesis of Bcl-3 in isolated human platelets. Isolated platelets were incubated in suspension with mAb D3GP3 (40 μg/ml), with fibrinogen (100 μg/ml), or with mAb D3GP3 plus fibrinogen for 1 h at 37°C and then processed for Western analysis for Bcl-3 (Materials and Methods and Fig. 3). In parallel, platelets were treated with thrombin (0.1 U/ml) or control buffer as positive and negative controls, respectively. In contrast to mAb D3GP3 (third and fifth lanes), a control antibody did not trigger Bcl-3 accumulation (not shown). A second experiment yielded similar results.
We then used an alternative strategy to ask if engagement of integrin αIIbβ3 delivers outside-in signals to the translation pathway that regulates synthesis of Bcl-3. mAb D3GP3 is directed against the β3 chain of the integrin αIIbβ3 heterodimer and induces a conformational change that makes the integrin competent to bind fibrinogen and mediate aggregation in the absence of thrombin or another stimulus (Kouns et al., 1990; Kouns and Jennings, 1991). We found that incubation of platelets with mAb D3GP3 resulted in their aggregation (not shown), as previously reported, and also triggered synthesis of Bcl-3 (Fig. 7 b). An isotype-matched antibody against another protein on the platelet plasma membrane, PECAM-1 (CD31), did not induce Bcl-3 synthesis (not shown). When examined by immunocytochemistry, Bcl-3 was predominantly located in aggregated platelets in suspensions treated with D3GP3, with few single platelets showing staining (not shown). The accumulation of Bcl-3 in platelets incubated with mAb D3GP3 was not as great as that in platelets stimulated with thrombin in parallel (Fig. 7 b), consistent with the fact that the antibody induces submaximal aggregation under these conditions (Kouns et al., 1990) (our experiments not shown). Platelets treated with mAb D3GP3 bind exogenously added fibrinogen in an enhanced fashion (Kouns et al., 1990). When we added soluble fibrinogen to the incubation, the accumulation of Bcl-3 was enhanced in platelets incubated with D3GP3 (Fig. 7 b). Exogenous soluble fibrinogen did not induce Bcl-3 synthesis in platelets incubated with the control mAb against PECAM-1 (not shown).
Collagen Triggers Bcl-3 Synthesis by Human Platelets
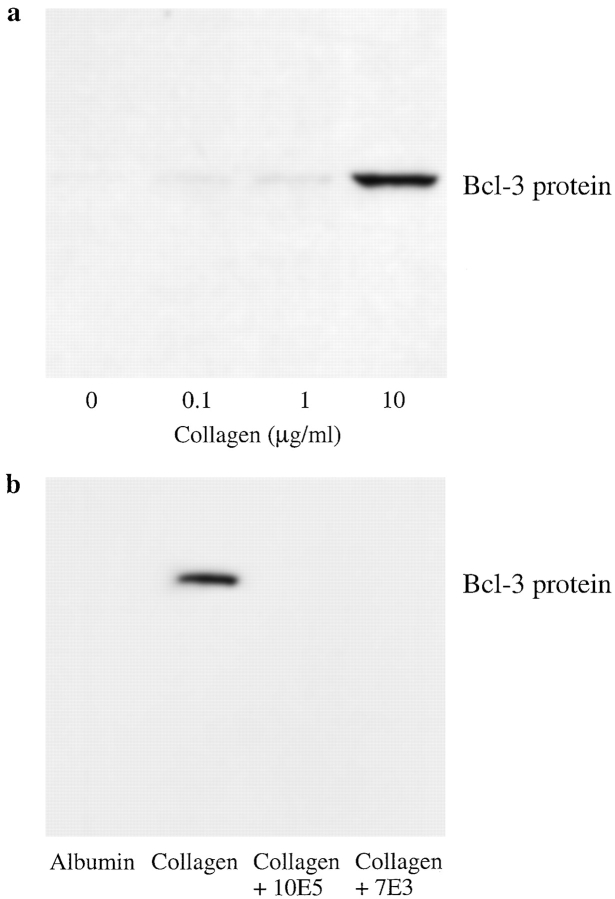
Collagen is recognized by integrin α2β1 as well as by other adhesion molecules on platelets, and treatment of isolated platelets with collagen in solution induces activation of intracellular kinases and aggregation equivalent in magnitude to that triggered by thrombin (Lipfert et al., 1992; Shattil et al., 1994). We found that collagen in solution induced synthesis of Bcl-3 in a concentration-dependent fashion (Fig. 8 a). In addition, we found that platelets adherent to immobilized collagen synthesized Bcl-3 (Fig. 8 b). The accumulation of Bcl-3 in platelets adherent to immobilized collagen was inhibited by a blocking antibody against the integrin α2 subunit (not shown) and also by mAb 7E3 (see above) (Fig. 8 b) but not by a control mAb against αvβ3 (not shown). The latter findings are consistent with a previous report that mAb 7E3 and an antibody against α2β1 integrin each inhibited outside-in signaling of platelets when the cells adhered to an immobilized collagen matrix (Haimovich et al., 1993). mAb 10E5 also blocked synthesis of Bcl-3 under similar conditions (Fig. 8 b). Thus, engagement of integrin α2β1 and/or other surface receptors for collagen together with integrin αIIbβ3 may mediate signaling of translational events leading to Bcl-3 synthesis in a costimulatory fashion.
Figure 8.
Collagen induces synthesis of Bcl-3 in human platelets. (a) Collagen in solution induces Bcl-3 synthesis by human platelets. Platelets were isolated as described in Materials and Methods and incubated in suspension with collagen in the concentrations shown for 1 h at 37°C. Synthesis of Bcl-3 protein was examined by Western analysis. Collagen in solution induced Bcl-3 expression in three additional experiments. (b) Adhesion of platelets to immobilized collagen induces Bcl-3 synthesis. Platelets were pretreated with control buffer, mAb 10E5, or mAb 7E3 as described in Fig. 4 and Materials and Methods and then were incubated on immobilized collagen or on immobilized albumin for 1 h at 37°C. Accumulation of Bcl-3 was examined by Western analysis as described in Materials and Methods. This result is representative of three experiments.
Discussion
The flow of genetic information is regulated at sequential checkpoints that, together, provide precise control of the expression of protein products (Darnell, 1982; Kozak, 1991). Here we show that integrin αIIbβ3 regulates translation of a marker protein, Bcl-3, in thrombin-stimulated aggregated platelets. Integrin αIIbβ3 is the principle integrin of human platelets and is a herald member of the integrin family that has yielded many insights into the structure and function of these adhesion proteins (Phillips et al., 1991; Hynes, 1992; Shattil et al., 1994; Shattil et al., 1998). We also found that outside-in signals delivered via integrin αIIbβ3 trigger Bcl-3 synthesis when the integrin heterodimer is engaged by immobilized ligand or a function-perturbing antibody. Our findings raise the possibility that a general mechanism by which integrins regulate gene expression is by interacting at posttranscriptional checkpoints, in addition to mediating nuclear signaling and transcriptional events. It has been suggested previously that β1 integrins on leukocytes may influence posttranscriptional steps (Mondal et al., 1995). Also, mechanical signaling via engaged β1 integrins may orchestrate local accumulation of mRNA and ribosomes in the region of focal adhesion complexes (Chicurel et al., 1998). However, our findings provide the first evidence that outside-in signaling via a specific integrin heterodimer regulates expression of protein products in the absence of nuclear effects.
Human platelets may be a particularly informative system in which to study adhesion-dependent signaling of translation in a primary cell type, and mechanisms elucidated in platelets may also reflect similar processes at the earlier, nucleated, megakaryocyte stage (Weyrich et al., 1998). Platelets have ribosomes and other components required for protein synthesis and carry stable mRNA transcripts (Warshaw et al., 1967; Morgenstern, 1980; Belloc et al., 1982; Kieffer et al., 1987; Newman et al., 1988; Roth et al., 1989; Power et al., 1995). Multiple new proteins are synthesized in thrombin-stimulated platelets and in platelets adherent to immobilized fibrinogen; this synthesis is interrupted by puromycin and cycloheximide and, for a subset of these proteins, by rapamycin (Weyrich, 1998; and our unpublished observations). To date, we have identified Bcl-3 as one of the newly synthesized products and five others as proteins that regulate or are involved in cytoskeletal interactions (Weyrich A.S., N.D. Tolley, M.L. Wade, T.M. McIntyre, S.M. Prescott, Z. Wu, and G.A. Zimmerman, manuscript in preparation). Although mitochondrial transcription occurs in platelets (Agam et al., 1976), Bcl-3 is translated from preformed mRNA and the transcriptional inhibitor actinomycin D does not prevent synthesis (Weyrich et al., 1998). Platelets contain key enzymes in the specialized mTOR pathway that controls translation of a subset of mRNAs with specific structural features (Brown and Schreiber, 1996; Thomas and Hall, 1997), including the mRNA for Bcl-3 (Weyrich et al., 1998). Current evidence from cell lines and transfected cell models indicate that activity in this pathway is initiated by a signal at the plasma membrane followed by a cascade involving PI3K and 3-phosphoinositide-dependent protein kinase 1 (PDK1) and culminating in phosphorylation of the translation repressor eIF4E-binding protein 1 (4EBP-1), causing it to dissociate from eukaryotic translation initiation factor 4E (eIF4E) and allowing cap-dependent translation to proceed (Sonenberg and Gingras, 1998). Phosphorylation and activation of the ribosomal S6 kinase, p70 S6 kinase (p70S6K), also occurs. In previous studies of lymphocytic cell lines and fibroblasts, this pathway was shown to be triggered by growth factors and mitogens (reviewed in Brown and Schreiber, 1996; Thomas and Hall, 1997; Peterson and Schreiber, 1998; Sonenberg and Gingras, 1998). In aggregating platelets, PI3K is triggered in an adhesion-dependent fashion and PDK1 is also present (Clark and Brugge, 1995; Banfic et al., 1998a,b). Inhibitors of PI3K block synthesis of Bcl-3 in thrombin-stimulated aggregated platelets (Weyrich et al., 1998) and in platelets adherent to immobilized fibrinogen (this study). In addition, p70S6K is present in human platelets (Papkoff et al., 1994) and is activated when they aggregate in response to thrombin (Weyrich, A.S., unpublished experiments). 4EBP-1 is also phosphorylated in thrombin-stimulated platelets and this event and the synthesis of Bcl-3 are blocked by inhibitors of PI3K and by rapamycin (Weyrich et al., 1998). Thus, platelets have critical enzymatic and regulatory molecules that are required for translation control, including components of the mTOR pathway, and the activities of these systems are influenced by outside-in signals.
Using blocking antibodies, competitive peptides, and deficient platelets from a subject with Glanzmann thrombasthenia, we found that engagement of integrin αIIbβ3 regulates Bcl-3 synthesis in aggregating platelets stimulated by thrombin. Thus, signals transmitted by integrin αIIbβ3 are linked to translation control pathways. We also found that ligation of integrin αIIbβ3 by immobilized fibrinogen or binding of a conformation-altering antibody induces synthesis of Bcl-3 in the absence of thrombin stimulation. These experiments and our previous observations (Weyrich et al., 1998) indicate that engagement of integrin αIIbβ3 is sufficient to signal activation of translational pathways and synthesis of a variety of proteins. Binding of fibrinogen to integrin αIIbβ3 in the presence of an activating “LIBS” anti-β3 antibody triggers PI3K and apparent PDK1 activities, responses that require platelet–platelet contact and aggregation to be maximal (Banfic et al., 1998a,b). Thus, engagement of integrin αIIbβ3 can activate key enzymes in the transduction cascade that relay signals from the plasma membrane to translational pathways (see above). Whether there are intracellular signaling cascades that are specific to integrin αIIbβ3 (Banfic et al., 1998a) is unknown. In other systems, integrins and growth factors or mitogens use common, rather than unique, intracellular mechanisms to trigger gene expression (Juliano, 1996; Howe et al., 1998). How integrin αIIbβ3 interfaces with downstream components of the translation control pathway that regulate phosphorylation of 4EBP1 and p70S6K activation is also currently unknown. In a previous study, adhesion of a cell line to immobilized fibronectin, laminin, or vitronectin activated p70S6K, implying that regulation of this enzyme is linked to engagement of integrins of both the β1 and β3 classes (Malik and Parsons, 1996). Additional experiments indicated that focal adhesion kinase was partially required for p70S6K activation. Whether focal adhesion kinase, which is signaled by integrin αIIbβ3 engagement (Lipfert et al., 1992; reviewed in Shattil et al., 1994; reviewed in Clark and Brugge, 1995; Lyman et al., 1997; and reviewed in Shattil et al., 1998), is involved in p70S6K activation and translational regulation in platelets remains to be explored.
In cell–cell interactions, signals delivered through adhesion molecules are integrated with signals from surface receptors, such as those for growth factors or chemokines, to yield qualitatively distinct responses (Weyrich et al., 1996; Zimmerman et al., 1996). Signals delivered by integrins and growth factors converge and are integrated in this fashion (reviewed in Schwartz et al., 1995; Juliano, 1996; Sastry and Horwitz, 1996). Our finding that Bcl-3 expression in platelets is induced by thrombin stimulation raises the possibility that outside-in signals transmitted by engagement of integrin αIIbβ3 interface and are integrated with those generated by ligation of the thrombin receptor resulting in translation of mRNAs. The thrombin receptor and integrin αIIbβ3 transmit convergent signals to other response pathways in human platelets (Ferrell and Martin, 1989; Golden et al., 1990; Clark et al., 1994; Shattil et al., 1994; Cichowski et al., 1996). In our experiments, expression of Bcl-3 in thrombin-activated platelets required engagement of integrin αIIbβ3 at concentrations of thrombin (0.01–0.1 U/ml) that triggered maximal or near-maximal platelet aggregation, and was blocked by inhibitory antibodies or peptides against αIIbβ3 (see Results). The results argue that at these concentrations ligation of the thrombin receptor is not sufficient to induce translation. At higher concentrations of thrombin, there appeared to be an αIIbβ3-independent mechanism of signaling when platelets from a subject with Glanzmann thrombasthenia were studied (Fig. 6). This is potentially due to differential signaling through other receptors on platelets that recognize thrombin (Schmidt et al., 1998). Alternatively, this may represent the activity of a small number of residual copies of integrin αIIbβ3 on the platelets that we used for this study (Jin et al., 1996).
In addition to integrating of signals delivered via receptors for mitogens and growth factors, integrins of different classes may also signal cooperatively (Schwartz et al., 1995; Juliano, 1996). Our experiments in which adhesion of platelets to immobilized collagen induced synthesis of Bcl-3 (Results and Fig. 8) indicate that integrin αIIbβ3 and integrin α2β1 cooperatively signal translation events. We found that an antibody against the α2 subunit of α2β1 integrin, which recognizes collagen, and mAb 7E3 and 10E5, which block ligand binding by integrin αIIbβ3, each inhibited Bcl-3 synthesis in platelets that adhered to immobilized collagen matrices (Results). One explanation for this experimental outcome is that adhesion of individual platelets to immobilized collagen caused outside-in signaling via integrin α2β1 and triggered degranulation and local secretion of fibrinogen, with secondary formation of microaggregates caused by binding of fibrinogen to integrin αIIbβ3 on adjacent platelets. Haimovich et al. (1993) reported that microaggregate formation occurs when platelet suspensions are incubated on immobilized collagen and that this is blocked by mAb 7E3. Thus, it is possible that engagement of integrin αIIbβ3 by endogenously released fibrinogen alone signals expression of Bcl-3 under these conditions. Alternatively, engagement of integrin α2β1 may signal directly to the mTOR pathway when platelets bind to immobilized collagen and additionally triggers local secretion of fibrinogen and engagement of integrin αIIbβ3, concomitantly inducing translation of Bcl-3. Although these two possibilities cannot be resolved yet, our results are consistent with cooperative interaction of the two platelet integrins in regulating translational events. Additional evidence for cooperative interaction between integrins αIIbβ3 and α2β1 has also been reported (Coller et al., 1989; Lipfert et al., 1992; Savage et al., 1998). Whether other receptors for collagen (Shattil et al., 1994; Savage et al., 1998; Watson and Gibbins, 1998) also signal translation in adherent platelets is unknown at this time.
Our finding that engagement of integrins regulates synthesis of proteins in platelets (Results and Weyrich et al., 1998) suggests that control of translation is a general mechanism by which integrins and other classes of adhesion molecules influence gene expression. Adhesion-dependent signaling, a process by which integrins and other adhesion molecules can specifically modulate or induce synthesis of particular gene products, adds spatial regulation to this process (Juliano, 1996; Schwartz et al., 1996; Zimmerman et al., 1996). In addition to spatial regulation, activation of translation pathways by outside-in signals delivered through integrins or other adhesion molecules can rapidly induce synthesis of proteins from preformed mRNA, influencing the temporal sequence of expression of gene products. Specific modulation by integrins of translation checkpoints, which are downstream of transcription, mRNA processing, nuclear export, mRNA degradation, also adds precision and variety in signaling of gene expression that would not be available if transcription were the only point of influence (Darnell, 1982). Our ongoing studies indicate that translation control occurs when other adhesion molecules besides integrins are engaged (Mahoney, T.S., A.S. Weyrich, G.A. Zimmerman, T.M. McIntyre, S.M. Prescott, manuscript in preparation), suggesting that signaling to translational control pathways is a general mechanism of adhesion-dependent regulation of gene expression.
Acknowledgments
We thank Jeanne Falk, Donnie Benson, and Wenhua Li for excellent technical assistance. We also thank Barry S. Coller, Lisa Jennings, Tom Gadek, Stanley Hollenbach, and Susan Tam for the gifts of important reagents. We are grateful to our colleagues at the CVRTI for their helpful comments and critical reading of the manuscript, to Cletus D'Souza for performing protein determinations, and Diana Lim and Richard Kuenzler for preparation of figures. We appreciate the help of Leona Montoya and Michelle Bills in preparation of the manuscript.
This work was supported by the Nora Eccles Treadwell Foundation, and the Richard A. and Nora Eccles Harrison Fund for Cardiovascular Research, the National Institutes of Health (HL44525), and the Wellcome Trust (UK). Dr. Ravinder Pabla is a Wellcome International Traveling Fellow (grant 046937/Z/96/Z/).
Abbreviations used in this paper
- Bcl-3
B cell lymphoma 3
- mTOR
mammalian target of rapamycin
- PDK1
3-phosphoinositide-dependent protein kinase 1
- PI3K
phosphatidylinositol-3-kinase
References
- Agam G, Bessler H, Djaldetti M. In vitroDNA and RNA synthesis by human platelets. Biochim Biophys Acta. 1976;425:41–48. doi: 10.1016/0005-2787(76)90214-8. [DOI] [PubMed] [Google Scholar]
- Banfic H, Tang X, Batty IH, Downes CP, Chen C, Rittenhouse SE. A novel integrin-activated pathway forms PKB/Akt-stimulatory phosphatidylinositol 3,4-biphosphate via phosphatidylinositol 3-phosphate in platelets. J Biol Chem. 1998a;273:13–16. doi: 10.1074/jbc.273.1.13. [DOI] [PubMed] [Google Scholar]
- Banfic H, Downes CP, Rittenhouse SE. Biphasic activation of PKBα/Akt in platelets. Evidence for stimulation both by phosphatidylinositol 3,4-biphosphate, produced via a novel pathway, and by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998b;273:11630–11637. doi: 10.1074/jbc.273.19.11630. [DOI] [PubMed] [Google Scholar]
- Barker PL, Bullens S, Bunting S, Burdick DJ, Chan KS, Deisher T, Eigenbrot C, Gadek TR, Gantzos R, Lifari MT. Cyclic RGD peptide analogues as anti-platelet anti-thrombotics. J Med Chem. 1992;35:2040–2048. doi: 10.1021/jm00089a014. [DOI] [PubMed] [Google Scholar]
- Belloc F, Hourdille P, Boisseau MRT, Bernard P. Protein synthesis in human platelets: correlation with platelet size. Nouv Rev Fr Hematol. 1982;24:369–373. [PubMed] [Google Scholar]
- Brown EJ, Schreiber SL. A signaling pathway to translational control. Cell. 1996;86:517–520. doi: 10.1016/s0092-8674(00)80125-7. [DOI] [PubMed] [Google Scholar]
- Chicurel NE, Singer RH, Meryer CJ, Ingbe DE. Integrin binding and mechanical tension induce movement of mRNA and ribosomes to focal adhesions. Nature. 1998;392:730–733. doi: 10.1038/33719. [DOI] [PubMed] [Google Scholar]
- Cichowski K, Brugge JS, Brass LF. Thrombin receptor and integrin engagement stimulate tyrosine phosphorylation of the proto-oncogene product, p95vav, in platelets. J Biol Chem. 1996;271:7544–7550. doi: 10.1074/jbc.271.13.7544. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Clark EA, Shattil SJ, Ginsberg MH, Bolen J, Brugge JS. Regulation of the protein tyrosine kinase pp72syk by platelet agonists and the integrin αIIbβ3 . J Biol Chem. 1994;269:28859–28864. [PubMed] [Google Scholar]
- Coller BS. A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet glycoprotein IIb/IIIa complex. J Clin Invest. 1985;76:101–108. doi: 10.1172/JCI111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller BS. Platelet GPIIb/IIIa antagonists. The first anti-integrin receptor therapeutics. J Clin Invest. 1997;100:S57–S60. [PubMed] [Google Scholar]
- Coller BS, Peerschke EI, Scudder LE, Sullivan CA. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoprotein IIb and/or IIIa. J Clin Invest. 1983;72:325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller BS, Beer JH, Scudder LE, Steinberg MH. Collagen-platelet interactions: evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIb/IIIa mediated by adhesive proteins. Blood. 1989;74:182–192. [PubMed] [Google Scholar]
- Darnell JE., Jr Variety in the level of gene control in eucaryotic cells. Nature. 1982;297:365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Du X, Plow EF, Frelinger AL, III, O'Toole TE, Loftus JC, Ginsberg MH. Ligands “activate” integrin αIIbβ3(platelet GPIIb-IIIa) Cell. 1991;65:409–416. doi: 10.1016/0092-8674(91)90458-b. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Jr, Martin GS. Tyrosine-specific protein phosphorylation is regulated by glycoprotein IIb-IIIa in platelets. Proc Natl Acad Sci USA. 1989;86:2234–2238. doi: 10.1073/pnas.86.7.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LA, Phillips DR. Calcium regulation of the platelet membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1985;260:11366–11374. [PubMed] [Google Scholar]
- Gawaz MP, Loftus JC, Bajt ML, Frojmovic MM, Plow EF, Ginsberg MH. Ligand bridging mediates integrin αIIbβ3(platelet GPIIB-IIIA) dependent homotypic and heterotypic cell–cell interactions. J Clin Invest. 1991;88:1128–1134. doi: 10.1172/JCI115412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JN, Caen JP, Nurden AT. Glanzmann's thrombasthenia: the spectrum of clinical disease. Blood. 1990;75:1383–1395. [PubMed] [Google Scholar]
- Golden A, Brugge JS, Shattil SJ. Role of platelet membrane glycoprotein IIb-IIIa in agonist-induced tyrosine phosphorylation of platelet proteins. J Cell Biol. 1990;111:3117–3127. doi: 10.1083/jcb.111.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich B, Lipfert L, Brugge JS, Shattil SJ. Tyrosine phosphorylation and cytoskeletal reorganization in platelets are triggered by interaction of integrin receptors with their immobilized ligands. J Biol Chem. 1993;268:15868–15877. [PubMed] [Google Scholar]
- Hamburger SA, McEver RP. GMP-140 mediates adhesion of stimulated platelets to neutrophils. Blood. 1990;75:550–554. [PubMed] [Google Scholar]
- Howe A, Aplin ARE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- Hynes RC. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jin Y, Dietz HC, Montgomery RA, Bell WR, McIntosh I, Coller B, Bray PF. Glanzmann thrombasthenia. Cooperation between sequence variants in Cis during splice site selection. J Clin Invest. 1996;98:1745–1754. doi: 10.1172/JCI118973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. Cooperation between soluble factors and integrin-mediated cell anchorage in the control of cell growth and differentiation. Bioessays. 1996;18:911–917. doi: 10.1002/bies.950181110. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyoshi Y, Dorschner A, Mallet AI, Christophers E, Schroder JM. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992;176:587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer N, Guichard J, Farcet J-P, Vainchenker W, Breton-Gorius J. Biosynthesis of major platelet proteins in human blood platelets. Eur J Biochem. 1987;164:189–195. doi: 10.1111/j.1432-1033.1987.tb11010.x. [DOI] [PubMed] [Google Scholar]
- Kouns WC, Jennings LK. Activation-independent exposure of the GPIIb/IIIa fibrinogen receptor. Thromb Res. 1991;63:343–354. doi: 10.1016/0049-3848(91)90137-l. [DOI] [PubMed] [Google Scholar]
- Kouns WC, Wall CD, White MM, Fox CF, Jennings LK. A conformation-dependent epitope of human platelet glycoprotein IIIa. J Biol Chem. 1990;265:20594–20601. [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991;115:887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipfert L, Haimovich B, Schaller MD, Cobb BS, Parsons JT, Brugge JS. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAKin platelets. J Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman S, Gilmore A, Burridge K, Gidwitz S, White GC., II Integrin-mediated activation of focal adhesion kinase is independent of focal adhesion formation or integrin activation. Studies with activated and inhibitory β3 cytoplasmic domain mutants. J Biol Chem. 1997;272:22538–22547. doi: 10.1074/jbc.272.36.22538. [DOI] [PubMed] [Google Scholar]
- Malik RK, Parsons JT. Integrin-dependent activation of the p70 ribosomal S6 kinase signaling pathway. J Biol Chem. 1996;271:29785–29791. doi: 10.1074/jbc.271.47.29785. [DOI] [PubMed] [Google Scholar]
- Modur V, Feldhaus MJ, Weyrich AS, Jicha DL, Prescott SM, Zimmerman GA, McIntyre TM. Oncostatin M is a proinflammatory mediator. In vivo effects correlate with endothelial cell expression of inflammatory cytokines and adhesion molecules. J Clin Invest. 1997;100:158–168. doi: 10.1172/JCI119508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal K, Lofquist AK, Watson JM, Morris JS, Price LK, Haskill JS. Adhesion and direct integrin engagement differentially regulate gene transcription, transcript stabilization and translation. Biochem Soc Trans. 1995;23:460–464. doi: 10.1042/bst0230460. [DOI] [PubMed] [Google Scholar]
- Morgenstern E. Ultracytochemistry of human blood platelets. Prog Histochem Cytochem. 1980;12:1–86. doi: 10.1016/s0079-6336(80)80006-4. [DOI] [PubMed] [Google Scholar]
- Newman, P.J., and M. Poncz. 1995. Inherited disorders of platelets. In The Metabolic and Molecular Bases of Inherited Disease. 7th edition. C.R. Scriver, A.L. Beaudet, W.S. Sly, D. Valle, J.B. Stanbury, J.B. Wyngaarden, and D.S. Fredrickson, editors. McGraw-Hill, Inc., New York. 3335–3366.
- Newman PJ, Gorski J, White GC, II, Gidwitz S, Cretney CJ, Aster RH. Enzymatic amplification of platelet-specific messenger RNA using the polymerase chain reaction. J Clin Invest. 1988;82:739–743. doi: 10.1172/JCI113656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Takimoto G, McKeithan TW. The candidate proto-oncogene Bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell. 1990;60:991–997. doi: 10.1016/0092-8674(90)90347-h. [DOI] [PubMed] [Google Scholar]
- Pan J, McEver RP. Regulation of the human P-selectin promoter by Bcl-3 and specific homodimeric members of the NF-κB/Rel family. J Biol Chem. 1995;270:23077–23083. doi: 10.1074/jbc.270.39.23077. [DOI] [PubMed] [Google Scholar]
- Papkoff J, Chen RH, Blenis J, Forsman J. P42 mitogen-activated protein kinase and p70 ribosomal S6 kinase are selectively phosphorylated and activated during thrombin-induced platelet activation and aggregation. Mol Cell Biol. 1994;14:463–472. doi: 10.1128/mcb.14.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RT, Schreiber SL. Translation control: connecting mitogens and the ribosome. Curr Biol. 1998;8:R248–R250. doi: 10.1016/s0960-9822(98)70152-6. [DOI] [PubMed] [Google Scholar]
- Phillips DR, Charo IF, Scarborough RM. GPIIb-IIIa: the responsive integrin. Cell. 1991;65:359–362. doi: 10.1016/0092-8674(91)90451-4. [DOI] [PubMed] [Google Scholar]
- Power CA, Clemetson JM, Clemetson KJ, Wells TNC. Chemokine and chemokine receptor mRNA expression in human platelets. Cytokine. 1995;7:479–482. doi: 10.1006/cyto.1995.0065. [DOI] [PubMed] [Google Scholar]
- Pursuit Trial Investigators. Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. N Engl J Med. 1998;339:436–443. doi: 10.1056/NEJM199808133390704. [DOI] [PubMed] [Google Scholar]
- Reverter JC, Béguin S, Kessels H, Kumar R, Hemker HC, Coller BS. Inhibition of platelet-mediated, tissue factor-induced thrombin generation by the mouse/human chimeric 7E3 antibody. Potential implications for the effect of c7E3 Fab treatment on acute thrombosis and clinical restenosis. J Clin Invest. 1996;98:863–874. doi: 10.1172/JCI118859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GJ, Hickey MJ, Chung DW, Hickstein DD. Circulating human blood platelets retain appreciable amounts of poly (A)+ RNA. Biochem Biophys Res Commun. 1989;160:705–710. doi: 10.1016/0006-291x(89)92490-x. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Horwitz AF. Adhesion-growth factor interactions during differentiation: an integrated biological response. Dev Biol. 1996;180:455–467. doi: 10.1006/dbio.1996.0319. [DOI] [PubMed] [Google Scholar]
- Savage B, Ruggeri ZM. Selective recognition of adhesive sites in surface-bound fibrinogen by glycoprotein IIb-IIIa on nonactivated platelets. J Biol Chem. 1991;266:11227–11233. [PubMed] [Google Scholar]
- Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–666. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- Scarborough RM, Rose JW, Hsu MA, Phillips DR, Fried VA, Campbell AM, Nannizzi L, Charo IF. Barbourin: a GPIIb-IIIa specific integrin antagonist from the venom of Sistrurus M. Barbouri. . J Biol Chem. 1991;266:9359–9362. [PubMed] [Google Scholar]
- Scarborough RM, Naughton MA, Teng W, Rose JW, Phillips DR, Nannizzi L, Arfsten A, Campbell AM, Charo IF. Design of potent and specific integrin antagonists. Peptide antagonists with high specificity for glycoprotein IIb-IIIa. J Biol Chem. 1993;268:1066–1073. [PubMed] [Google Scholar]
- Schmidt VA, Nierman WC, Maglott DR, Cupit LD, Moskowitz KA, Wainer JA, Bahou WR. The human proteinase-activated receptor-3 (PAR-3) gene. Identification within a PAR gene cluster and characterization in vascular endothelial cells and platelets. J Biol Chem. 1998;273:15061–15068. doi: 10.1074/jbc.273.24.15061. [DOI] [PubMed] [Google Scholar]
- Schulman SP, Goldschmidt-Clermont PJ, Topol EJ, Califf RM, Navetta FI, Willerson JT, Chandra NC, Guerci AD, Ferguson JJ, Harrington RA, et al. Effects of integrilin, a platelet glycoprotein IIb/IIIa receptor antagonist, in unstable angina. A randomized multicenter trial. Circulation. 1996;94:2083–2089. doi: 10.1161/01.cir.94.9.2083. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Toksoz D, Khosrari-Far R. Transformation by Rho exchange factor oncogenes is mediated by activation of an integrin-dependent pathway. EMBO (Eur Mol Biol Organ) J. 1996;15:6525–6530. [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ, Ginsberg MH. Integrin signaling in vascular biology. J Clin Invest. 1997;100:1–5. [PubMed] [Google Scholar]
- Shattil SJ, Brass LF, Bennett JS, Pandhi P. Biochemical and functional consequences of dissociation of the platelet membranes glycoprotein IIb-IIIa complex. Blood. 1985;66:92–98. [PubMed] [Google Scholar]
- Shattil SJ, Ginsberg MH, Brugge JS. Adhesive signaling in platelets. Curr Opin Cell Biol. 1994;6:695–704. doi: 10.1016/0955-0674(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, Kashiwagi H, Pampori N. Integrin signaling: the platelet paradigm. Blood. 1998;91:2645–2657. [PubMed] [Google Scholar]
- Sonenberg N, Gingras A-C. The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol. 1998;10:268–275. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- Thomas G, Hall MN. TOR signalling and control of cell growth. Curr Opin Cell Biol. 1997;9:782–787. doi: 10.1016/s0955-0674(97)80078-6. [DOI] [PubMed] [Google Scholar]
- Wang R, Shattil SJ, Ambruso DR, Newman PJ. Truncation of the cytoplasmic domain of β3 in a variant form of Glanzmann thrombasthenia abrogates signaling through the integrin αIIbβ3complex. J Clin Invest. 1997;100:2393–2403. doi: 10.1172/JCI119780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw AL, Laster L, Schulman NR. Protein synthesis by human platelets. J Biol Chem. 1967;242:2094–2097. [PubMed] [Google Scholar]
- Watson SP, Gibbins J. Collagen receptor signaling in platelets: extending the role of the ITAM. Immunol Today. 1998;19:260–264. doi: 10.1016/s0167-5699(98)01267-5. [DOI] [PubMed] [Google Scholar]
- Weyrich AS, Ma X-L, Lefer DJ, Albertine KH, Lefer AM. In vivo neutralization of P-selectin protects feline heart and endothelium in myocardial ischemia and reperfusion injury. J Clin Invest. 1993;91:2620–2629. doi: 10.1172/JCI116501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH, Prescott SM, Zimmerman GA. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyrich AS, Dixon DA, Pabla R, Elstad MR, McIntyre TM, Prescott SM, Zimmerman GA. Signal-dependent translation of a regulatory protein, Bcl-3, in activated human platelets. Proc Natl Acad Sci USA. 1998;95:5556–5561. doi: 10.1073/pnas.95.10.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MJ, Du X, Loftus JC, Ginsberg MH. Platelet adhesion receptors. Semin Cell Biol. 1995;6:305–314. doi: 10.1006/scel.1995.0040. [DOI] [PubMed] [Google Scholar]
- Zimmerman GA, McIntyre TM, Prescott SM. Adhesion and signaling in vascular cell–cell interactions. J Clin Invest. 1996;98:1699–1702. doi: 10.1172/JCI118967. [DOI] [PMC free article] [PubMed] [Google Scholar]