Abstract
CLIP-170 is a cytoplasmic linker protein that localizes to plus ends of microtubules in vivo. In this study, we have characterized the microtubule-binding properties of CLIP-170, to understand the mechanism of its plus end targeting. We show that the NH2-terminal microtubule-interacting domain of CLIP-170 alone localizes to microtubule plus ends when transfected into cells. Association of CLIP-170 with newly-formed microtubules was observed in cells microinjected with biotinylated tubulin, used as a tracer for growing microtubules. Using in vitro assays, association of CLIP-170 with recently polymerized tubulin is also seen. Cross-linking and sedimentation velocity experiments suggest association of CLIP-170 with nonpolymerized tubulin. We conclude from these experiments that the microtubule end targeting of CLIP-170 is closely linked to tubulin polymerization.
Keywords: CLIP-170, MAPs, microtubules, polymerization, targeting
The ability of cells to establish and maintain a specific organization of their cytoplasmic components is mainly dependent on their interphase microtubule network, which is involved in defining the position and mediating the transport of organelles (for review see Cole and Lippincott-Schwartz, 1995). Important for these functions are the dynamic properties of microtubules, which enable them to explore rapidly the cytoplasmic space and may allow selective stabilization in response to localized cues (Kirschner and Mitchison, 1986). The intrinsic assembly properties of tubulin are modulated by interactions with other proteins (for review see Mandelkow and Mandelkow, 1995; Desai and Mitchison, 1997) which are responsible for important differences seen between the assembly properties of microtubules in vitro and in vivo (Cassimeris et al., 1988; Sammak and Borisy, 1988; Schulze and Kirschner, 1988), and for their interaction with intracellular organelles (Rickard and Kreis, 1996; Goodson et al., 1997). Identification and characterization of these proteins are important for an understanding of the role of microtubules in cellular organization.
CLIP-170 has a localization to the plus ends of a subset of microtubules in vivo that is unique among microtubule-binding proteins (MBPs)1 so far described (Rickard and Kreis, 1990; Rickard, 1998) although another MBP, the APC protein, has a localization at plasma membrane sites which is dependent on intact microtubules (Näthke et al., 1996). CLIP-170 has been shown to be involved in binding of endocytic carrier vesicles to microtubules in vitro (Pierre et al., 1992), and has also been localized to prometaphase kinetochores (Dujardin et al., 1998). Mutational analysis of CLIP-170 showed that the NH2-terminal domain can bind to microtubules (Pierre et al., 1992, 1994). The COOH-terminal domain is important in targeting it to kinetochores in prometaphase cells (Dujardin et al., 1998) and in directing it to patchy structures which appear in interphase cells on overexpression (Pierre et al., 1994). Since early endosomes usually form near the plasma membrane close to microtubule plus ends, and prometaphase kinetochores establish interactions with microtubule plus ends, it is possible that targeting of CLIP-170 to microtubule plus ends may be determined by its interaction with these organelles.
An alternative mechanism for the plus end localization of CLIP-170 could be related to the assembly properties of tubulin alone. During microtubule assembly, the GTP bound to β-tubulin is hydrolyzed. Because this hydrolysis is not mechanistically coupled to assembly, there is formation of a small cap of terminal GTP- or GDP–Pi-bound tubulin subunits, conformationally distinct from the GDP polymer (Carlier and Pantaloni, 1981; Melki et al., 1990, 1996). Stochastic loss of this cap causes destabilization and rapid disassembly of a subpopulation of microtubules, a behavior termed dynamic instability (Mitchison and Kirschner, 1984a). Using the GTP analogue GMPCPP, which appears to stabilize the GTP conformation of the tubulin lattice (Vale et al., 1994; Hyman et al., 1995), it has been shown that kinesin moves microtubules polymerized with GMPCPP more quickly than microtubules polymerized with GTP (Vale et al., 1994), and kinetochores bind preferentially to GMPCPP-microtubules (Severin et al., 1997), suggesting that MBPs may distinguish between the different conformations of the polymer. This property could allow targeting of an MBP to the elongating end. Alternatively, interaction of MBPs with tubulin subunits or oligomers before assembly has been suggested as a mechanism for increasing the polymerization rate (Erickson and Pantaloni, 1981; Carlier et al., 1984; Drechsel et al., 1992; Pedrotti and Islam, 1994). In the case of XMAP215, a protein purified from Xenopus oocytes, increase in the polymerization rate is restricted specifically to microtubule plus ends (Gard and Kirschner, 1987; Vasquez et al., 1994), the main assembly site in vivo. Such an interaction would also promote association of the MBP preferentially at the elongating end. Localization of an MBP to the plus ends of microtubules by either of these mechanisms could allow both regulation of assembly of microtubules and promotion of interaction of microtubule ends with cytoplasmic organelles.
In this study, we have examined the association of CLIP-170 with microtubules, to characterize the basis for its in vivo localization. We have found that the NH2-terminal microtubule-binding domain of CLIP-170 alone can localize to microtubule plus ends in cells, indicating that interactions of the tail domain with other structures are not essential for its correct targeting. We show that the association of CLIP-170 with the plus ends of microtubules is coordinated with polymer assembly, since the protein localizes preferentially to growing microtubules, both in vivo and in vitro. Finally, we provide evidence suggesting that of the two possible mechanisms for the plus end targeting of the protein that we considered, the more likely is copolymerization of CLIP-170 with tubulin oligomers, rather than specific recognition of a transient conformational cap at the ends of microtubules. The specific localization of CLIP-170 with the dynamic plus ends of microtubules raises the possibility that it may assist microtubules to explore the cellular space, helping them to find and capture organelles for subsequent transport.
Materials and Methods
Materials
Paclitaxel was a gift of the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment, National Cancer Institute (Bethesda, MD). Nocodazole (Sigma) and paclitaxel were stored as stock solutions in DMSO at −20°C. GTP and guanosine-5′- O-(3-thiotriphosphate) tetralithium salt (GTPγS) came from Boehringer Mannheim and were stored as stock solutions in water at −20°C. MES was purchased from Calbiochem-Novabiochem. Pipes, EGTA, DTT, and SDS were from Sigma. Guanylyl-(α,β)-methylene-diphosphonate (GMPCPP) was a gift of A. Hyman (European Molecular Biological Laboratory, Heidelberg, Germany). All other reagents were of analytical grade.
Cell Culture and Transfections
HeLa cells were grown as described (Allan and Kreis, 1986) except that 10% FCS was used. HeLa cells were transfected with cDNA encoding the first 350 amino acids of CLIP-170 (H1) tagged at its NH2 terminus with the myc epitope (Pierre et al., 1994) in the eukaryotic expression vector pSG5 (Green et al., 1988) using calcium phosphate as described (Pierre et al., 1994). Cells in a 60-mm dish were transfected with 5 μg of DNA for 12 h and then fixed immediately after washing; the short transfection time was necessary to avoid overexpression of the protein that leads to loss of its microtubule end localization.
Protein Purification
For all the in vitro experiments except the analytical centrifugation, GDP-tubulin was produced as described by Hyman et al. (1991). In brief, tubulin prepared from pig brain by two cycles of temperature-dependent polymerization followed by phosphocellulose chromatography was polymerized, sedimented, and then the pellet was depolymerized in 80 mM K Pipes, 1 mM EGTA, 1 mM MgCl2, pH 6.8 (BRB80 buffer), in the absence of free nucleotide to give GDP-bound tubulin (Hyman et al., 1991) which was stored at −80°C. After thawing, this tubulin was incubated with GTP or other nucleotide analogues as described in Results. Alternatively, for the analytical centrifugation experiments, GTP-tubulin was prepared from pig brain as described by Melki et al. (1996); after the final gel filtration into 100 mM K Pipes, 1 mM EGTA, 3 mM MgCl2, pH 6.9 (P buffer), fractions containing tubulin were pooled and tubulin and GTP concentrations were adjusted to 10 and 50 μM, respectively. GDP-tubulin was prepared by polymerizing this tubulin at 37°C, pelleting the microtubules at 300,000 g for 15 min at 30°C, disassembling the polymers in P buffer at 4°C and clarifying this solution by ultracentrifugation. The final concentrations of tubulin and GDP were 10 and 50 μM, respectively.
Purification and characterization of H1 and H2 recombinant proteins will be reported elsewhere (Scheel, J., P. Pierre, J.E. Rickard, G.S. Diamantopoulos, C. Valetti, F.G. van der Goot, M. Häner, U. Aebi, and T.E. Kreis, manuscript in preparation). In brief, the cDNAs encoding H1 and the first 481 amino acids of CLIP-170 (H2) were cloned in the pET19 vector (Novagen) for expression in bacteria with an NH2-terminal histidine tag. The histidine-tagged fusion proteins were purified from bacterial lysates using nickel chelate chromatography according to the Novagen protocol. The cDNA encoding MAP2C cloned in the pET3d vector (Novagen) was kindly provided by A. Matus (Friedrich Miescher Institute, Basel, Switzerland). The protein expressed in bacteria was purified using its property of heat stability (Takeuchi et al., 1992). In brief, the bacterial lysates were boiled for 5 min and then left on ice for another 10 min. Heat-stable MAP2C remained in the supernatant after centrifugation of the boiled sample at 90,000 g for 30 min and was then concentrated, followed by dialysis.
Porcine brain MAP2 was prepared according to Drubin and Kirschner (1986) with a slight modification at the gel filtration step, which was performed on Sepharose CL-4B using an XK16 (16-mm diam, 1-m length) column (Pharmacia) pumped at a rate of 0.15 ml/min to separate the high molecular mass MAP2 from the lower molecular mass tau protein. Native CLIP-170 was purified from human placenta as described elsewhere (Diamantopoulos et al., 1998). In brief, a monoclonal antibody affinity column (Rickard and Kreis, 1991) was used to isolate CLIP-170 from a high speed supernatant of human placenta and the protein was further purified and concentrated by a microtubule binding step. H1, H2, MAP2C, MAP2 and CLIP-170 were all dialyzed overnight against BRB80 buffer and stored at −80°C before use. All proteins were analyzed on SDS-PAGE using the Laemmli buffer system (Laemmli, 1970).
Preparation of Rhodamine and Biotin-labeled Tubulin
Tubulin was labeled with rhodamine (rh) or biotin (bt) as described by Hyman et al. (1991), using 5- (and-6) -carboxy-X-rhodamine, succinimidyl ester (C-1309) and biotin-XX, succinimidyl ester (B-1606), both purchased from Molecular Probes. Labeled tubulin was stored at −80°C in a GDP-bound form; GTP or analogues as described were added immediately after thawing. Rh-tubulin was diluted to the concentrations indicated in BRB80 buffer for in vitro assembly experiments. Bt-tubulin, used for the microinjection experiments, was diluted to 1 mg/ml in 50 mM potassium glutamate, 0.5 mM glutamic acid, 0.5 mM MgCl2, pH 6.5 (microinjection buffer).
Microtubule Regrowth Off Centrosomes
Centrosomes were purified from a human lymphoblast cell line (KE37) as described by Bornens et al. (1987). Polymerization of microtubules from centrosomes was performed according to the method of Mitchison and Kirschner (1984b) with some modifications. Tubulin at a concentration of 1 mg/ml in BRB80 plus 1 mM GTP was polymerized from centrosomes at 37°C for 9 min before a second addition of tubulin pre-equilibrated with GTP, GTPγS, or GMPCPP. For this pre-equilibration, GDP-tubulin, in some cases labeled with rhodamine, was incubated for 15 min at 0°C with 5 mM GTP, 12 mM GTPγS, or 0.6 mM GMPCPP; dilution into the polymerization reaction gave final concentrations of 0.3 mg/ml (GTP- or GTPγS-tubulin) or 0.1 mg/ml (GMPCPP-tubulin) additional tubulin and 1.2 mM GTP, 2 mM GTPγS or 0.1 mM GMPCPP. For the latter two analogues, 1 mM GTP remains in the reaction from the first incubation. The affinity of tubulin for these analogues compared with GTP is approximately twofold less for GTPγS (Roychowdhury and Gaskin, 1986) and four- to eightfold less for GMPCPP (Hyman et al., 1992). A low concentration of GMPCPP was used because higher concentrations were found to promote strong self nucleation of microtubule assembly in the absence of H2; however, it should be noted that there will be significant exchange for GTP on the soluble tubulin, leading to incorporation of both GDP and GMPCPP into the polymer. After a 3-min incubation, H2, CLIP-170, or MAP2 was added at the concentrations indicated in the figure legends. After a further incubation of 3 min, the samples were fixed in 0.25% glutaraldehyde at room temperature for 4 min. For the simultaneous addition of tubulin and H2 described in Fig. 6 (e and f), microtubule asters were polymerized with 1 mg/ml tubulin in the presence of 1 mM GTP at 37°C for 9 min. Tubulin (0.3 mg/ml) and H2 (50 μg/ml) were preincubated at 37°C for 2 min and then diluted fivefold into the asters. The samples were fixed 2 min later in glutaraldehyde. All samples were then sedimented onto coverslips as described by Mitchison and Kirschner (1984b) before immunofluorescence labeling.
Figure 6.
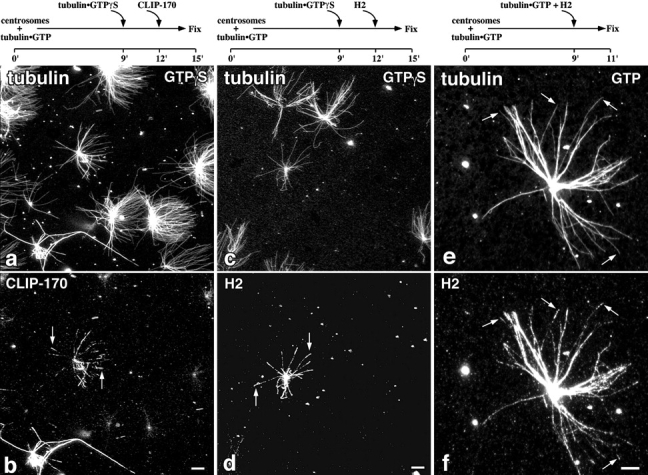
Effect of GTPγS-tubulin or preincubation of H2 with GTP-tubulin on H2 localization. (a–d) Microtubule asters were made as described in the legend to Fig. 4, except that the second tubulin addition was of unlabeled tubulin (0.3 mg/ml) equilibrated with GTPγS. The final GTPγS concentration was 2 mM. After a further 3 min of incubation, 18 μg/ml of CLIP-170 (a and b) or 40 μg/ml of H2 (c and d) were added. 3 min later the asters were fixed and labeled for tubulin (a and c), CLIP-170 (b), or H2 (d) as described for Fig. 4. Arrows in b and d indicate plus ends of the centrosomal microtubules that are labeled for CLIP-170 and H2, respectively. (e and f) Microtubule asters were polymerized from centrosomes with GTP-tubulin for 9 min. Separately, GTP-tubulin (∼0.3 mg/ml) and H2 (50 μg/ ml) were preincubated at 37°C for 2 min and then diluted fivefold into the asters. The samples were fixed 2 min later, sedimented onto coverslips and labeled for H2 (e) or tubulin (f). Arrows, plus ends of the centrosomal microtubules that are labeled with H2; only a small fraction of asters exhibited this labeling pattern (quantitated in Table III). Experimental time lines are indicated above relevant panels. Bar, 10 μm.
Microinjection
Glass capillary microinjection of bt-tubulin into cells was performed with an automated microinjection system (Zeiss) as described elsewhere (Pepperkok et al., 1993) in 30-mm dishes containing 2 ml of MEM without carbonate, supplemented with nonessential amino acids, 1% glutamine, 10% FCS, and then buffered with 10 mM Hepes, pH 7.4. The cells were microinjected at room temperature during 3 min, immediately permeabilized in 80 mM K Pipes, 5 mM EGTA, 1 mM MgCl2, 0.5% Triton X-100, pH 6.8, as described (Kreis, 1987), and then fixed in methanol at −20°C for 5 min. Only the last cells injected before fixation exhibited short bt-tubulin segments and were used for analysis.
Immunofluorescence
The following antibodies were used for immunofluorescence labeling: monoclonal anti-myc, 9E10 (Evan et al., 1985), for labeling of HeLa cells transfected with myc-tagged mutated CLIP-170; a mixture of two monoclonal antibodies against CLIP-170, 2D6 and 4D3 (Rickard and Kreis, 1991) for labeling of microinjected HeLa cells; monoclonal and rabbit polyclonal antibodies against tyrosinated α-tubulin, 1A2, and αT-13, respectively (Kreis, 1987); a rabbit peptide antibody against CLIP-170, αKRKV (Pierre et al., 1992), and a monoclonal antibody recognizing MAP2, M3A5 (Allan and Kreis, 1986). FITC-streptavidin was from Pierce. Coverslips with labeled cells or in vitro polymerized microtubules were mounted in Mowiol on glass slides before analysis using the 63 or 100× Planapo objective on a Zeiss inverted fluorescence microscope (model Axiovert TV 135). Images were recorded with a cooled charge-coupled device camera (model CH250, 1,317 × 1,035 pixels; Photometrics), controlled by a Power Macintosh 8100/100 (Apple). Images were recorded with the software package IPLab spectrum V2.3 (Signal Analytics) and processed using Adobe Photoshop 3.0 (Adobe Systems).
Cross-linking Experiments
Reactions containing proteins (2.5 μM) as indicated in Fig. 7 were mixed on ice in BRB80 buffer containing 1 mM GTP before addition of the cross-linker 1-ethyl-3-(3-[dimethyl-amino]propyl) carbodiimide as previously reported (Song and Mandelkow, 1993). Tubulin was cycled immediately before use in this assay, and both cycled tubulin and other proteins were centrifuged at 150,000 g for 20 min to remove aggregates before cross-linking. After 2 h on ice, reactions were quenched by the addition of 10 mM hydroxylamine followed by dilution in standard SDS sample buffer containing DTT, and separated by SDS-PAGE using the Laemmli buffer system (Laemmli, 1970) except that the separating gel buffer was at pH 9.5 to maximize separation of the tubulin subunits (Melki et al., 1991). Cross-linked products were detected with specific antibodies against H1 (anti-KRKV and anti-GSIK; Pierre et al., 1992), α-tubulin (1A2), or β-tubulin (JDR.3B8; Sigma) followed by horseradish peroxidase-labeled second antibodies and enhanced chemiluminescence detection (Amersham).
Figure 7.
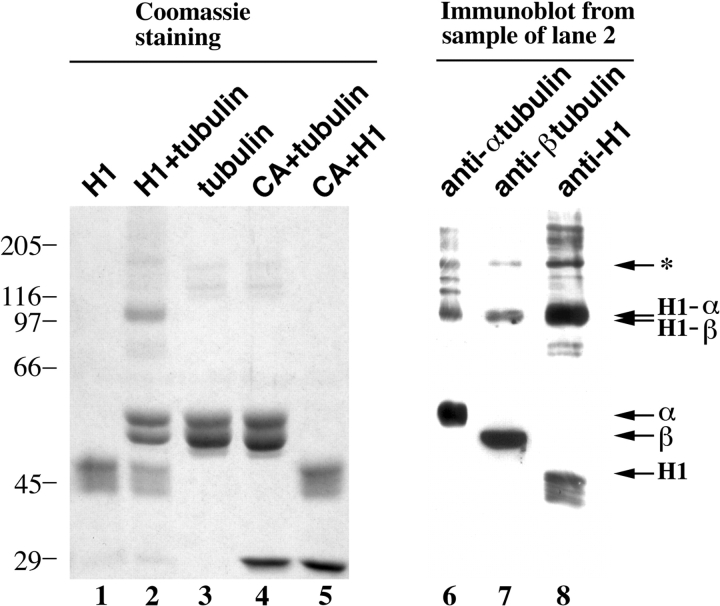
CLIP-170 H1 fragment can be cross-linked to tubulin dimer in vitro. H1, tubulin and carbonic anhydrase, each at 2.5 μM, incubated alone and together, were cross-linked as described in Materials and Methods before separation of the cross-linked products by SDS-PAGE. The Coomassie-stained gel shows cross-linked H1 alone (lane 1), H1 + tubulin (lane 2), tubulin alone (lane 3), tubulin + carbonic anhydrase (CA, lane 4), and H1 + carbonic anhydrase (lane 5). Lanes 6–8 are immunoblots of the sample shown in lane 2 probed with antibodies specific for: α-tubulin (lane 6), β-tubulin (lane 7), and H1 (lane 8). Arrows, relative positions of uncross-linked α-tubulin, β-tubulin, and H1 fragment, as well as cross-linked apparent α-H1, β-H1. Asterisk, higher mol wt cross-linked products which could correspond to trimers of H1–α-β-tubulin and/or H1–α-α-tubulin plus H1– β-β-tubulin. Numbers at the left, position of molecular weight standards (10−3).
Sedimentation Velocity
Sedimentation velocity experiments were carried out with a Beckman Optima XL-A analytical ultracentrifuge equipped with an 60 Ti four-hole rotor and cells with two-channel 12-mm path length centerpieces. Samples in 400 μl P buffer were centrifuged either at 25,000 or 60,000 rpm at 4°C. Radial scans of absorbance at 290 nm were taken at 10-min intervals. The total absorbance of the sample was measured at the start of the run, and values for each sedimenting species expressed as a percentage of this value. Data were analyzed to provide the apparent distribution of sedimentation coefficients by means of the programs DCDT (Stafford, 1992) and SVEDBERG (Philo, 1994).
Results
CLIP-170 Associates with Growing Microtubule Ends In Vivo
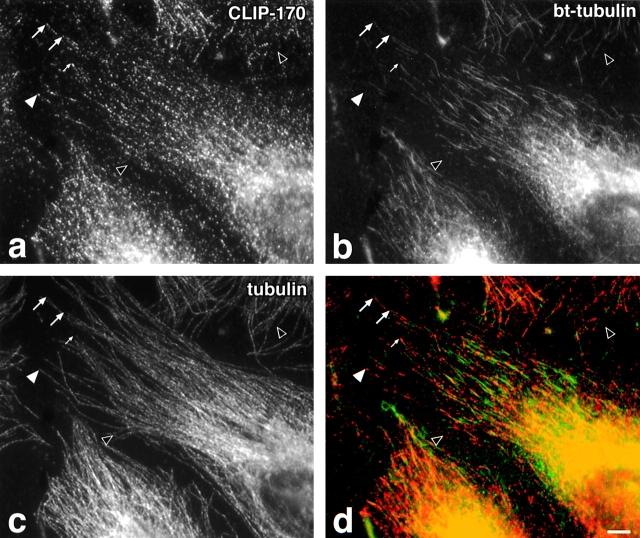
To investigate whether CLIP-170 associates with the subset of growing microtubule ends, bt-tubulin, a tracer for recently assembled microtubules (Schulze and Kirschner, 1986), was microinjected into HeLa cells at room temperature, the cells were fixed within 3 min, and then triple-labeled for CLIP-170, bt, and total tubulin (Fig. 1). Since it is difficult to find individual microtubules in the centrosomal area, we only examined the microtubules at the periphery of the cell, where their plus ends are more distinct (Fig. 1 c). We quantified the labeling pattern on these microtubules in 20 cells, and found that 61% of the microtubules had polymerized bt-tubulin at their plus ends (Fig. 1 b, summarized in Table I). With its characteristic patchy distribution at the plus ends of microtubules (Rickard and Kreis, 1990), two types of labeling for CLIP-170 on microtubules (Fig. 1 a) could be distinguished: labeling along a significant length, or a small dot of labeling at the tip. Quantification revealed that more than 90% of the bt-positive microtubules were also positive for CLIP-170 (Table I). Approximately 60% of these microtubules showed a dotty labeling of CLIP-170 along a significant length (Fig. 1, large arrows), whereas 34% exhibited only a single dot labeling of CLIP-170 at their plus end (Fig. 1, small arrow) and 6% had no CLIP-170 (Table I). The majority (63%) of the bt-negative microtubules was not labeled for CLIP-170 (Fig. 1, black arrowheads; Table I). Of the remaining bt-negative microtubules, 10 and 26.4% showed extended CLIP-170 labeling and single dot labeling (Fig. 1, white arrowhead) at their plus ends, respectively (Table I). The high correlation of CLIP-170 labeling with bt-labeled microtubule ends seen in this experiment suggests that CLIP-170 marks growing microtubules in vivo.
Figure 1.
Colocalization of CLIP-170 with newly polymerized bt-tubulin microinjected into HeLa cells. HeLa cells were microinjected at room temperature during 3 min with bt-tubulin (∼1 mg/ml in microinjection buffer) and then pre-extracted with detergent before fixation in methanol at −20°C for 5 min. Cells were labeled for (a) CLIP-170 using two mouse monoclonal antibodies followed by Cy3 anti-mouse, (b) bt-tubulin using FITC-streptavidin, and (c) total tubulin using a rabbit anti-tyrosinated tubulin followed by Cy5 anti-rabbit. (d) An overlay of a, in red, and b, in green. Large arrows, microtubule ends where CLIP-170 colocalizes with bt-tubulin over an extended region. Small arrow, bt-positive microtubule with labeling for CLIP-170 only at the tip. Closed arrowhead, microtubule plus end that is positive for CLIP-170 but not for bt; open arrowheads, ends that are negative for both bt-tubulin and CLIP-170. Bar, 10 μm.
Table I.
Codistribution of CLIP-170 at the Plus Ends of Microtubules with Microinjected Bt-Tubulin
| Biotin-labeled microtubules 61% | Biotin-free microtubules 39% | |||
|---|---|---|---|---|
| % | % | |||
| CLIP-170 labeled | 94.4 | 37.1 | ||
| Extended labeling | 60.4 | 10.7 | ||
| Tip labeling | 34.0 | 26.4 | ||
| No labeling | 5.6 | 62.9 |
HeLa cells were injected for a period of 3 min with bt-tubulin, fixed, and then labeled for bt, tubulin, and CLIP-170 as described in Fig. 1. 218 microtubules with distinct plus ends, in 23 cells from a total of three experiments, were quantified for bt incorporation and CLIP-170 labeling. 61% of microtubules had incorporated bt. CLIP-170 labeling was classified as extended, tip labeling, or no labeling (for details see Fig. 1 and text).
H1, the NH2-terminal Microtubule-binding Domain of CLIP-170, Localizes to the Plus Ends of Microtubules In Vivo
We have shown previously that CLIP-170 transiently overexpressed in cells formed large patches, whereas mutant protein with the COOH-terminal tail domain deleted bound along the length of microtubules, suggesting a role for the COOH-terminal sequence in defining its localization (Pierre et al., 1994). To establish whether the microtubule plus end–targeting function of CLIP-170 resides in the microtubule-binding domain, we reinvestigated the localization of this domain at lower levels of expression. H1, a construct encoding the first 350 amino acids of CLIP-170 and lacking any coiled-coil sequence, was transfected into HeLa cells. At low expression levels, H1 clearly localized to the plus ends of a subset of the microtubules (Fig. 2, arrows), exhibiting a distribution very similar to endogenous CLIP-170 (Rickard and Kreis, 1990). It is important for observation of this distribution to fix cells within 12 h of transfection, when most of the cells have a low level of expression of the transfected protein. This is a much shorter time than used previously (24 h), when H1 was expressed at higher levels and was found to bind along the length of microtubules (Pierre et al., 1994). Since H1 expressed and purified from bacteria is monomeric (Scheel, J., P. Pierre, J.E. Rickard, G.S. Diamantopoulos, C. Valetti, F.G. van der Goot, M. Häner, U. Aebi, and T.E. Kreis, manuscript in preparation), it should be unable to dimerize with endogenous CLIP-170, and should therefore behave independently of endogenous protein. The dimeric protein, missing just the COOH terminus, was also found to localize to microtubule plus ends at low expression levels (data not shown), and both mutants labeled along the length of microtubules at higher expression levels (Pierre et al., 1994). We conclude that targeting of CLIP-170 to microtubule plus ends derives from the NH2-terminal domain alone.
Figure 2.
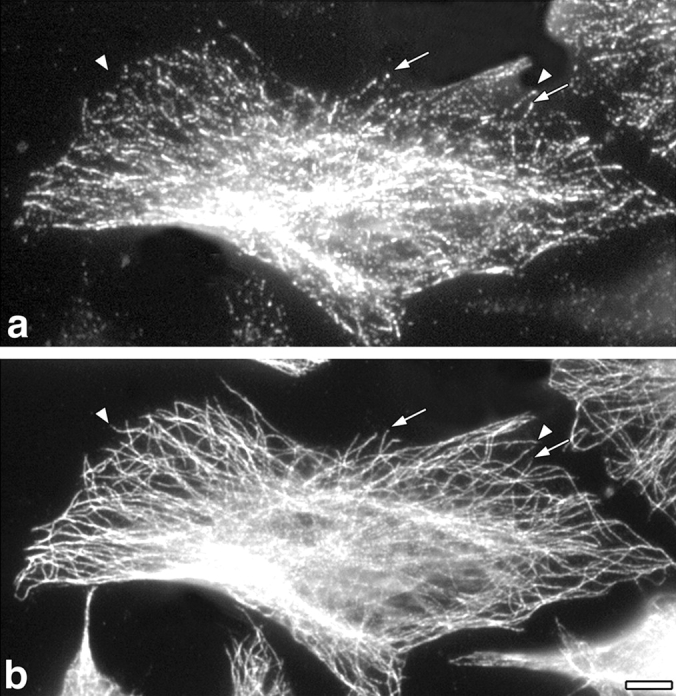
The head domain of CLIP-170, H1, localizes to microtubule plus ends in HeLa cells. HeLa cells were transfected with a pSG5 construct encoding the head domain of CLIP-170 (H1) tagged with the myc epitope. 12 h after transfection, the cells were fixed in cold methanol without pre-extraction and the myc tag and tubulin were localized by indirect immunofluorescence. (a) H1 labeling visualized by a monoclonal anti-myc antibody (9E10) and (b) tubulin labeling using a polyclonal anti-tubulin (αT-13). Arrows and arrowheads, microtubule ends with or without myc labeling, respectively. Bar, 10 μm.
CLIP-170 Binds to Newly Polymerized Microtubules Formed In Vitro in the Presence of GMPCPP or GTP
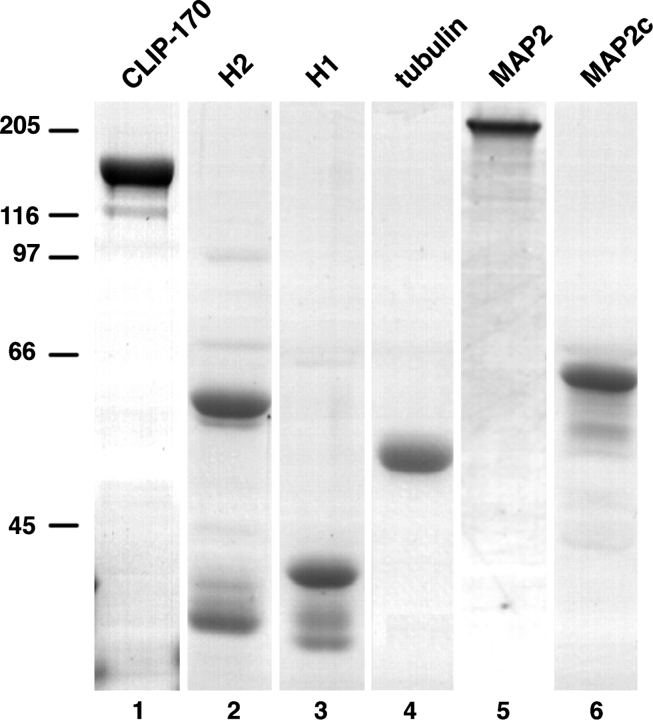
We considered two possible mechanisms related to microtubule assembly that may account for targeting of CLIP-170 to the plus ends of microtubules: the protein may have a higher affinity for the GTP conformation of tubulin at the polymerizing ends of the polymer (Vale et al., 1994; Severin et al., 1997) or it may interact with and stabilize precursors of assembly to copolymerize with them, as has been suggested for other microtubule-associated proteins (MAPs; Burns and Islam, 1984; Carlier et al., 1984; Pedrotti and Islam, 1994; Vasquez et al., 1994). Either of these mechanisms could target CLIP-170 to microtubule ends, although it is likely that phosphorylation of CLIP-170 (Rickard and Kreis, 1991) plays a role in maintaining its distribution in cells. To examine these mechanisms, we used an assay in which microtubules are assembled from centrosomes, and the distribution of CLIP-170 along the polymer is visualized by immunofluorescence. We used native CLIP-170 purified from human placenta, as well as a bacterially expressed protein, H2, encoding the head domain plus a short region of coiled coil. Although H1 can target to microtubule plus ends, we preferred to use H2 because it is dimeric (Scheel, J., P. Pierre, J.E. Rickard, G.S. Diamantopoulos, C. Valetti, F.G. van der Goot, M. Häner, U. Aebi, and T.E. Kreis, manuscript in preparation) as is native CLIP-170 (Pierre et al., 1992). Representative examples of purified proteins used in these experiments analyzed by SDS-PAGE are shown in Fig. 3. CLIP-170 (Fig. 3, lane 1) was purified from human placenta to apparent homogeneity (Diamantopoulos et al., 1998). Bacterially expressed H2, H1, and MAP2C (Fig. 3, lanes 2, 3, and 6 respectively) preparations contained some degradation products, together with a low amount of contaminating bacterial protein at a mol wt ∼70,000 D, which does not bind to microtubules (data not shown). Porcine brain tubulin (Fig. 3, lane 4) and MAP2 (Fig. 3, lane 5) were homogeneous. The experimental time line for production of microtubule asters is summarized at the top of Fig. 4. Asters were polymerized using GTP-tubulin for 9 min and then supplemented with a small amount of tubulin equilibrated with either GTP or GMPCPP. Rh-tubulin was used for this second addition, to trace subsequent polymerization. After a further 3 min of incubation, H2 was added and the asters were fixed 3 min later.
Figure 3.
Purified proteins analyzed by SDS-PAGE. CLIP-170 purified from human placenta (lane 1), bacterially expressed H2 (lane 2), H1 (lane 3), and MAP2C (lane 6), and porcine brain tubulin (lane 4) and MAP2 (lane 5) were separated on reducing 8% SDS-polyacrylamide gels and stained with Coomassie blue. Numbers at the left, position of molecular weight standards (10−3).
Figure 4.
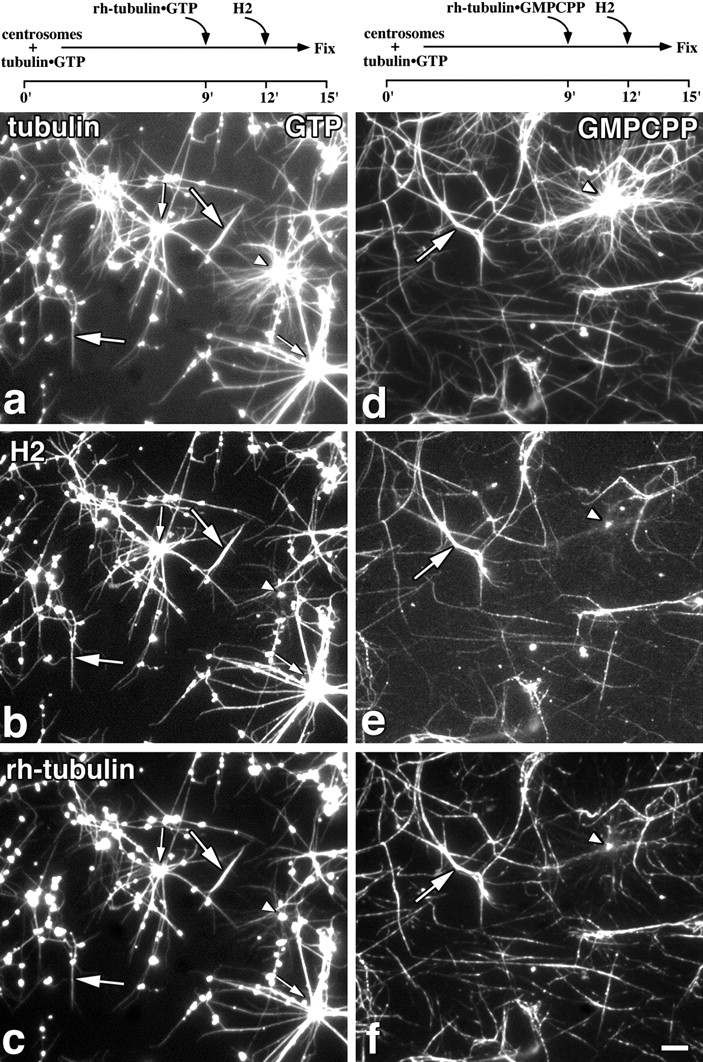
CLIP-170 binds preferentially to newly polymerized microtubules in vitro. Tubulin (∼1 mg/ml) was polymerized from centrosomes in the presence of 1 mM GTP for 9 min before a second addition of rh-tubulin, at 0.3 mg/ml equilibrated with GTP (a–c) or at 0.1 mg/ml equilibrated with GMPCPP (d–f). The final nucleotide concentrations were 1.2 mM GTP for a–c and 1.0 mM GTP, 0.1 mM GMPCPP for d–f. After a further 3 min of incubation, H2 was added at 40 μg/ml and the asters were fixed 3 min later (experimental time line above relevant panels). The fixed microtubules were sedimented onto coverslips as described in Materials and Methods and labeled for tubulin (a and d) using a monoclonal antibody (1A2) or H2 (b and e) using an anti-peptide antibody (anti-KRKV). Rh-tubulin is shown in c and f. Arrowheads, asters that have not incorporated rh-tubulin, and are not labeled for H2; small and large arrows, rh-labeled asters and rh-labeled free microtubules, respectively, which are also labeled for H2. Bar, 10 μm.
In control experiments, where GTP–rh-tubulin was added after 9 min and the asters fixed after 12 or 15 min, the rh labeling showed that the fraction of asters with elongated microtubules increases with time (summarized in Table II). No microtubules were seen with rh labeling along their length, indicating that very little new nucleation from the centrosomes occurs after the second addition of tubulin. If H2 is added at t = 12 min (Fig. 4, a–c), the fraction of asters incorporating rh-tubulin at their microtubule ends at the time of fixation (t = 15 min) is less than for the control (Table II) and there is an additional number of asters with rh-tubulin along their length (Fig. 4 c, small arrows), suggesting nucleation of new microtubules. There is also noticeable nucleation of free microtubules containing rh-tubulin (Fig. 4 c, large arrows). As a control, the effect of MAP2 was also tested in this assay; in this case, rh-tubulin was only found incorporated at the ends of microtubules (data not shown). In this case a higher proportion of asters incorporated rh-tubulin compared with the control (Table II), presumably due to the assembly promoting effect of MAP2 (Bré and Karsenti, 1990). The nucleation of new assembly both spontaneously and from centrosomes, therefore, appears to be a property of H2 but not MAP2.
Table II.
The Effect of H2 and MAP2 on the Sites of Incorporation of Tubulin into Microtubules
| Experiment | Percent of asters in- corporating rh-tubulin | Formation of free microtubules‡ | ||||
|---|---|---|---|---|---|---|
| At ends | Along length | |||||
| % | % | |||||
| 1 Fix t = 12′ | 40 | 0 | − | |||
| 2 . . . . . . . . . . . . . Fix t = 15′ | 78* | 0 | − | |||
| 3 H2 t = 12′ Fix t = 15′ | 31 | 32 | +++ | |||
| 4 MAP2 t = 12′ Fix t = 15′ | 92 | 0 | − | |||
Centrosomes were incubated with GTP-tubulin at 1 mg/ml for 9 min before addition of rh-GTP–tubulin to final 0.3 mg/ml. For experiments 3 and 4, H2 or MAP2 were added at 12 min to final concentrations of 40 or 17 μg/ml, respectively. Asters were fixed at times indicated before analysis as described for Fig. 4.
Much longer segments of end labeling compared to experiment 1.
Cannot be quantitated, this is a qualitative assessment.
When the distribution of H2 was examined in these experiments, it was found all along the length of microtubules, but on only a subset of asters, with a significant number of asters remaining negative for H2 (Fig. 4 a). Quantitation showed that H2 associates with all the asters that contain rh-tubulin but only 30% of those asters that do not contain rh-tubulin. The free microtubules, the assembly of which is promoted by H2 (Table II), were positive for both H2 and rhodamine (Fig. 4, a–c, large arrows). This suggested that H2 was associating preferentially with more recently polymerized microtubules. It also suggested that a fraction of the centrosomes is less active (Mitchison and Kirschner, 1984b) and nucleates assembly only after addition of further tubulin and H2. The morphology of the asters that were labeled with H2 (Fig. 4, a and b, small arrows) was different from those not labeled for the protein (Fig. 4, a and b, arrowheads). The latter apparently contained many more centrosomal microtubules, which appear very fine and are probably single microtubules, whereas aster microtubules labeled for H2 appear more bundled (Fig. 4, a and b). The reason for the bundling of microtubules in the presence of H2 is not clear, but we also observe it with the monomeric H1 (data not shown), so it does not depend on dimerization of the protein. Observation of the rh-tubulin (Fig. 4 c) shows that the asters with fine microtubules were polymerized before the second addition of tubulin, whereas the more bundled asters labeled for H2 also contain rh-tubulin (Fig. 4, a–c, small arrows). There is also noticeable formation of aggregates of H2 and tubulin, although the amount varied between different experiments, as well as an increase in the formation of free microtubules compared with the control with no H2 (Table II). Formation of these aggregates may be related to oligomer formation (see below). Labeling for H2 only at the ends of microtubules was never seen in these experiments; this experiment, therefore, did not mimic the behavior of CLIP-170 in cells. However, a conformational cap on the microtubules may be very small under these polymerization conditions (Walker et al., 1991). To test further the possibility that CLIP-170 may be targeted to the cap, we therefore repeated the experiment using tubulin equilibrated with a slowly hydrolyzable analogue of GTP, GMPCPP (Hyman et al., 1992, 1995; Vale et al., 1994), as the second tubulin addition in the hope of creating an extended GTP cap.
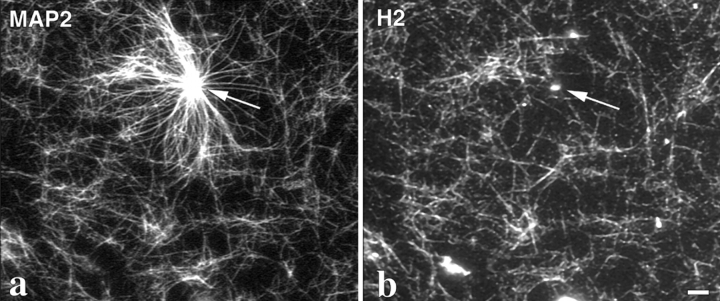
Preliminary experiments in which rh-tubulin complexed with GMPCPP was added to preformed GDP-microtubule asters showed that the rh-tubulin capped the ends of the existing microtubules (data not shown). However, when this was followed by addition of H2, there was a striking change in the appearance of the microtubules compared with the experiment with GTP-tubulin. There was a reduction of 85% in the number of asters seen after GMPCPP-tubulin addition (Fig. 4 d) relative to the experiment with GTP (Fig. 4 a). There was also a noticeable increase in the number of non-centrosomal microtubules (Fig. 4, a and d; summarized in Table III). The rh-tubulin signal (Fig. 4 f) showed that these free microtubules were more recently polymerized, whereas the asters were negative for rh-tubulin. This free nucleation is consistent with the strong nucleation-promoting activity of GMPCPP (Hyman et al., 1992), but control experiments show that it is enhanced in the presence of H2 (data not shown), as was also found with GTP-tubulin (Table II). H2 was found preferentially associated with these free microtubules formed in the presence of GMPCPP and was excluded from the asters (Fig. 4 e). As a control for whether the non-uniform labeling by H2 of the microtubule population seen in these experiments could be due to an artefact of our mixing protocol, and also to determine whether this could be a general property of MAPs, we repeated this experiment with a mixture of H2 and MAP2. MAP2 bound both to individual microtubules positive for H2 and to the asters presumably formed before the GMPCPP-tubulin addition (Fig. 5 a, arrow), whereas H2 labeled only the free microtubules (Fig. 5 b). This result excludes the possibility of mixing artefacts, and shows that the preference of H2 for these free microtubules is real and is a property not shared by MAP2.
Table III.
The Effect of GTP Analogues on Sites of Binding of H2 on Microtubules
| Experiment | Free microtubules* | Percent of asters labeled for H2 | Percent of free microtubules labeled for H2 | |||||
|---|---|---|---|---|---|---|---|---|
| At ends | Along length | |||||||
| 1 GTP-tubulin‡ | +++ | 0 | 32 | 100 | ||||
| 2 GMPCPP-tubulin | ++++++ | 0 | 0 | 100 | ||||
| 3 GTPγS-tubulin | − | 9 | 17 | NA§ | ||||
Centrosomes were incubated with GTP-tubulin at 1 mg/ml for 9 min before addition of tubulin equilibrated with the analogues shown to final 0.3 mg/ml (GTP- or GTPγS-tubulin) or 0.1 mg/ml (GMPCPP-tubulin). H2 was added at 12 min to a final concentration of 40 μg/ml and samples fixed at 15 min before analysis as described in Fig. 4. For further details see text and figure legend.
Cannot be quantitated, this is a qualitative assessment.
This is the same as experiment 3 of Table II.
Not applicable.
Figure 5.
CLIP-170 binding to new microtubules in vitro is specific and different from the binding of MAP2. Asters were made following the same protocol as in Fig. 4, d–f (i.e., with GMPCPP-tubulin in the second addition), in the presence of nonlabeled tubulin, and with the addition of a mixture of MAP2 (5 μg/ml) and H2 (20 μg/ml). The asters were fixed and sedimented onto coverslips as already described. Double immunofluorescence was performed for MAP2 (a) and H2 (b) using a monoclonal (M3A5) and a polyclonal (anti-KRKV) antibody, respectively. Asters (arrow) are labeled for MAP2 but have no detectable H2. Bar, 10 μm.
In this experiment, H2 was found on free microtubules formed after the second addition of tubulin, which could be due to a dynamic recognition of polymerizing microtubules, or to recognition of GMPCPP-tubulin. Indeed, even though the amount of added GMPCPP compared with GTP is very low (Materials and Methods), these microtubules are expected to contain some GMPCPP-bound subunits.
CLIP-170 Can Be Targeted to the Plus Ends of Centrosomal Asters in the Presence of GTPγS
To test further the possibility of association of CLIP-170 with a GTP conformation without the problem of spontaneous nucleation of tubulin assembly, we repeated the experiment described in Fig. 4 using tubulin equilibrated with GTPγS, an analogue known to decrease the spontaneous assembly (Roychowdhury and Gaskin, 1986). In this case, a small fraction of asters (<10%) had microtubules labeled for CLIP-170 (Fig. 6 b, arrows) or H2 (Fig. 6 d, arrows) predominantly at their plus ends. However, there were also asters with labeling for CLIP-170 along the length of microtubules, whereas the majority of asters, visualized by tubulin staining (Fig. 6, a and c), remained negative for CLIP-170 or H2, as was seen before with GTP (summarized in Table III). Although, only a small number of asters showed this end labeling for CLIP-170, the localization of CLIP-170 at the ends of microtubules in the presence of GTPγS left open the possibility that the protein may have a higher affinity with the GTP conformation of microtubules. We therefore examined by a different assay the interaction of H2 with GMPCPP-microtubules. Segmented microtubules, in which the GMPCPP and GDP parts of the polymer were labeled with faint and bright rh-tubulin, respectively (Hyman, 1991; Severin et al., 1997) were incubated with different concentrations of H2, which was then visualized by immunofluorescence. The protein was found to bind uniformly along these mixed microtubules, even at the lowest detectable concentrations of H2 (data not shown).
The results of these aster experiments show that H2 enhances spontaneous nucleation of GTP-tubulin assembly, even in the presence of centrosomes. This effect is enhanced with GMPCPP-tubulin, and suppressed with GTPγS-tubulin (Table III), consistent with the known effects of these analogues on microtubule assembly (Roychowdhury and Gaskin, 1986; Hyman et al., 1992). In all cases where free microtubules form, they are always labeled for H2, implying their formation is promoted by H2 and that association of H2 with microtubules is closely linked to the assembly process.
CLIP-170 Can Localize to the Plus Ends of Centrosomal Microtubules after Preincubation with GTP-Tubulin
The results using GTP analogues could be explained by association of CLIP-170 with tubulin subunits before assembly, as has been suggested for other MAPs (Carlier et al., 1984; Erickson and Pantaloni, 1981; Pedrotti and Islam, 1994; Vasquez et al., 1994), if we consider the differences between the two analogues. In the absence of glycerol, spontaneous assembly of tubulin is favored in the presence of GMPCPP (Hyman et al., 1992), whereas it is very slow in the presence of GTPγS (Roychowdhury and Gaskin, 1986). If CLIP-170 interacts with GTPγS-tubulin, it will have a stronger likelihood to add to the ends of pre-existing microtubules than to promote free nucleation. To test further this idea, we polymerized asters as before with GTP-tubulin, but for the second addition we preincubated at 37°C H2 with low concentrations of GTP-tubulin before addition to the asters, in the hope of promoting an interaction between H2 and tubulin to form a complex that would associate with microtubule ends. When this preincubation mix alone was sedimented onto coverslips, no microtubules were detected by immunofluorescence (data not shown). The distribution of H2 on asters polymerized according to this protocol is shown in Fig. 6 (e and f). The asters are labeled for H2 (Fig. 6 f) either all along centrosomal microtubules or at their plus ends (Fig. 6, e and f, arrows). These data indicate that the targeting of CLIP-170 to the plus ends of microtubules in vitro is not dependent on the presence of a nonhydrolyzable GTP analogue. Nevertheless, in order for H2 to be targeted to plus ends when microtubules are polymerized with GTP-tubulin, we had to preincubate the protein with tubulin, which supports the idea that CLIP-170 may interact with GTP-tubulin before polymerization.
Interaction of CLIP-170 with Tubulin Oligomers
If CLIP-170 interacts with tubulin before assembly, it may be possible to detect this interaction in vitro. We initially carried out chemical cross-linking experiments, which have been used previously to document tubulin interaction with MBPs, for example kinesin (Song and Mandelkow, 1993). For these experiments, we used the monomeric H1 polypeptide to avoid problems of interdimer cross-linking. Using the zero length cross-linker 1-ethyl-3-(3-[dimethylamino]propyl) carbodiimide (Song and Mandelkow, 1993), we detected interaction of H1 with both the α and β chains of the tubulin heterodimer (Fig. 7). By Coomassie staining we find a species with a mol wt of ∼100,000 D after cross-linking of H1 and tubulin (Fig. 7, lane 2) which does not appear with either protein alone (Fig. 7, lanes 1 and 3). A control protein, carbonic anhydrase, did not show significant interaction with either tubulin or H1 using this protocol (Fig. 7, lanes 4 and 5). This 100-kD species (Fig. 7, lane 2) reacts with antibodies against H1, α-, and β-tubulin (Fig. 7), consistent with it being a mixture of H1–α-tubulin and H1–β-tubulin dimers. A larger species with mol wt ∼150,000 D, which reacts with all three antibodies (Fig. 7, asterisk), could be trimers of H1–α-β-tubulin and/or H1–α-α-tubulin plus H1–β-β-tubulin.
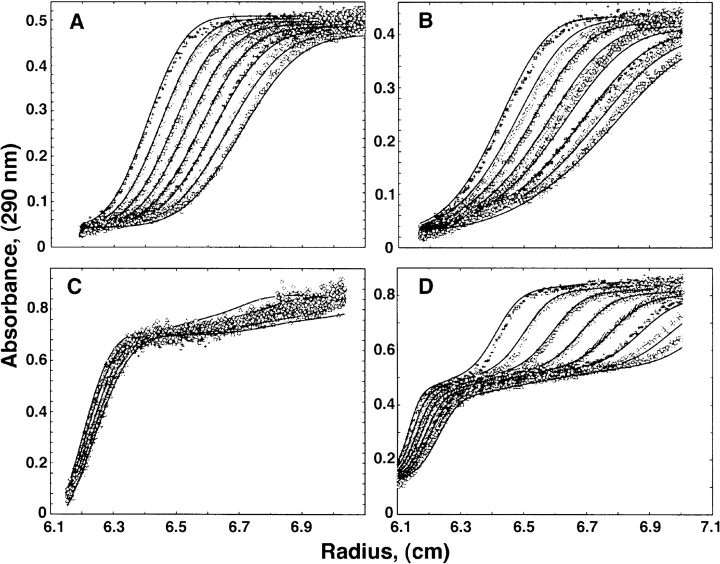
This cross-linking experiment alone does not allow us to distinguish between the interaction of H1 with tubulin heterodimers or higher order oligomers that are believed to behave as precursors in microtubule assembly (Carlier et al., 1984; Melki et al., 1989). To examine more precisely whether CLIP-170 may interact with tubulin heterodimers or oligomers, and to characterize the oligomers that form, we carried out sedimentation velocity experiments at 4°C (Fig. 8). Typical sedimentation boundaries at a series of equally spaced times are shown for H2 (Fig. 8 A) and GDP- or GTP-tubulin alone (Fig. 8 B). The solid lines represent the fitting of the data using a model involving single species of 3.2 (Fig. 8 A) or 6.2 S (Fig. 8 B). These values are consistent with those measured previously for dimeric H2 (Scheel, J., P. Pierre, J.E. Rickard, G.S. Diamantopoulos, C. Valetti, F.G. van der Goot, M. Häner, U. Aebi, and T.E. Kreis, manuscript in preparation) and tubulin (Howard and Timasheff, 1986). Typical sedimentation boundaries at a series of equally spaced times are also shown for 1.25 μM H2 and 10 μM GTP-tubulin (Fig. 8 C) or 1.25 μM H2 and 10 μM GDP-tubulin (Fig. 8 D). The solid lines represent the fitting of the data using models involving either three species of 5.6, 28, and 47 S (Fig. 8 C) or two species of 5.6 and 48 S (Fig. 8 D). The sedimentation coefficients and the proportion of the different species formed at constant tubulin concentration and increasing H2 concentrations are summarized in Table IV. At H2 concentrations higher than 5 μM, i.e., H2/tubulin ratios higher than 1:2, large oligomers that have a sedimentation coefficient of ∼70 S were observed (data not shown). The proportion of the 5.6 S species decreases with increasing H2 concentration, although this effect is not marked for the GTP-tubulin samples. Since this species will contain unresolved free H2 and tubulin, this may suggest that the stoichiometry of tubulin to H2 is higher in the 26–28 S species formed in GTP, compared with the 45–48 S species, so that with GTP-tubulin, less H2 is depleted from the available pool. It also suggests that formation of the 26–28 S species reaches saturation at the lowest H2 concentration used, and that higher concentrations of H2 lead to increased formation of the 45–48 S species as well as larger aggregates. We conclude from these results that H2 interacts with unassembled tubulin, and either stabilizes spontaneously formed oligomers, or promotes oligomerization. It has been reported previously that MAPs may promote tubulin oligomer formation (Marcum and Borisy, 1978; Carlier et al., 1984), and we indeed observed formation of similar, although not identical, oligomers with MAP2C under similar conditions. With MAP2C, a protein of similar mol wt to H2 (Garner and Matus, 1988), at high molar ratio to tubulin (0.43:1), more of the protein remained in the 5.6 S form, and only one oligomeric species formed, of 19.5 S in GTP (21% of the protein) or 28.5 S in GDP (26% of the protein). Thus, formation of oligomers with tubulin is not unique to H2, but the characteristics of the oligomers formed with each protein differ, and our assembly experiments described above suggest that only H2 may have a strong preference to use this interaction for coassembly to new microtubules. It is also interesting that in the case of H2, the species formed depend strongly on the nature of the nucleotide bound to the tubulin: when either GTP or GDP is bound, a 45–48 S species forms, whereas the 26–28 S species forms only in the presence of GTP (Table IV). In addition, the formation of these oligomers occurs at a lower molar ratio of H2:tubulin when GTP is bound compared with GDP, suggesting a preferential interaction of H2 with GTP-tubulin.
Figure 8.
Sedimentation behavior of H2, αβ-tubulin heterodimer, H2-GTP-tubulin, and H2-GDP-tubulin complexes. H2, tubulin and mixtures of the two proteins were analyzed by ultracentrifugation as described in Materials and Methods. Typical sedimentation velocity data are shown for: A, H2 (5 μM); B, tubulin heterodimer (10 μM); C, H2-GTP–tubulin complexes ([tubulin] = 10 μM, [H2] = 2.5 μM); D, H2-GDP–tubulin complexes ([tubulin] = 10 μM, [H2] = 2.5 μM). Rotor speed was 60,000 (A and B) or 25,000 rpm (C and D). Data points show the position of the boundary recorded at 10-min intervals; absorbance was recorded at 290 nm. Solid line, fitted data curves.
Table IV.
Sedimentation Properties of Species Formed by GTP-tubulin and GDP-tubulin in the Absence or in the Presence of H2
| H2 (μm) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.25 | 5 | |||||||||||||
| GTP | GDP | GTP | GDP | GTP | GDP | GTP | GDP | |||||||||
| 1 5.6 S | 100 | 100 | 63 | 98 | 65 | 86 | 48 | 58 | ||||||||
| 2 26–28 S | — | — | 26 | — | 21 | — | 32 | — | ||||||||
| 3 45–48 S | — | — | 10 | — | 14 | 11 | 19 | 40 | ||||||||
Sedimentation properties of species formed by 10 μM of tubulin in the presence of increasing concentrations of H2, calculated from the sedimentation profiles as described for Fig. 8 and in the text. Tubulin was complexed with GTP or GDP as indicated. Numbers express the proportion of protein in each species as a percentage of the total amount of protein measured by absorbance at 290 nm.
Discussion
We have examined the mechanism that may control the intracellular distribution of CLIP-170 towards microtubule plus ends and find that the binding of CLIP-170 to microtubules follows very rapidly the polymerization of microtubules in vivo. We furthermore demonstrate that CLIP-170 localization in vitro is also coupled to microtubule assembly. The NH2-terminal microtubule-binding domain of CLIP-170 is sufficient to obtain plus end localization. Our results provide no evidence that targeting of CLIP-170 to the plus ends of microtubules depends on the recognition of a conformational GTP cap on the assembled microtubule. They rather suggest that CLIP-170 can interact with tubulin to form oligomeric complexes that act as carrier molecules to incorporate CLIP-170 into the assembly sites of microtubules.
Two Possible Mechanisms for Targeting of CLIP-170 to the Plus Ends of Microtubules
Localization of CLIP-170 to microtubule plus ends in vivo correlates with new polymerization marked by microinjection of bt-tubulin. Localization of CLIP-170, therefore, is linked to the dynamics of microtubule assembly and closely follows elongating ends. The CLIP-170 microtubule-binding domain is necessary and sufficient for end localization in vivo, suggesting that the mechanism for end targeting does not depend on interaction of CLIP-170 with cargo. We therefore considered two possible mechanisms related to the dynamic properties of microtubules to explain this targeting. Firstly, CLIP-170 may have a higher affinity for a distinct conformation of microtubules at their plus ends. The dynamic instability mechanism for microtubule assembly states that the terminal subunits of the polymer must be conformationally distinct from the internal subunits (Mitchison and Kirschner, 1984a). There may exist proteins which can recognize such structural differences (Vale et al., 1994; Severin et al., 1997), and it is possible that CLIP-170, in order to be initially targeted to the plus ends of microtubules, could recognize and preferentially bind to such a specific conformation. Alternatively, CLIP-170 may associate preferentially with nonpolymerized GTP- tubulin to form complexes that subsequently incorporate at the assembly sites of growing microtubules. In this way, CLIP-170 would use tubulin heterodimers or oligomers as carrier molecules to be targeted to the plus ends of the polymer. Other processes related to tubulin assembly or regulation of CLIP-170 in vivo may be necessary to promote dissociation from older polymer, to achieve the localization found in cells.
To distinguish between these mechanisms, we tested the interaction of CLIP-170 with microtubules polymerized from centrosomes in vitro. Microtubules polymerized in the presence of GTP are labeled along their length by CLIP-170 when the protein is added during their polymerization (Fig. 4) (data not shown). Chrétien et al. (1995) reported that the ends of growing microtubules undergo a series of conformational changes, first forming sheets and then closing into tubes. Since we used similar experimental conditions and found no end only localization of CLIP-170, this suggests that CLIP-170 has no preference for such transitional steps. However since a possible GTP cap may be very small in vitro (Walker et al., 1991; Drechsel and Kirschner, 1994; Caplow and Shanks, 1996), we also used slowly hydrolyzable GTP analogues to create conformationally distinct microtubules. Again, the results of these experiments did not indicate that CLIP-170 might recognize specifically a distinct microtubule conformation. In addition, there was no preferential binding of CLIP-170 to the GMPCPP-containing part of mixed microtubules. The association of CLIP-170 with newly formed microtubules would therefore be more easily explained by interaction of CLIP-170 with tubulin before assembly, which will target the protein to growing ends. Accordingly, targeting of H2 to plus ends of microtubules was observed when H2 was preincubated with tubulin before addition to polymerizing asters. In addition, we obtained evidence for interaction of CLIP-170 with nonassembled tubulin at 4°C, leading to formation or stabilization of oligomeric species, the size of which depend on the tubulin-bound nucleotide. We find here that H2 induces or stabilizes GTP-tubulin oligomer species of 28 S and at increasing concentrations there is also formation of a larger 45–48 S species. The 26–28 S oligomers, which form efficiently at lower concentrations, and only in GTP-tubulin, may represent functional complexes of GTP-tubulin and CLIP-170 that can be assembled at the polymerization sites of microtubules and target CLIP-170 to the plus ends, but this remains to be shown. The possibility that MAPs can copolymerize with tubulin has already been proposed; in vitro experiments have suggested that some MAPs can associate with tubulin oligomers (Carlier et al., 1984; Drechsel et al., 1992; Pedrotti and Islam, 1994; Vasquez et al., 1994). These oligomers are thought to be precursors of microtubules and their stabilization by MAPs could enhance the assembly of the polymer (Melki et al., 1989; Drechsel et al., 1992; Vasquez et al., 1994). In the case of MAP1A, it has been reported that incorporation of oligomers into microtubules only occurs at high GTP concentrations (Pedrotti and Islam, 1994), and we found that the 26–28S oligomer formed in the presence of H2 is specific to GTP-tubulin. Such a mechanism of binding could also allow the targeting of other MAPs to the assembly site of microtubules in vivo. However, in contrast to MAP2, our results show a strong preference of CLIP-170 for binding to newly assembled microtubules, and so far CLIP-170 is unique in being regulated to maintain a predominantly plus end localization in cells.
In a dynamic system where both polymerized microtubules and free tubulin are present, we postulate that CLIP-170 is preferentially associated with free tubulin (or oligomers of it) before coassembly at the plus ends of microtubules. This could be due to a higher affinity for oligomeric complexes, as well as to molar excess of accessible free subunit. This model does not necessarily require a difference in affinity of CLIP-170 for copolymerized and prepolymerized microtubules, and indeed is consistent with the fact that CLIP-170 interacts strongly with both copolymerized and prepolymerized microtubules in sedimentation assays (data not shown). According to this mechanism, our results using GTP analogues in vitro can also be explained by their different effects on tubulin assembly. In the presence of GTPγS, spontaneous assembly of tubulin is inhibited (Roychowdhury and Gaskin, 1986) whereas it is very strongly favored by GMPCPP (Hyman et al., 1992). CLIP-170 also increases spontaneous nucleation of assembly of GTP-tubulin (Diamantopoulos, G.S., and R. Melki, unpublished observations). The nucleating effect of CLIP-170 would be enhanced in the presence of GMPCPP and the protein will be found on the free microtubules that form. With GTPγS, spontaneous assembly would be inhibited and tubulin and CLIP-170 would coassemble at the ends of already formed centrosomal microtubules. Although the sum of our results favor this oligomer assembly mechanism, it remains possible that the preference for oligomer assembly is due to an inherent preference of CLIP-170 for GTP-tubulin, and that the nucleotide analogues used here may not allow exact mimicking of the correct conformational state.
In our in vitro assays, localization of CLIP-170 at microtubule ends, although observed, was not seen on the majority of microtubules, making it less likely that restriction of the protein to the ends is intrinsic to the mechanism of assembly. Since binding of CLIP-170 to microtubules can be inhibited by phosphorylation in vitro, and nocodazole treatment of cells leads to dephosphorylation of CLIP-170 in vivo (Rickard and Kreis, 1991), whereas inducing binding along the length of the remaining microtubules (Rickard and Kreis, 1990), it is likely that this posttranslational modification regulates localization of CLIP-170 along microtubules in vivo. Experiments on living cells indeed show that CLIP-170 dynamically interacts with microtubules in vivo, being rapidly released from the older part of microtubules to achieve its steady-state plus end localization (Perez et al., 1999). Although we found that transfected H1 localized to microtubule plus ends at low expression levels, the overexpressed protein binds along the length of microtubules (Pierre et al., 1994), suggesting that regulation of the localization of the protein is affected by the COOH-terminal part of the protein.
In our experiments using rh-tubulin, it was also notable that, in the presence of CLIP-170, polymerization did not occur equally from all microtubule plus ends. After addition of H2, tubulin was apparently no longer incorporated at the extremity of preformed aster microtubules but efficiently incorporated into newly nucleated CLIP-170–positive polymer. No such effect was observed in the presence of MAP2, since in this case tubulin was incorporated at the end of more than 90% of preexisting asters. This suggests that there could be preferential binding of CLIP-170– tubulin complexes to CLIP-170–positive microtubule ends as compared with those which are CLIP-170–negative, and that a possible function of this is to promote persistence of microtubule assembly or to orientate microtubule growth in vivo. Such a preferential binding to CLIP-170–positive ends could also explain the low frequency of addition of H2 to microtubule ends in vitro, since addition to H2-negative ends would be of low probability, but once initiated would continue efficiently.
Microtubule Plus End Targeting Depends on the Microtubule-binding Domain of CLIP-170
So far CLIP-170 is unique among MBPs studied in being localized to the plus ends of microtubules in vivo (Rickard and Kreis, 1990). The microtubule-binding motif of CLIP-170 (Pierre et al., 1992) differs from other MBPs studied, which may explain its unique properties. We indeed found a difference in behavior between H2 and MAP2, a neuronal MAP with a different microtubule-binding motif; H2 was found preferentially associated with new microtubules, whereas MAP2 bound to both newly formed microtubules and old centrosomal microtubules. We have no in vivo evidence about the importance of this observation, but it is possible that the difference in the microtubule-binding domain between the two proteins may influence their in vivo distribution on microtubules. Although MAP2 is neuronally expressed, it shares a microtubule-binding domain with a ubiquitously expressed protein, MAP4 (Aizawa et al., 1991; Chapin and Bulinski, 1991; West et al., 1991), and we have found that MAP2 and MAP4 bind to sites on microtubules different from CLIP-170 (Diamantopoulos, G.S., and R. Melki, unpublished observations).
There is an increasing number of proteins that share the microtubule-binding motif found in CLIP-170, but the properties of their microtubule association are not well investigated. A brain-specific protein, CLIP-115 (De Zeeuw et al., 1997), which contains two microtubule-binding motifs, as does CLIP-170, has been found to bind all along the length of microtubules in cultured hippocampal neurones and in sections of rat brain (De Zeeuw et al., 1997). It is also enriched on a new membranous organelle, the dendritic lamellar body (De Zeeuw et al., 1995), that has a microtubule-dependent distribution (De Zeeuw et al., 1997). It is possible, therefore, that CLIP-115 may play a similar role to CLIP-170 in mediating interaction of specific membranous organelles with microtubules. The p150glued subunit of the dynactin complex (Swaroop et al., 1987; Holzbaur et al., 1991), which has been shown to associate with microtubules in vivo and in vitro (Waterman-Storer et al., 1995), has one copy of the microtubule-binding motif of CLIP-170. There are no published reports that it may preferentially localize to microtubule plus ends, and its major in vivo localization as part of the dynactin complex appears to be mainly on membranes (Schroer et al., 1996). The yeast protein Bik1p was identified as a karyogamy mutant and has effects on microtubule assembly in vivo (Berlin et al., 1990) but no direct studies of the interaction of Bik1p and microtubules in vitro have been reported. Two other proteins containing this motif, named cofactors B and E, have been shown to participate in the folding pathways of α- and β-tubulin (Lewis et al., 1997). In addition to its interaction with unassembled tubulin, cofactor E also seems to behave as a MAP, since it coassembles with microtubules (Tian et al., 1996). It may therefore play separate roles, in tubulin folding and in modulating microtubule behavior. It is interesting that both of these factors bind to non-polymerized tubulin, and as folding factors may sense changes in the conformation of tubulin. It would be interesting to compare further the effects of CLIP-170 and these other proteins on microtubule assembly. It may be that the presence of two copies of the microtubule-binding motif confers specific properties; alternatively, CLIP-170 alone may be tightly regulated in the cell to maintain its microtubule plus end distribution.
In Vivo Function of Microtubule Plus End Targeting of CLIP-170
It has been suggested that CLIP-170 may facilitate the microtubule-dependent transport of endosomal vesicles between early and late endosomes (Pierre et al., 1992; Rickard and Kreis, 1996). CLIP-170 has also been localized to prometaphase kinetochores and appears to play a role in correct spindle assembly (Dujardin et al., 1998). The preferential accumulation of CLIP-170 at the elongating extremities of microtubules in vivo would be consistent with such roles, considering the distribution of early endosomes at the cell periphery and the interaction of kinetochores with microtubule plus ends. One possibility suggested by our data is that following its targeting to the plus ends of microtubules, CLIP-170 will remain attached in order to find and capture a cargo. This association may be regulated at the level of a receptor that may be a protein complex and/or a specific lipid composition. Possible candidates for such interactions may be proteins of the dynactin complex (Schroer et al., 1996) and there is evidence for a colocalization in vivo of CLIP-170 with the Arp1 subunit of dynactin (Dujardin et al., 1998) (Valetti, C., T.E. Kreis, and T.A. Schroer, manuscript submitted for publication). However, since this interaction has not been biochemically confirmed, it is not clear whether it represents direct association or whether additional factors are involved, and it is likely that the interaction is transient and tightly regulated. Interaction of CLIP-170 with membranes may have the additional function of capturing and stabilizing microtubule ends to regulate their dynamic behavior and to provide more stable tracks for organelle movements. In addition, the association of CLIP-170 with kinetochores is seen very early in prometaphase, and mutants lacking the microtubule-binding domain can bind to the kinetochore (Dujardin et al., 1998). Thus, association of CLIP-170 with membranous organelles may also occur independently of interaction with microtubules. Therefore, although CLIP-170 clearly has the property of targeting to microtubule plus ends, it is unclear whether binding of CLIP-170 to a target organelle before interactions with microtubules is also important for its function. Further investigation of the interaction of CLIP-170 with both microtubules and organelles is necessary to answer these questions.
Acknowledgments
We thank A. Hyman for the gift of GMPCPP, A. Matus for the gift of MAP2C expression construct, and the National Cancer Institute for paclitaxel. We are grateful to the Lausanne abattoir (Switzerland) for provision of pig brains, and to the staff of the Maternity Hospital (Geneva, Switzerland) for help in obtaining placentas. We thank G. Charly and R. Leguy (both from Centre National de la Recherche Scientifique [CNRS], Gif-sur-Yvette, France) for preparation of tubulin used for the ultracentrifugation experiments. We are grateful to S. Scales (Stanford University Medical School, Stanford, CA) and M-F. Carlier (CNRS) for comments on the manuscript.
This work was supported by grants from the Fonds National Suisse to J.E. Rickard and T.E. Kreis, and from the Canton de Genève to T.E. Kreis. Work done in the laboratory of R. Melki is supported by the Centre National de la Recherche Scientifique to the group of D. Pantaloni, and the Association pour la Recherche sur le Cancer.
Abbreviations used in this paper
- bt
biotin
- CLIP
cytoplasmic linker protein
- GMPCPP
guanylyl-(α,β)-methylene-diphosphonate
- GTPγS
guanosine 5′-O-(3-thiotriphosphate) tetralithium salt
- MAP
microtubule-associated protein
- MBP
microtubule-binding protein
- rh
rhodamine
Footnotes
J.E. Rickard's present address is Department of Mental Health, University Medical School, Foresterhill, Aberdeen AB25 2ZD, Scotland, UK.
References
- Aizawa H, Emori Y, Mori A, Murofushi H, Sakai H, Suzuki K. Functional analyses of the domain structure of microtubule-associated protein-4 (MAP-U) J Biol Chem. 1991;266:9841–9846. [PubMed] [Google Scholar]
- Allan VJ, Kreis TE. A microtubule-binding protein associated with membranes of the Golgi apparatus. J Cell Biol. 1986;103:2229–2239. doi: 10.1083/jcb.103.6.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin V, Styles CA, Fink GR. BIK1, a protein required for microtubule function during mating and mitosis in Saccharomyces cerevisiae, colocalizes with tubulin. J Cell Biol. 1990;111:2573–2586. doi: 10.1083/jcb.111.6.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornens M, Paintrand M, Berges J, Marty M-C, Karsenti E. Structural and chemical characterization of isolated centrosomes. Cell Motil Cytoskel. 1987;8:238–249. doi: 10.1002/cm.970080305. [DOI] [PubMed] [Google Scholar]
- Bré M-H, Karsenti E. Effects of brain microtubule-associated proteins on microtubule dynamics and the nucleating activity of centrosomes. Cell Motil Cytoskel. 1990;15:88–98. doi: 10.1002/cm.970150205. [DOI] [PubMed] [Google Scholar]
- Burns RG, Islam K. Direct incorporation of microtubule oligomers at high GTP concentrations. FEBS (Fed Eur Biochem Soc) Lett. 1984;173:67–74. doi: 10.1016/0014-5793(84)81019-4. [DOI] [PubMed] [Google Scholar]
- Caplow M, Shanks J. Evidence that a single monolayer tubulin-GTP cap is both necessary and sufficient to stabilize microtubules. Mol Biol Cell. 1996;7:663–675. doi: 10.1091/mbc.7.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M-F, Pantaloni D. Kinetic analysis of guanosine 5′-triphosphate hydrolysis associated with tubulin polymerization. Biochemistry. 1981;20:1918–1924. doi: 10.1021/bi00510a030. [DOI] [PubMed] [Google Scholar]
- Carlier M-F, Simon C, Pantaloni D. Polymorphism of tubulin oligomers in the presence of microtubule-associated proteins. Implications in microtubule assembly. Biochemistry. 1984;23:1582–1590. doi: 10.1021/bi00302a037. [DOI] [PubMed] [Google Scholar]
- Cassimeris L, Pryer NK, Salmon ED. Real-time observations of microtubule dynamic instability in living cells. J Cell Biol. 1988;107:2223–2231. doi: 10.1083/jcb.107.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin SJ, Bulinski JC. Non-neuronal 210 × 103 M rmicrotubule-associated protein (MAP4) contains a domain homologous to the microtubule-binding domains of neuronal MAP2 and tau. J Cell Sci. 1991;98:27–36. doi: 10.1242/jcs.98.1.27. [DOI] [PubMed] [Google Scholar]
- Chrétien D, Fuller SD, Karsenti E. Structure of growing microtubule ends: two-dimensional sheets close into tubes at variable rates. J Cell Biol. 1995;129:1311–1328. doi: 10.1083/jcb.129.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole NB, Lippincott-Schwartz J. Organization of organelles and membrane traffic by microtubules. Curr Opin Cell Biol. 1995;7:55–64. doi: 10.1016/0955-0674(95)80045-x. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Hertzberg EL, Mugnaini E. The dendritic lamellar body: a new neuronal organelle putatively associated with dendrodendritic gap junctions. J Neurosci. 1995;15:1587–1604. doi: 10.1523/JNEUROSCI.15-02-01587.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw CI, Hoogenraad CC, Goedknegt E, Hertzberg E, Neubauer A, Grosveld F, Galjart N. CLIP-115, a novel brain-specific cytoplasmic linker protein, mediates the localization of dendritic lamellar bodies. Neuron. 1997;19:1187–1199. doi: 10.1016/s0896-6273(00)80411-0. [DOI] [PubMed] [Google Scholar]
- Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- Diamantopoulos GS, Kreis TE, Rickard JE. Purification and assay of CLIP-170. Methods Enzymol. 1998;298:197–206. doi: 10.1016/s0076-6879(98)98019-3. [DOI] [PubMed] [Google Scholar]
- Drechsel DN, Kirschner MW. The minimum GTP cap required to stabilize microtubules. Curr Biol. 1994;4:1053–1061. doi: 10.1016/s0960-9822(00)00243-8. [DOI] [PubMed] [Google Scholar]
- Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin D, Kirschner M. Purification of tau protein from brain. Methods Enzymol. 1986;134:156–160. doi: 10.1016/0076-6879(86)34084-9. [DOI] [PubMed] [Google Scholar]
- Dujardin D, Wacker UI, Moreau A, Schroer TA, Rickard JE, De Mey JR. Evidence for a role of CLIP-170 in the establishment of metaphase chromosome alignment. J Cell Biol. 1998;141:849–862. doi: 10.1083/jcb.141.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP, Pantaloni D. The role of subunit entropy in cooperative assembly. Nucleation of microtubules and other two-dimensional polymers. Biophys J. 1981;34:293–309. doi: 10.1016/S0006-3495(81)84850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DW, Kirschner MW. A microtubule-associated protein from Xenopuseggs that specifically promotes assembly at the plus end. J Cell Biol. 1987;105:2203–2215. doi: 10.1083/jcb.105.5.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner CC, Matus A. Different forms of microtubule-associated protein 2 are encoded by separate mRNA transcripts. J Cell Biol. 1988;106:779–783. doi: 10.1083/jcb.106.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson HV, Valetti C, Kreis TE. Motors and membrane traffic. Curr Opin Cell Biol. 1997;9:18–28. doi: 10.1016/s0955-0674(97)80147-0. [DOI] [PubMed] [Google Scholar]
- Green S, Issemann I, Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988;16:369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur ELF, Hammarback JA, Paschal BM, Kravit NG, Pfister KK, Vallee RB. Homology of a 150 K cytoplasmic dynein-associated polypeptide with the Drosophila gene Glued. . Nature. 1991;351:579–583. doi: 10.1038/351579a0. [DOI] [PubMed] [Google Scholar]
- Howard WD, Timasheff SN. GDP state of tubulin: stabilization of double rings. Biochemistry. 1986;25:8292–8300. doi: 10.1021/bi00373a025. [DOI] [PubMed] [Google Scholar]
- Hyman AA. Preparation of marked microtubules for the assay of the polarity of microtubule-based motors by fluorescence. J Cell Sci Suppl. 1991;14:125–127. doi: 10.1242/jcs.1991.supplement_14.25. [DOI] [PubMed] [Google Scholar]
- Hyman AA, Salser S, Drechsel DN, Unwin N, Mitchison TJ. Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolyzable analogue, GMPCPP. Mol Biol Cell. 1992;3:1155–1167. doi: 10.1091/mbc.3.10.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A, Drechsel D, Kellogg D, Salser S, Sawin K, Steffen P, Wordeman L, Mitchison T. Preparation of modified tubulins. Methods Enzymol. 1991;196:478–485. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- Hyman AA, Chrétien D, Arnal I, Wade RH. Structural changes accompanying GTP hydrolysis in microtubules: information from a slowly hydrolyzable analogue guanylyl-(α,β)-methylene-diphosphonate. J Cell Biol. 1995;128:117–125. doi: 10.1083/jcb.128.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M, Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Kreis TE. Microtubules containing detyrosinated tubulin are less dynamic. EMBO (Eur Mol Biol Organ) J. 1987;6:2597–2606. doi: 10.1002/j.1460-2075.1987.tb02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of the bacteriphage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Tian G, Cowan NJ. The α- and β-tubulin folding pathways. Trends Cell Biol. 1997;7:479–484. doi: 10.1016/S0962-8924(97)01168-9. [DOI] [PubMed] [Google Scholar]
- Mandelkow E, Mandelkow EM. Microtubules and microtubule-associated proteins. Curr Opin Cell Biol. 1995;7:72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- Marcum JM, Borisy GG. Characterization of microtubule protein oligomers by analytical ultracentrifugation. J Biol Chem. 1978;253:2825–2833. [PubMed] [Google Scholar]
- Melki R, Carlier M-F, Pantaloni D, Timasheff SN. Cold depolymerization of microtubules to double rings: geometric stabilization of assemblies. Biochemistry. 1989;28:9143–9152. doi: 10.1021/bi00449a028. [DOI] [PubMed] [Google Scholar]
- Melki R, Carlier M-F, Pantaloni D. Direct evidence for GTP and GDP-Piintermediates in microtubule assembly. Biochemistry. 1990;29:8921–8932. doi: 10.1021/bi00490a007. [DOI] [PubMed] [Google Scholar]
- Melki R, Kerjan P, Waller J-P, Carlier M-F, Pantaloni D. Interaction of microtubule-associated proteins with microtubules: yeast lysyl- and valyl-tRNA synthetases and t 218-235 synthetic peptide as model systems. Biochemistry. 1991;30:11536–11545. doi: 10.1021/bi00113a008. [DOI] [PubMed] [Google Scholar]
- Melki R, Fievez S, Carlier M-F. Continuous monitoring of Pirelease following nucleotide hydrolysis in actin or tubulin assembly using 2-amino-6-mercapto-7-methylpurine ribonucleoside and purine-nucleoside phosphorylase as an enzyme-linked assay. Biochemistry. 1996;35:12038–12045. doi: 10.1021/bi961325o. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984a;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 1984b;312:232–237. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]
- Näthke IS, Adams CL, Polakis P, Sellin JH, Nelson WJ. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J Cell Biol. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrotti B, Islam K. Purified native microtubule associated protein MAP1A: kinetics of microtubule assembly and MAP1A/tubulin stoichiometry. Biochemistry. 1994;33:12463–12470. doi: 10.1021/bi00207a013. [DOI] [PubMed] [Google Scholar]
- Pepperkok R, Scheel J, Horstmann H, Hauri HP, Griffiths G, Kreis TE. β-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993;74:71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- Perez, F., G.S. Diamantopoulos, R. Stalder, and T.E. Kreis. 1999. CLIP-170 highlights growing microtubule ends in vivo. Cell. In press. [DOI] [PubMed]
- Philo, J.S. 1994. Measuring sedimentation, diffusion, and molecular weights of small molecules by direct fitting of sedimentation velocity concentration profiles. In Modern Analytical Ultracentrifugation. T.M. Schuster and T.M. Laue, editors. Birkhäuser, Boston, MA. 156–170.
- Pierre P, Scheel J, Rickard JE, Kreis TE. CLIP-170 links endocytic vesicles to microtubules. Cell. 1992;70:887–900. doi: 10.1016/0092-8674(92)90240-d. [DOI] [PubMed] [Google Scholar]
- Pierre P, Pepperkok R, Kreis TE. Molecular characterization of two functional domains of CLIP-170 in vivo. J Cell Sci. 1994;107:1909–1920. doi: 10.1242/jcs.107.7.1909. [DOI] [PubMed] [Google Scholar]
- Rickard, J.E. 1998. CLIP-170. In Guidebook to the Cytoskeletal and Motor Proteins. T.E. Kreis and R.D. Vale, editors. Oxford University Press, Oxford, UK. In press.
- Rickard JE, Kreis TE. Identification of a novel nucleotide-sensitive microtubule-binding protein in HeLa cells. J Cell Biol. 1990;110:1623–1633. doi: 10.1083/jcb.110.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard JE, Kreis TE. Binding of pp170 to microtubules is regulated by phosphorylation. J Biol Chem. 1991;266:17597–17605. [PubMed] [Google Scholar]
- Rickard JE, Kreis TE. CLIPs for organelle-microtubule interactions. Trends Cell Biol. 1996;6:178–183. doi: 10.1016/0962-8924(96)10017-9. [DOI] [PubMed] [Google Scholar]
- Roychowdhury S, Gaskin F. Magnesium requirements for guanosine 5′-O-(3-thiotriphosphate) induced assembly of microtubule protein and tubulin. Biochemistry. 1986;25:7847–7853. doi: 10.1021/bi00372a010. [DOI] [PubMed] [Google Scholar]
- Sammak PJ, Borisy GG. Direct observation of microtubule dynamics in living cells. Nature. 1988;332:724–726. doi: 10.1038/332724a0. [DOI] [PubMed] [Google Scholar]
- Schroer TA, Bingham JB, Gill SR. Actin-related protein 1 and cytoplasmic dynein-related motility—what's the connection? . Trends Cell Biol. 1996;6:212–215. doi: 10.1016/0962-8924(96)20014-5. [DOI] [PubMed] [Google Scholar]
- Schulze E, Kirschner M. Microtubule dynamics in interphase cells. J Cell Biol. 1986;102:1020–1031. doi: 10.1083/jcb.102.3.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E, Kirschner M. New features of microtubule behaviour observed in vivo. Nature. 1988;334:356–359. doi: 10.1038/334356a0. [DOI] [PubMed] [Google Scholar]
- Severin FF, Sorger PK, Hyman AA. Kinetochores distinguish GTP from GDP forms of the microtubule lattice. Nature. 1997;388:888–891. doi: 10.1038/42270. [DOI] [PubMed] [Google Scholar]
- Song YH, Mandelkow E. Recombinant kinesin motor domain binds to beta-tubulin and decorates microtubules with a B surface lattice. Proc Natl Acad Sci USA. 1993;90:1671–1675. doi: 10.1073/pnas.90.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford WF., III Boundary analysis in sedimentation transport experiments: a procedure for obtaining sedimentation coefficient distributions using the time derivative of the concentration profile. Anal Biochem. 1992;203:295–301. doi: 10.1016/0003-2697(92)90316-y. [DOI] [PubMed] [Google Scholar]
- Swaroop A, Swaroop M, Garen A. Sequence analysis of the complete cDNA and encoded polypeptide for the Glued gene of Drosophila melanogaster. . Proc Natl Acad Sci USA. 1987;84:6501–6505. doi: 10.1073/pnas.84.18.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Hisanaga S, Umeyama T, Hirokawa N. The 72-kD microtubule-associated protein from porcine brain. J Neurochem. 1992;58:1510–1516. doi: 10.1111/j.1471-4159.1992.tb11372.x. [DOI] [PubMed] [Google Scholar]
- Tian G, Huang Y, Rommelaere H, Vandekerckhove J, Ampe C, Cowan NJ. Pathway leading to correctly folded β-tubulin. Cell. 1996;86:287–296. doi: 10.1016/s0092-8674(00)80100-2. [DOI] [PubMed] [Google Scholar]
- Vale RD, Coppin CM, Malik F, Kull FJ, Milligan RA. Tubulin GTP hydrolysis influences the structure, mechanical properties, and kinesin-driven transport of microtubules. J Biol Chem. 1994;269:23769–23775. [PubMed] [Google Scholar]
- Vasquez RJ, Gard DL, Cassimeris L. XMAP from Xenopuseggs promotes rapid plus end assembly of microtubules and rapid microtubule polymer turnover. J Cell Biol. 1994;127:985–993. doi: 10.1083/jcb.127.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RA, Pryer NK, Salmon ED. Dilution of individual microtubules observed in real time in vitro: evidence that cap size is small and independent of elongation rate. J Cell Biol. 1991;114:73–81. doi: 10.1083/jcb.114.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Karki S, Holzbaur EL. The p150Gluedcomponent of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1) Proc Natl Acad Sci USA. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RR, Tenbarge KM, Olmsted JB. A model for microtubule-associated protein 4 structure. J Biol Chem. 1991;266:21886–21896. [PubMed] [Google Scholar]