Abstract
Immunoglobulin heavy chain-binding protein (BiP) is a member of the hsp70 family of chaperones and one of the most abundant proteins in the ER lumen. It is known to interact transiently with many nascent proteins as they enter the ER and more stably with protein subunits produced in stoichiometric excess or with mutant proteins. However, there also exists a large number of secretory pathway proteins that do not apparently interact with BiP. To begin to understand what controls the likelihood that a nascent protein entering the ER will associate with BiP, we have examined the in vivo folding of a murine λI immunoglobulin (Ig) light chain (LC). This LC is composed of two Ig domains that can fold independent of the other and that each possess multiple potential BiP-binding sequences. To detect BiP binding to the LC during folding, we used BiP ATPase mutants, which bind irreversibly to proteins, as “kinetic traps.” Although both the wild-type and mutant BiP clearly associated with the unoxidized variable region domain, we were unable to detect binding of either BiP protein to the constant region domain. A combination of in vivo and in vitro folding studies revealed that the constant domain folds rapidly and stably even in the absence of an intradomain disulfide bond. Thus, the simple presence of a BiP-binding site on a nascent chain does not ensure that BiP will bind and play a role in its folding. Instead, it appears that the rate and stability of protein folding determines whether or not a particular site is recognized, with BiP preferentially binding to proteins that fold slowly or somewhat unstably.
Keywords: BiP, endoplasmic reticulum, chaperone, protein folding
Proteins that traverse the mammalian secretory pathway are translated on polyribosomes attached to the endoplasmic reticulum (ER) membrane (Walter and Johnson, 1994). This allows cotranslational translocation of the nascent chain into the ER as an extended or unfolded polypeptide. Upon entering the lumen of the ER, the nascent protein encounters a variety of molecular chaperones and folding enzymes and begins to fold, in some cases, even before translation is complete (Hammond et al., 1994). One of the molecular chaperones encountered by newly synthesized polypeptides is BiP/ GRP78, the ER cognate of the hsp70 family (Haas, 1991; Gething and Sambrook, 1992). In yeast, immunoglobulin heavy chain-binding protein (BiP)1 is required for translocation and may act to pull the nascent chain into the ER through cycles of binding and release (Vogel et al., 1990). Mammalian BiP is present at the translocon where it serves to maintain the permeability barrier of the ER during the early stages of targeting nascent chains into the translocon (Hamman et al., 1998). However, there is no clear evidence that mammalian BiP plays a direct role in pulling proteins into the ER, and this point remains unresolved (Gorlich and Rapoport, 1993; Nicchitta and Blobel, 1993).
BiP, like all hsp70 family members, binds to unfolded nascent polypeptides (Landry et al., 1992; Simons et al., 1995; Hendershot et al., 1996), although exactly what BiP recognizes on a newly synthesized protein and how this binding contributes to protein folding in not clearly understood. Several in vitro (Munro and Pelham, 1986; Hendrick and Hartl, 1993; Palleros et al., 1993) and in vivo (Hendershot et al., 1995; Simons et al., 1995) studies demonstrated that hsp70 release and polypeptide folding are dependent on ATP binding to hsp70. In vitro peptide-binding studies revealed that BiP recognizes heptapeptides and prefers those with aliphatic residues (Flynn et al., 1991). Flynn et al. (1991) speculated that sequences of this type would appear approximately every 16 residues in the average globular protein. A somewhat similar recognition motif was identified when purified BiP was used to affinity pan a library of peptides displayed on bacteriophages (Blond-Elguindi et al., 1993). Further analysis of these peptides revealed that the aliphatic residues were preferred only for alternating residues, suggesting that if a protein containing this sequence was extended, the hydrophobic residues would all face the same direction and perhaps fit in to the BiP polypeptide-binding pocket (Blond-Elguindi et al., 1993). Although sequences of this type can indeed be found in most proteins entering the ER, it appears that some secretory pathway proteins do not normally bind to BiP (Hurtley et al., 1989; Graham et al., 1990; Morris et al., 1997). This is perplexing if these proteins are entering the ER in an extended conformation and BiP, which is present at the translocon at least initially (Hamman et al., 1998), “scans” nascent chains as they enter the ER. It is possible that for some proteins BiP binding is very transient and limited to chains that are not fully translated, or conversely that the presence of a potential BiP-binding sequence does not in itself dictate whether BiP will bind.
The algorithm generated by phage display analysis to identify BiP binding sites (Blond-Elguindi et al., 1993) has been applied to immunoglobulin (Ig) domain sequences (Knarr et al., 1995). As predicted by Flynn et al. (1991), multiple “BiP-binding sites” were identified in both the variable and constant region domains of the heavy and light chains subjected to this analysis. Peptides corresponding to these sequences were generated and shown to stimulate the ATPase activity of BiP (Knarr et al., 1995), which is presumed to be an indication of their binding to the protein-binding domain of BiP. It is unclear which, if any, of these sites are used in vivo and how BiP binding to various “hydrophobic sequences” might be regulated during translocation and folding.
Ig heavy and light chains are comprised of a series of tandemly aligned Ig domains (four or five and two, respectively) that can fold independent of each other in vitro (Goto et al., 1979; Goto and Hamaguchi, 1982) into a compact structure composed of two twisted β sheets stabilized by a single disulfide bond (Amzel and Poljak, 1979). Ig LCs bind transiently to BiP and can be secreted without heavy chains, making them a relatively simple protein for in vivo folding analyses. In a recent study, we coexpressed murine λ light chain (LC) in COS cells together with either wild-type hamster BiP or BiP ATPase mutants that bind substrate proteins but do not release them (Wei et al., 1995). The LCs were synthesized in the presence of dithiothreitol (DTT), which is a fully reversible reducing agent in vivo (Braakman et al., 1992a,b). This treatment prevents LC oxidation and maximizes the binding of LCs to both wild-type and mutant BiP. Removing the DTT, allows LCs expressed with wild-type BiP to fold and form intradomain disulfide bonds in both domains, whereas those coexpressed with mutant BiP were only capable of oxidizing a single domain (Hendershot et al., 1996). In spite of the structural similarity between the variable (V) and constant (C) domain of the LC and the prediction of multiple BiP-binding sites per Ig domain, these data argue that only a single LC domain binds to BiP in vivo. This suggests that either the algorithm is not a good detector of BiP-binding sites or that these two domains have different requirements for folding in vivo, and that the simple presence of a BiP-binding sequence does not ensure that it will be used.
In the present study, we confirmed that BiP binds to a single λ LC domain in vivo and determined which one it is. Then using a combination of in vivo and in vitro BiP-binding and protein-folding assays, we examined the reason for the differential association of BiP with these domains in the ER.
Materials and Methods
Construction of λI LC Mutants
Before producing LC cysteine mutants, it was necessary to obtain a cDNA clone of the λI LC gene. The pJDEλI genomic clone (gWTλ) originally obtained from the murine myeloma HOPC 2020 (Bernard et al., 1978) and provided by Dr. Y. Argon (University of Chicago, Chicago, IL) was transiently expressed in COS 1 cells. RNA was isolated (RNAzol B; Tel-Test) and λ-specific mRNA was amplified by reverse transcription PCR using (CTGACCAATCTAGAAAAGAATAG) as the 5′ primer and (CCTCTGTGGATCCAATGAAGGTT) as a 3′ primer. The PCR product was digested with XbaI and BamHI and ligated into the pTZ vector. The entire cDNA clone was sequenced before recloning it into the pSVL eukaryotic expression vector (cWTλ) for use in transfection studies.
The variable region cysteines (C41 and C109) and constant region cysteines (C156 and C215) responsible for forming the intradomain disulfide bonds were mutated to serine by overlap extension PCR as described (Ho et al., 1989). The final PCR products were digested with XbaI and BbsI (Vmut) or BbsI and BamHI (Cmut) and inserted into pTZ-cWTλ in place of the corresponding wild-type sequence. The alterations of the cysteine residues to serine and the absence of other mutations were verified by DNA sequencing before the mutants were subcloned into the pSVL vector.
An ER-targeted constant region domain was created by removing 107 amino acids (Val22–Leu128) which encode the variable region of the λI LC. Oligonucleotides were designed to give a PCR product that joined the sequence encoding Ala21 (the second amino acid of the mature variable region) to Gly129 (the first amino acid of the constant region). The resulting cDNA (ER-Cλ) encodes a protein with the ER-targeting signal sequence, the first two amino acids of the variable domain (to ensure proper cleavage of the signal sequence after translocation), and the entire constant region domain. The cDNA product was sequenced to verify the deletion and to ensure the constant region was not altered before inserting it into pSVL, a eukaryotic expression vector.
Construction of Recombinant Variable and Constant Domains
For in vitro protein folding studies, individual domains were produced in bacteria and purified using the 6× histidine tag methodology (QIAGEN). A BamHI site followed by a factor Xa site (Ile-Glu-Gly-Arg) was introduced immediately 5′ of the sequence encoding the NH2 terminus of the mature variable domain (Glu20) or the NH2 terminus of the constant domain (Gly129). Additionally, a stop codon was inserted at the end of the variable region coding sequence (Leu128). Each modified domain was excised and ligated into the QE-10 vector for expression in bacteria cells. The final coding sequence for the variable domain was MRGSHHHHHHTDPIEGRQ20–L128 and for the constant domain MRGSHHHHHHTDPIEGRG129–S234. This construction allowed for purification on Ni2+-agarose columns and would allow us, if necessary, to cleave the tag with factor Xa leaving an intact Ig domain.
Recombinant Protein Isolation
Bacteria transformed with the individual Ig domains were grown to an OD595 of 0.7 and protein expression was induced for 2 h with 1 mM IPTG. Initial experiments showed that neither construct (particularly the variable domain) gave high yields of soluble protein when the standard isolation protocol was followed (Wei and Hendershot, 1995). In an attempt to increase the yield of soluble protein, the bacterial pellets were resuspended in 16 ml (5 ml/g of bacteria) of buffer A (0.1 M NaH2PO4, 0.01 M Hepes, 6 M GdmCl, pH 8) containing 1 mM DTT. The cell lysate was stirred at room temperature for 40 min and then 10 mM 2-mercaptoethanol (2-ME) was added and the sample was stirred for an addition 20 min. The lysate was sonicated and centrifuged at 10,000 rpm. Batch adsorption was performed by adding 1 ml Ni-NTA-agarose (QIAGEN) equilibrated in buffer A containing 10 mM 2-ME to the supernatant. The sample was stirred for 90 min at room temperature and loaded into a polypropylene disposable column. The resin was extensively washed with buffer A containing 2-ME, and the bound protein was allowed to refold on the column through successive washes with the following solutions: (a) buffer B (0.1 M NaH2PO4, 0.01 M Hepes, 0.5 M NaCl, pH 7.5) containing 3 M GdmCl and 10 mM 2-ME; (b) buffer B containing 1 M GdmCl and 3 mM 2-ME; (c) buffer B containing 0.3 M GdmCl and 1 mM 2-ME; (d) buffer B; (e) buffer B containing 10% (vol/vol) glycerol. Proteins were eluted with 2 ml of buffer B containing 10% glycerol and 250 mM imidazole. The sample was loaded on a 5-ml polyacrylamide desalting column (Pierce) equilibrated with 20 mM Hepes, 300 mM NaCl, 10% glycerol, pH 7.5, to remove the imidazole.
Thermal Unfolding Curves
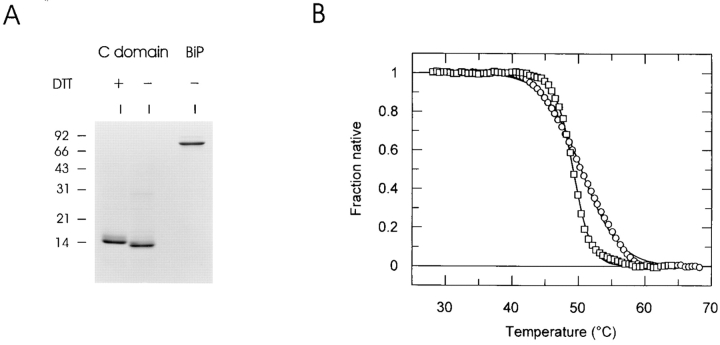
Thermal unfolding curves were obtained for recombinant BiP and for DTT-reduced C domain and analyzed as described (Vanhove et al., 1995). The experiments were performed in 20 mM Hepes, pH 7.5, 300 mM NaCl, 10% glycerol, 10 mM DTT. Protein concentrations were 2 μM for the C domain and 4 μM for BiP, and temperature was increased at a rate of 1°C/ min. Unfolding of the protein was monitored by fluorescence spectroscopy and data are presented as the fraction of native protein remaining at each temperature.
BiP Score Algorithm
Potential BiP-binding sites on the V and C domain were identified by a computer algorithm we developed using the BiP-binding scores determined by Blond-Elguindi et al. (1993). These scores reflected the probability of finding particular amino acids at specific positions within octomeric peptides that bound to BiP. For each of the 20 amino acids, numerical scores ranging from negative to positive values were assigned based on the likelihood that the amino acid was excluded or included at positions 1–8 in BiP-binding peptides. The LC sequence was analyzed eight amino acids at a time, and the algorithm determined scores associated with each overlapping octomer and reported the values on a position by position basis. This provided an indication of the propensity of the following octomer to interact with BiP.
COS Transfections
For in vivo analysis of BiP binding to the various λI LC mutants, COS-1 cells were cotransfected with the various λ constructs along with cDNAs for either wild-type (WT) hamster BiP (obtained from A.S. Lee, University of Southern California, Los Angeles, CA) or a BiP ATPase mutant created by changing Thr37 to Gly37 (G37) (Gaut and Hendershot, 1993; Wei et al., 1995) as described previously (Hendershot et al., 1995). In all cases, the transfected cells were analyzed 40 h after transfection, but the constructs and labeling conditions varied for the individual experiments and are detailed in the results section and figure legends. Transfected cells were biosynthetically labeled with [35S] Translabel (ICN). For pulse–chase experiments, cells were preincubated in met−–cys− labeling media for 45 min before the isotope was added. When labeled proteins were analyzed under nonreducing conditions, cells were washed first with PBS containing 20 mM NEM before lysing. NEM and apyrase were included in the lysing buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 0.5% DOC, and 0.5% NP-40). Labeled lysates were reacted with a polyclonal rabbit anti–rodent BiP antiserum (Hendershot et al., 1995) or with a polyclonal goat anti– mouse λ antiserum (Southern Biotechnology), and immune complexes were precipitated by binding to protein A–Sepharose beads. Proteins were resolved on SDS-polyacrylamide gels under either reducing or nonreducing conditions and visualized by fluorography using the Amplify reagent (Amersham).
Results
BiP ATPase Mutants Block Folding of Only One λ LC Domain
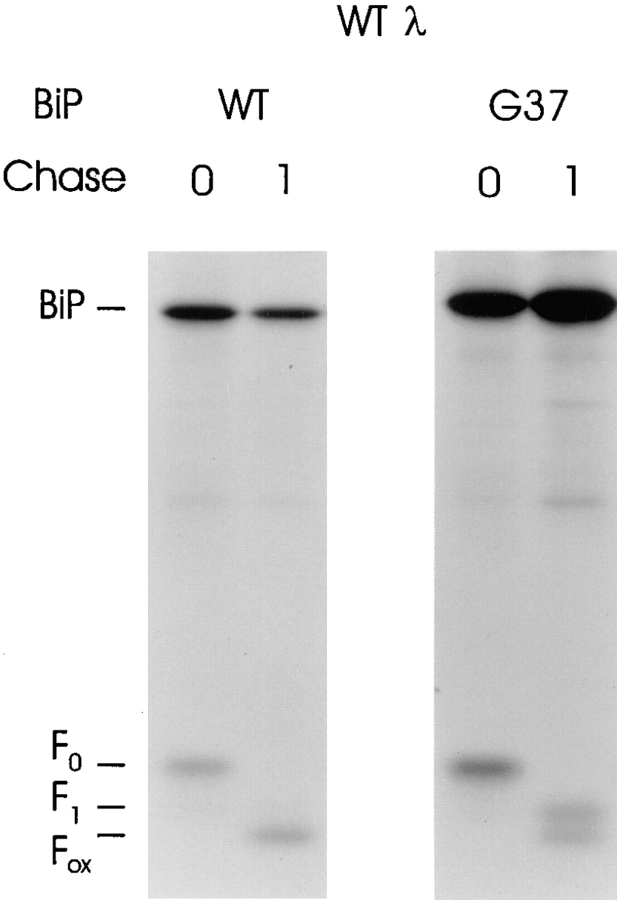
Previous studies using a genomic λI LC clone suggested that only a single LC domain binds to BiP and is unable to fold in the presence of BiP ATPase mutants (Hendershot et al., 1996). As a first step toward identifying the domain that BiP binds, we produced a λI LC cDNA clone (cWTλ) that would serve as a template for producing LC domain mutants. This construct was transiently coexpressed with either wild-type hamster BiP or a BiP ATPase mutant, G37, which binds stably to target proteins in vivo (Wei et al., 1995). As was observed with the genomic clone, the cDNA produced a λ LC that was expressed at high levels and bound to both wild-type and mutant BiP when synthesized under reducing conditions (Fig. 1). Subsequent removal of DTT allows the formation of disulfide bonds in both domains, which is dependent on LC folding to bring the intradomain cysteine residues into the correct position (Goto and Hamaguchi, 1981). This oxidation causes an increase in the mobility of the protein that, unlike protein folding itself, can be monitored on SDS-polyacrylamide gels (Braakman et al., 1992a; Hendershot et al., 1996). When DTT was removed, the LC expressed with wild-type BiP folded completely and disulfide bonds were formed in both domains (Fig. 1). When LCs coexpressed with mutant BiP were analyzed similarly, approximately half of the LCs underwent complete folding and oxidation. This is presumably due to the fact that the endogenous wild-type BiP present in these cells competes with the mutant BiP for binding to the reduced LCs (Hendershot et al., 1996). When DTT is removed, those LC bound to the endogenous BiP are capable of folding completely. The other half of the LC (i.e., those bound to the mutant BiP), underwent partial folding and oxidization as determined by their intermediate mobility. If both domains contained BiP-binding sites that could be used simultaneously, a portion of the LC should have remained completely reduced. Thus, it appears that either a single domain expresses BiP-binding sites or that only a single domain can bind to BiP at a time.
Figure 1.
Characterization of λI LC cDNA clone. COS-1 cells were transfected with the wild-type λ cDNA clone along with either wild-type or ATPase mutant (G37) hamster BiP. Cells were metabolically labeled in the presence of 10 mM DTT for 30 min and then chase for 0 or 1 h in complete media without DTT. Labeled lysates were immunoprecipitated with anti-λ antiserum and analyzed on nonreducing SDS-polyacrylamide gels.
Production of LC Domain Mutants That Lack Cysteine Residues Required for Intrachain Disulfide Bonds
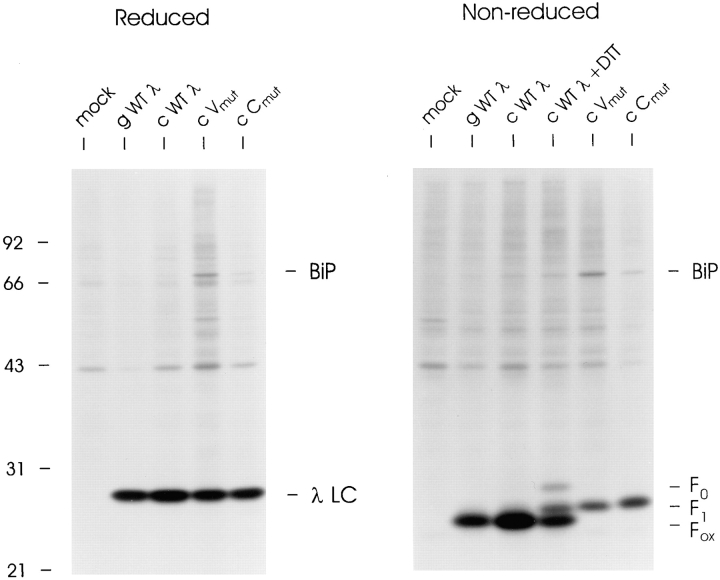
To determine which LC domain was bound to BiP and unable to fold in the presence of BiP mutants, we mutated the cysteine pairs that form the intradomain disulfide bond to serine in each of the domains separately. Both λ LC mutants were transiently expressed in COS cells and the resultant LC proteins were compared with wild-type λ LC. On reducing gels, the V and C domain mutants migrated exactly the same as wild-type LC produced from either the genomic (gWTλ) or cDNA (cWTλ) clone (Fig. 2). These labeled LCs were also analyzed under nonreducing conditions to determine their oxidation status. During the final 10 min of biosynthetic labeling, DTT was added to a dish transfected with cWTλ in order to produce a mixture of completely reduced (F0), partially oxidized (F1), and completely oxidized (Fox) intermediates of λ LC for comparison. The genomic and cDNA clones of wild-type λ produced completely oxidized LC that comigrated with the fastest migrating band in the DTT-treated sample (Fig. 2). Both the V and C domain cysteine mutants migrated with an intermediate mobility (F1), which was expected if one domain folded and formed an intradomain disulfide bond but the other did not. This demonstrated that mutation of the cysteines prevented disulfide bond formation in the targeted domain but did not alter folding and disulfide bond formation in the nonmutated domain.
Figure 2.
Characterization of V and C region intradomain cysteine mutants. COS-1 cells were transfected with genomic (gWTλ) or cDNA (cWTλ, cVmutλ, and cCmutλ) LC expression vectors. Cells were pulse labeled for 30 min, lysed in the presence of 20 mM NEM, immunoprecipitated with anti-λ antiserum, and then analyzed on either reducing or nonreducing SDS-polyacrylamide gels. A plate transfected with cWTλ had DTT added for the last 10 min of the 30 min label. This sample serves as a control on the nonreduced gel for the migration of reduced (F0), partially oxidized (F1), and fully oxidized (Fox) λ LCs.
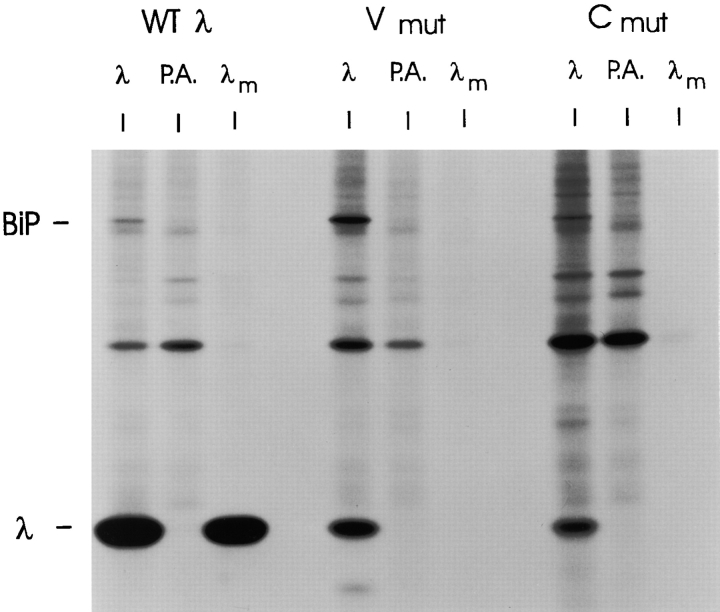
We examined the effects of genetically interrupting disulfide bond formation on secretion of the two LC mutants. Under labeling conditions where the wild-type LC was readily secreted, we observed no secretion of either the Vmutλ or the Cmutλ (Fig. 3). Thus, disulfide bond formation in both domains is required for LC secretion. All LC possess a COOH-terminal cysteine that remains unpaired in LCs that have not formed homodimers or assembled with heavy chains. To determine if a thiol-mediated retention mechanism (Alberini et al., 1990; Fra et al., 1993) was responsible for the lack of secretion, we treated cells with 2-β mercaptoethanol. This did not induce the secretion of either LC mutant (data not shown), suggesting that the thiol-mediated retention system (Alberini et al., 1990; Fra et al., 1993) is not solely responsible for keeping these LCs in the cell.
Figure 3.
Neither the V or C domain mutants are transport competent. COS-1 cells were transfected with the cDNA clones for WTλ, Vmutλ, and Cmutλ. Cells were metabolically labeled for 3 h. Culture supernatants were saved and immunoprecipitated with anti-λ (λm), and lysates were prepared, divided, and then immunoprecipitated with either anti-λ followed by protein A–Sepharose (λ) or protein A–Sepharose (P.A.) alone (to control for nonspecific binding). Samples were analyzed on SDS gels under reducing conditions.
Expression of a BiP ATPase Mutant Had No Additional Effect on the Folding of the λ Vmut, Whereas It Further Inhibited the Folding of λ Cmut
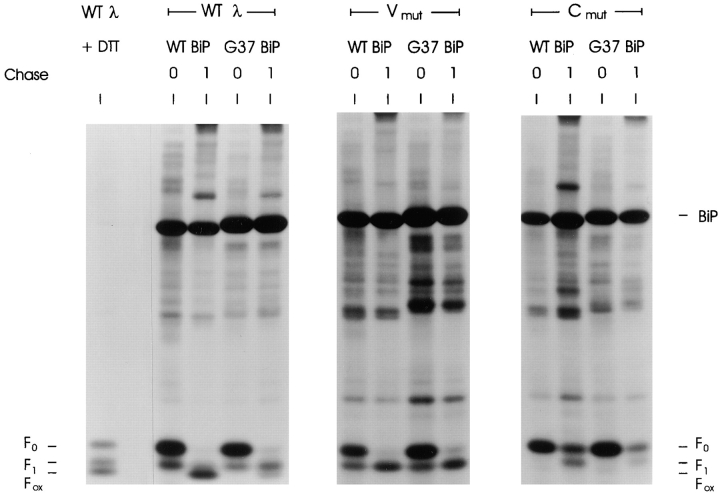
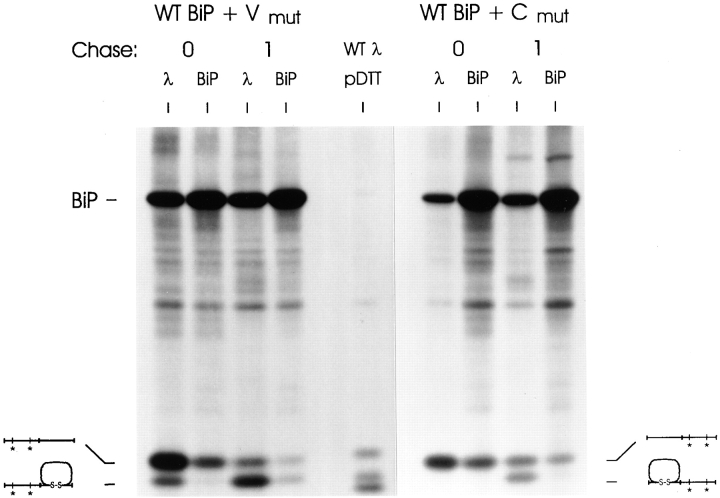
If BiP binds to a single LC domain, expression of the BiP ATPase mutant should only further affect oxidation of the LC mutant that had cysteine changes in the non–BiP-binding domain. Conversely, if both domains were capable of binding to BiP but only one BiP molecule could bind to the LC at a time, the mutant BiP should either further prevent the oxidation (i.e., from F1 to F0) of both LC mutants or have no further effect on either, depending on whether BiP was already associated with the nondisulfide-bonded domains. Either way, BiP mutant expression should affect both domains equally. Wild-type λ and both λ mutants were coexpressed with either wild-type or G37 mutant BiP. Transfected cells were pulse labeled with [35S]methionine and [35S]cysteine in the presence of DTT to inhibit disulfide bond formation and then chased in the absence of DTT to allow the LC domains to fold and form disulfide bonds. As in previous experiments, wild-type λ LCs were oxidized completely during the 1-h chase when expressed with wild-type BiP, but about half of the LC expressed with mutant BiP failed to be oxidized completely as evidenced by the presence of the F1 form of the LC (Fig. 4). There is some variability in the percentage of LC in the F1 and Fox forms between experiments, which may relate to the relative pool size of transfected mutant BiP and endogenous WT BiP. The Vmutλ migrated with an intermediate mobility (F1) when coexpressed with wild-type BiP. This was anticipated because the V region cannot form a disulfide bond due to mutation of its cysteine residues, and the other domain was shown to fold independently when synthesized in the absence of DTT (Fig. 2). Coexpression of the BiP ATPase mutant with Vmutλ did not significantly inhibit the LC from forming the F1 intermediate. When the Cmutλ was analyzed in the same way, we found that in the presence of wild-type BiP, it oxidized to the F1 intermediate after 1 h of chase, although not as efficiently as either the wild-type λ LC or the Vmutλ (Fig. 4). However, when the Cmutλ was coexpressed with the BiP ATPase mutant it was almost completely inhibited in its ability to form the F1 intermediate indicating that in the absence of BiP release both the VL and CL domain remained unoxidized. This strongly suggests that BiP binds to only a single domain in the λ LC and that it is the V domain.
Figure 4.
Mutant BiP expression further inhibits folding of the C domain mutant but has not further effect on the folding of the V domain mutant. COS-1 cells were cotransfected with one of the λ LC cDNA clones (WTλ, Vmutλ, or Cmutλ) and either wild-type (WT) or mutant (G37) BiP. Transfected cells were incubated in met−–cys− media for 45 min, pulse labeled for 45 min in the presence of 10 mM DTT, and then chased for 0 or 1 h in complete media without DTT. Cells were lysed in buffer containing 20 mM NEM and 10 U apyrase. Labeled proteins were immunoprecipitated with anti-λ and analyzed under nonreducing conditions. Again, a plate transfected with WTλ had DTT added for the final 10 min of labeling to serve as a control for the mobility of the various LC folding intermediates (WTλ + DTT).
There are several other interesting observations in this experiment. First, the wild-type and Vmut LC appear to be more resistant to DTT-mediated reduction than the Cmut, because in the pulse material (0) a portion of both these LC are partially oxidized (F1 form). Since the oxidized domain in the Vmut can only be the constant region domain, this suggests that the C domain is more resistant to reduction. In keeping with this, when the C domain cannot form disulfide bonds (Cmut), the V domain is readily reduced (i.e., no evidence of a partially oxidized, F1 form). Thus, although the folded tertiary structure is very similar for both domains, their ability to bind BiP and the susceptibility of their disulfide bonds to reducing agents are not the same. Previous studies have shown that buried disulfide bonds in Ig domains are fairly resistant to reducing agents. Thus, it is reasonable to suggest that during biosynthesis the C domain is folding and burying its disulfide bond so rapidly that a portion of it is resistant to DTT. The second interesting finding is that coexpression of mutant BiP diminished the post-DTT recovery of both wild-type and Cmut LC but did not greatly change the amount of Vmut LC (Fig. 4). The significance of this observation is not clear but may relate to the fact that mutant BiP expression would adversely affect the folding of both wild-type and Cmut LC by preventing the oxidation of the V domain. However, irreversible binding of the BiP mutant to the V domain would not have any further affect on the Vmut, because this domain was already unable to fold.
BiP Only Binds to the V domain of the λ LC In Vivo
The data in the previous experiment strongly suggest that BiP binds to the unoxidized variable region domain but does not associate with the unoxidized constant region domain. To examine this directly, both Vmut and Cmut LCs were coexpressed with wild-type BiP. Transfected cells were metabolically labeled in the presence of DTT and then chased without DTT. Lysates were immunoprecipitated with antibodies specific for λ LC and for rodent BiP. Immediately after labeling (0 chase), both the unfolded Vmut and Cmut LCs were coprecipitated with BiP (Fig. 5). After a 1-h chase, a portion of the Vmut had folded to the F1 intermediate. Both this intermediate and the completely reduced (F0) form bound to BiP and could be coprecipitated with the anti-BiP antiserum. Interestingly, BiP appeared to bind better to the completely reduced form than to the partially oxidized LC, as suggested by the fact that a larger proportion of the F0 form was coprecipitated with BiP than the F1 form. Although we do not understand the reason for this difference, it is possible that if BiP binds to V region sequences that are close to the C domain, folding and oxidation of the C domain could somehow weaken this interaction and make it less stable during immunoprecipitation. Examination of the Cmut LC revealed that although half of the LC achieved the F1 folding intermediate, they did not bind to BiP. Only the completely reduced (F0) form (in which the V domain was also unoxidized) was capable of associating with BiP (Fig. 5). Thus, BiP only binds to the unoxidized V domain of the full-length LC in vivo.
Figure 5.
BiP binds to the unoxidized V domain but not to the unoxidized C domain. COS-1 cells were transfected, labeled, and then lysed as in Fig. 4. Lysates from both the 0- and 1-h chase were divided and immunoprecipitated with either a polyclonal anti-rodent BiP antiserum or anti-mouse λ. The WTλ with partial DTT treatment is include in the middle of the gel as a control. The samples were analyzed under nonreduced conditions.
ER-targeted C Domain Does Not Associate with BiP
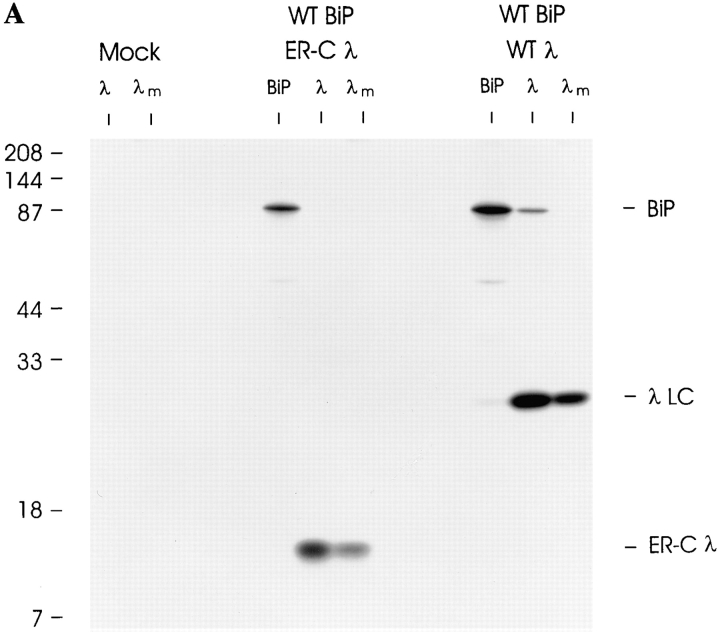
It is possible that because the V domain enters the ER first, BiP binds to this domain, and then sterically hinders a second BiP molecule from binding to the C domain as it enters. To examine this possibility, we produced a λI constant domain that could be translocated into the ER lumen (ER-Cλ) by engineering the cleavable ER signal sequence from the original full-length λ LC (Bernard et al., 1978) onto the NH2 terminus of the C domain. Both full-length wild-type λ LC and the ER-directed C domain were transiently expressed in COS cells. Metabolic labeling revealed that similar to WTλ LC, the ER-Cλ was synthesized and secreted from the transfected cells (Fig. 6 A). This demonstrated that, not only did the signal sequence target this domain to the ER, but that the signal sequence was efficiently cleaved and the isolated C domain was transport competent. Under steady-state labeling, some BiP was coprecipitated with wild-type λ LC and vice versa. However, when the C domain was analyzed in the same way, there was no evidence of BiP coprecipitating with the C domain or of the C domain coprecipitating with BiP (Fig. 6 A).
Figure 6.
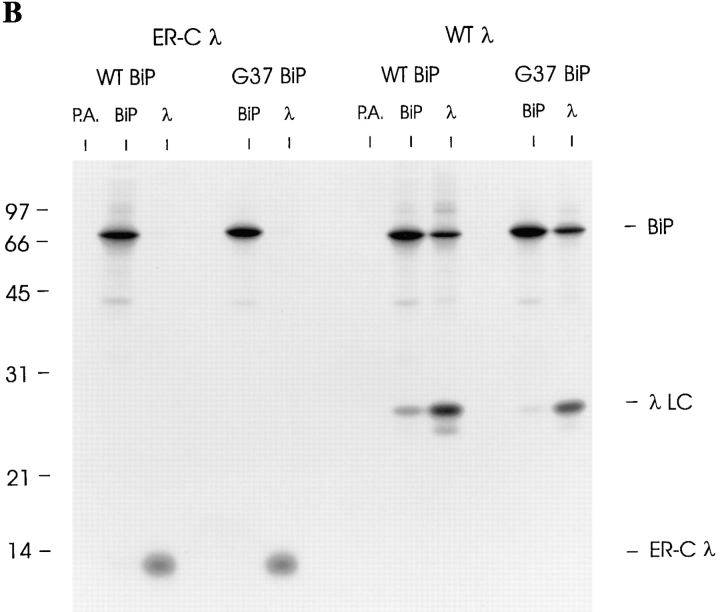
ER-targeted C domain is efficiently secreted and does not interact with BiP. (A) Cells were transfected with no DNA (Mock), the ER-targeted constant region gene (ER-Cλ), or wild-type λ cDNA along with wild-type hamster BiP. Transfected cells were labeled for 2 h, culture supernatants were saved, and then lysates were prepared. The supernatants were precipitated with anti-λ (λm) and the lysates were divided and precipitated with either anti-rodent BiP or anti-λ. Samples were analyzed on 12% SDS-gels under reducing conditions. (B) COS-1 cells were cotransfected with one of the λ LC cDNA clones (WTλ or ER-Cλ) and either wild-type (WT) or mutant (G37) BiP. Cells were pulse-labeled as in Fig. 4 and lysed immediately in the presence of NEM and apyrase. Lysates were divided and immunoprecipitated with anti-mouse λ, anti-rodent BiP, or protein A–Sepharose alone. Samples were analyzed on 12% SDS-gels under reducing conditions.
Steady-state labeling is not the most sensitive way to detect BiP association with proteins because the interactions are often transient or unstable. Therefore, we reexamined the ability of the isolated C domain to associate with BiP by coexpressing it with either wild-type or mutant BiP and pulse labeling under reducing conditions to inhibit folding of the domain. Under these conditions, the WTλ LC was easily detected in association with BiP (Fig. 6 B). Conversely, even under the most sensitive conditions for demonstrating BiP association with target proteins, there was no detectable evidence for BiP interacting with the ER-Cλ in vivo.
BiP-binding Sites Are Predicted to Occur in Both the V and C Domain of the λI LC
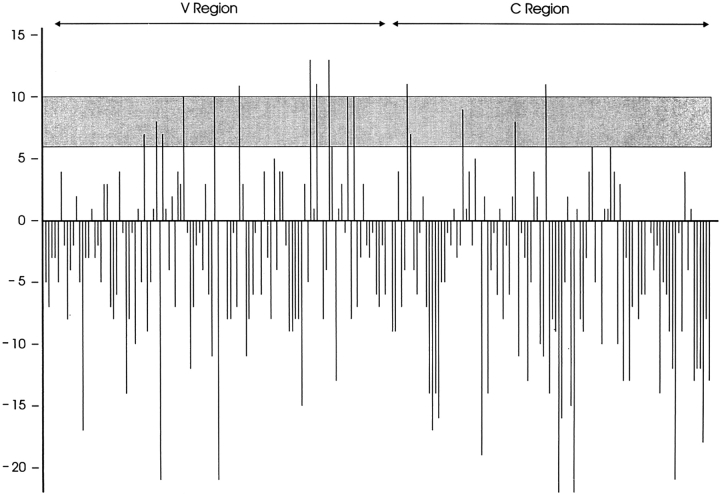
Our inability to detect BiP complexed to the C domain by any of the methods described above could mean the C domain does not possess BiP-binding sites. Alternatively, potential BiP-binding sites might be present but the C domain could fold too rapidly and stably, even in the absence of disulfide bond formation, to express them. A BiP-binding site algorithm (Blond-Elguindi et al., 1993) was applied to the λI LC. Peptide scores of between six and 10 were previously shown to be good predictors of peptides that were able to stimulate the ATPase activity of BiP and scores of above 10 were considered to be excellent predictors (Knarr et al., 1995). The relevance of a peptide to stimulate the ATPase activity of BiP is not clear, but it is generally assumed to indicate the ability of a peptide to bind BiP (Flynn et al., 1991; Blond Elguindi et al., 1993; Knarr et al., 1995). Although the variable domain clearly possesses more peptides with high “BiP scores,” numerous peptides scoring above 6 were found in the constant domain (Fig. 7). Two peptides had BiP scores of above 10 in the constant domain compared with four such sequences in the variable domain. Because we were unable to detect BiP binding to either an isolated constant domain or the cysteine-substituted constant domain in vivo, we must conclude that either the algorithm is not a good predictor of BiP-binding sites, or the constant domain folds so rapidly and stably that BiP does not have the opportunity to interact with it.
Figure 7.
Both the V and C domain of the HOPC 2020 λ LC possess potential BiP-binding sites. The BiP score algorithm was applied to the amino acid sequence and predicts a value for each eight amino acids. The value is assigned to the first amino acid of the octapeptide. Values of between six and 10 are predictive of possible BiP-binding sites and values above 10 are considered highly predictive. Negative values identify sequences that are very unlikely to stimulate the ATPase activity of BiP.
Thermal Unfolding of Native and Reduced C Domain
To address these two possibilities, recombinant λ LC V domain and C domain were produced in bacteria, purified, and then analyzed on reducing SDS gels. The C domain was relatively pure (Fig. 8 A), but much smaller quantities of the V domain were obtained and our preparations continuously had a major contaminating band (data not shown). The band corresponding to the anticipated size of the C domain was microsequenced to confirm its identity. The C domain migrated as a monomer on nonreducing gels and appeared to have formed the intradomain disulfide bond based on its decreased mobility after DTT treatment (Fig. 8 A). The contaminating protein in the V domain sample prevented us from using it in thermal unfolding assays, but the C domain was sufficiently pure for this analysis. The intrinsic fluorescence of a protein changes as it unfolds. Thus, a protein's stability can be assessed by monitoring its fluorescence at increasing temperatures. Thermal unfolding curves were generated for the C domain in the presence of 10 mM DTT. This was sufficient to reduce the intradomain disulfide bond, because the recombinant C domain migrated slower after DTT treatment (Fig. 8 A). The protein exhibited a single, cooperative unfolding transition as the temperature increased (Fig. 8 B). The melting temperature (T m) (i.e., the temperature at which 50% of the protein is unfolded) of the C domain under reducing conditions was calculated to be 50.4°C. Interestingly, even after reduction, the C domain was still completely folded at 37°C and was slightly more stable than the molecular chaperone BiP (T m = 49.2°C). This precluded in vitro studies to examine BiP association with an unfolded C domain since we were not able to find conditions where the C domain would be unfolded and the BiP still functional. Unfortunately it was not possible to establish a thermal unfolding curve for the C domain in the absence of DTT, presumably because the COOH-terminal cysteine, which pairs with a cysteine in the Ig heavy chain, contributes to aggregate formation under these experimental conditions (data not shown). Thus, although the exact contribution of the intradomain disulfide bond to the stability of the C domain could not be determined, it is clear that the C domain remains remarkably stable even in the absence of this bond. This may explain the lack of BiP binding to the C domain in vivo, even when LCs are synthesized in the presence of reducing agents.
Figure 8.
The recombinant C domain folds very stably even in the presence of reducing agents. (A) Recombinant BiP and C domain of λ LC were isolated and examined for purity and folding status. The C domain was incubated for 1 h at 37°C in the presence (+) or absence of DTT and then alkylated with a 10-fold molar excess of NEM. Samples were analyzed by SDS-PAGE under nonreducing conditions. (B) Thermal denaturation curves generated for purified hamster BiP (□) and for the purified C domain under reducing conditions (○). Fraction of native protein remaining was calculated at each temperature.
Discussion
The Ig domain is one of the elementary structures that serve as building blocks for many proteins. Although the sequences of these domains vary between different proteins, most are composed of seven β strands that fold into an antiparallel barrel structure that is stabilized by a single disulfide bond. The data presented here demonstrate that in spite of the strong structural similarities between Ig domains, the involvement of molecular chaperones in their folding in vivo can be quite different. All Ig domains that have been examined thus far possess potential BiP-binding sites, as detected by a BiP-binding algorithm (Knarr et al., 1995). It should be stressed that there are currently no data to demonstrate that any of these sites can be recognized in an intact protein, nor has an actual in vivo BiP-binding site been mapped on any protein. As a result, there is not a consensus in the field as to what is the best method for identifying potential BiP-binding sites. However, despite some differences in the sequences identified by the various hsp70-binding site algorithms, all of them detect multiple peptides within a given protein that have the potential to bind an hsp70 family member (Flynn et al., 1991; Blond-Elguindi et al., 1993; Takenaka et al., 1995; Rudiger et al., 1997). From the present study, at least in the case of BiP, it is clear that this does not necessarily mean they will be used in vivo during the folding of the protein. Although the NH2-terminal variable domain examined here is clearly bound to BiP before its complete folding and oxidation, we were unable to demonstrate BiP binding to the COOH-terminal constant region domain by any method. This included both the use of reducing agents or mutagenesis of cysteine residues to destabilize domain folding and the expression of BiP ATPase mutants to trap and stabilize complexes. All of these methods readily allowed us to detect BiP bound to the V domain.
Hsp70 family members interact with substrate proteins in an ATP-dependent fashion. In vitro experiments have demonstrated that the ATP-bound form of hsp70 binds proteins more rapidly than the ADP-bound form, but hydrolysis of the bound ATP to ADP is required to stabilize this binding (Bukau and Horwich, 1998). The G37 BiP mutant used in this study binds ATP as well as wild-type BiP, but it is unable to undergo the appropriate conformation change upon binding to ATP and therefore resembles the ADP-bound form of BiP (Wei et al., 1995). Thus, it might be argued that our BiP mutant is less able to complete with endogenous BiP for substrate proteins and therefore would not act as a kinetic trap for the C domain. However, the G37 mutant is able to compete well with endogenous wild-type BiP for binding to the V domain of the LC (this study), the CH1 domain of the Ig heavy chain (Gaut and Hendershot, 1993), and factor VIII, a component of the blood coagulation cascade (Morris et al., 1997). Nevertheless, it is conceivable that BiP has a much lower affinity for the C domain and the mutant BiP drops below a threshold of detectable binding. This would not be a factor in the experiments where reducing agents or cysteine mutants were employed. In this case, there was no evidence that either the endogenous BiP or the transfected wild-type BiP were capable of binding to the unoxidized C domain. So, at the very least, one must conclude that if BiP binds to the C domain it must be at a very reduced affinity so that mutant BiP can no longer compete, and this binding must be very transient and not prolonged by preventing the oxidation of the C domain. Clearly this is in contrast to BiP's interaction with the V domain.
Our data suggest that it is not the presence of BiP-binding sites per se that determines whether or not a protein will associate with BiP. Instead it appears that the folding pathway and rate at which folding occurs control whether an individual protein, or region of a protein, will bind to BiP. If the “BiP-binding” sequences are rapidly sequestered within the interior of the newly synthesized protein, they may never be exposed long enough to allow BiP to bind. The resistance of a portion of newly synthesized C domain to reducing agents strongly suggests that this domain folds rapidly, and the thermal stability of the reduced C domain demonstrates that it does not require a disulfide bond to stabilize this folding state. Consistent with this idea, in vitro folding studies on an isolated human C λ domain revealed that the conformation of the C domain is not greatly affected by reducing agents (Goto and Hamaguchi, 1981).
In spite of the fact that we were readily able to detect the V domain bound to BiP when it was reduced, we are not sure how much of the wild-type V domain binds to BiP when it is produced and folded under physiological conditions. Although clearly some of it can be trapped with the BiP mutants, we get a much higher proportion binding to BiP when they are synthesized in the presence of reducing agents (Hendershot et al., 1996), which destabilizes this domain. We interpret this to suggest that instead of BiP playing an active role in folding these proteins, that it may be standing by to “catch” proteins that are either unable or slow to fold and prevent them from aggregating. Thus, only proteins that have difficulties in folding or perhaps multisubunit proteins would require BiP to obtain their mature conformation. This conclusion is consistent with data showing that alterations in the levels of wild-type BiP can change the rate of folding of some proteins (Dorner et al., 1988; Braakman et al., 1991). Perhaps by greatly increasing the levels of BiP in the ER, low-affinity sites on proteins that are not normally occupied by BiP would be.
It is interesting to speculate on why these two domains might have evolved to have different folding stabilities and chaperone requirements. The variable domains of both Ig heavy and light chains contain the antigen-binding site and as such must accommodate a large variety of amino acid changes to recognize a nearly infinite number of antigens. Thus, the stability or rate of folding of variable regions may vary greatly and in some cases require more help to fold. Recently another LC, the nonsecreted NS-1 κ LC, was shown to bind to BiP via its unfolded V domain (Skowronek et al., 1998). Unlike the V domains, the constant domains do not contribute directly to the antigen-binding site and do not vary in sequence within a given isotype. The single constant domain of the LC (CL) pairs with the first constant domain (CH1) of the heavy chain, which possesses a stable BiP-binding site (Hendershot et al., 1987). The LC in some as yet undefined way must displace BiP from the heavy chain so Ig assembly can proceed (Lee, Y.-K., J.W. Brewer, R. Hellman, and L.M. Hendershot, manuscript submitted for publication). Thus, the conserved C domain may have evolved to fold rapidly and stably in order to assist the folding of the CH1 domain. In support of this role for CL, domain pairing appears to play a role in the folding of some of the other constant domains of the heavy chain (Kaloff and Haas, 1995).
In conclusion, the studies described here demonstrate that the two structurally similar Ig domains of the λI LC behave very differently in respect to their association with BiP during folding even though both domains possess potential BiP-binding sites. The data suggest that the simple presence of BiP-binding sites on a nascent chain does not ensure that BiP will bind and play a role in its folding. Instead, our data support a model in which BiP preferentially recognizes proteins that are slower to fold to a stable conformation.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM-54068), the Cancer Center CORE (P30 CA21765), and the American Lebanese Syrian Associated Charities of St. Jude Children's Research Hospital.
Abbreviations used in this paper
- 2-ME
2-mercaptoethanol
- BiP
immunoglobulin heavy chain-binding protein
- C
constant
- CH1
first constant domain of the heavy chain
- CL
single constant domain of the light chain
- cWTλ
pSVλ eukaryotic expression vector
- ER-Cλ
a λ1 constant domain that could be translocated to the ER lumen
- gWTλ
pJDEλI genomic clone
- LC
light chain
- V
variable
- WT
wild-type
References
- Alberini CM, Bet P, Milstein C, Sitia R. Secretion of immunoglobulin M assembly intermediates in the presence of reducing agents. Nature. 1990;347:485–487. doi: 10.1038/347485a0. [DOI] [PubMed] [Google Scholar]
- Amzel LM, Poljak RJ. Three-dimensional structure of immunoglobulins. Annu Rev Immunol. 1979;48:961–997. doi: 10.1146/annurev.bi.48.070179.004525. [DOI] [PubMed] [Google Scholar]
- Bernard O, Hozumi N, Tonegawa S. Sequences of mouse immunoglobulin light chain genes before and after somatic changes. Cell. 1978;15:1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Blond-Elguindi S, Cwirla SE, Dower WJ, Lipshutz RJ, Sprang SR, Sambrook JF, Gething MJ. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 1993;75:717–728. doi: 10.1016/0092-8674(93)90492-9. [DOI] [PubMed] [Google Scholar]
- Braakman I, Hoover H, Litty, Wagner KR, Helenius A. Folding of influenza hemagglutinin in the endoplasmic reticulum. J Cell Biol. 1991;114:401–411. doi: 10.1083/jcb.114.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I, Helenius J, Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO (Eur Mol Biol Organ) J. 1992a;11:1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I, Helenius J, Helenius A. Role of ATP and disulphide bonds during protein folding in the endoplasmic reticulum. Nature. 1992b;356:260–262. doi: 10.1038/356260a0. [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Dorner AJ, Krane MG, Kaufman RJ. Reduction of endogenous GRP78 levels improves secretion of a heterologous protein in CHO cells. Mol Cell Biol. 1988;8:4063–4070. doi: 10.1128/mcb.8.10.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn GC, Pohl J, Flocco MT, Rothman JE. Peptide-binding specificity of the molecular chaperone BiP. Nature. 1991;353:726–730. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- Fra AM, Fagioli C, Finazzi D, Sitia R, Alberini CM. Quality control of ER synthesized proteins: an exposed thiol group as a three-way switch mediating assembly, retention and degradation. EMBO (Eur Mol Biol Organ) J. 1993;12:4755–4761. doi: 10.1002/j.1460-2075.1993.tb06164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaut JR, Hendershot LM. Mutations within the nucleotide binding site of immunoglobulin-binding protein inhibit ATPase activity and interfere with release of immunoglobulin heavy chain. J Biol Chem. 1993;268:7248–7255. [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gorlich D, Rapoport TA. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 1993;75:615–630. doi: 10.1016/0092-8674(93)90483-7. [DOI] [PubMed] [Google Scholar]
- Goto Y, Hamaguchi K. Formation of the intrachain disulfide bond in the constant fragment of the immunoglobulin light chain. J Mol Biol. 1981;146:321–340. doi: 10.1016/0022-2836(81)90391-0. [DOI] [PubMed] [Google Scholar]
- Goto Y, Hamaguchi K. Unfolding and refolding of the reduced constant fragment of the immunoglobulin light chain. J Mol Biol. 1982;156:911–926. doi: 10.1016/0022-2836(82)90147-4. [DOI] [PubMed] [Google Scholar]
- Goto Y, Azuma T, Hamaguchi K. Refolding of the immunoglobulin light chain. J Biochem. 1979;85:1427–1438. doi: 10.1093/oxfordjournals.jbchem.a132470. [DOI] [PubMed] [Google Scholar]
- Graham KS, Le A, Sifers RN. Accumulation of the insoluble PiZ variant of human alpha 1-antitrypsin within the hepatic endoplasmic reticulum does not elevate the steady-state level of grp78/BiP. J Biol Chem. 1990;265:20463–20468. [PubMed] [Google Scholar]
- Haas IG. BiP—a heat shock protein involved in immunoglobulin chain assembly. Curr Top Microbiol Immunol. 1991;167:71–82. doi: 10.1007/978-3-642-75875-1_4. [DOI] [PubMed] [Google Scholar]
- Hamman BD, Hendershot LM, Johnson AE. BiP maintains the permeability barrier of the ER membrane by sealing the lumenal end of the translocon pore before and early in translocation. Cell. 1998;92:747–758. doi: 10.1016/s0092-8674(00)81403-8. [DOI] [PubMed] [Google Scholar]
- Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot L, Bole D, Kohler G, Kearney JF. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J Cell Biol. 1987;104:761–767. doi: 10.1083/jcb.104.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot LM, Wei J-Y, Gaut JR, Lawson B, Freiden PJ, Murti KG. In vivo expression of mammalian BiP ATPase mutants causes disruption of the endoplasmic reticulum. Mol Biol Cell. 1995;6:283–296. doi: 10.1091/mbc.6.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot L, Wei J, Gaut J, Melnick J, Aviel S, Argon Y. Inhibition of immunoglobulin folding and secretion by dominant negative BiP ATPase mutants. Proc Natl Acad Sci USA. 1996;93:5269–5274. doi: 10.1073/pnas.93.11.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins. Annu Rev Immunol. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hurtley SM, Bole DG, Hoover H, Litty, Helenius A, Copeland CS. Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP) J Cell Biol. 1989;108:2117–2126. doi: 10.1083/jcb.108.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaloff CR, Haas IG. Coordination of immunoglobulin chain folding and immunoglobulin chain assembly is essential for the formation of functional IgG. Immunity. 1995;2:629–637. doi: 10.1016/1074-7613(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Knarr G, Gething MJ, Modrow S, Buchner J. BiP binding sequences in antibodies. J Biol Chem. 1995;270:27589–27594. doi: 10.1074/jbc.270.46.27589. [DOI] [PubMed] [Google Scholar]
- Landry SJ, Jordan R, McMacken R, Gierasch LM. Different conformations for the same polypeptide bound to chaperones DnaK and GroEL. Nature. 1992;355:455–457. doi: 10.1038/355455a0. [DOI] [PubMed] [Google Scholar]
- Morris JA, Dorner AJ, Edwards CA, Hendershot LM, Kaufman RJ. Immunoglobulin binding protein (BiP) function is required to protect cells from endoplasmic reticulum stress but is not required for the secretion of selective proteins. J Biol Chem. 1997;272:4327–4334. doi: 10.1074/jbc.272.7.4327. [DOI] [PubMed] [Google Scholar]
- Munro S, Pelham HR. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986;46:291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Nicchitta CV, Blobel G. Lumenal proteins of the mammalian endoplasmic reticulum are required to complete protein translocation. Cell. 1993;73:989–998. doi: 10.1016/0092-8674(93)90276-v. [DOI] [PubMed] [Google Scholar]
- Palleros DR, Reid KL, Shi L, Welch WJ, Fink AL. ATP-induced protein-Hsp70 complex dissociation requires K+ but not ATP hydrolysis. Nature. 1993;365:664–666. doi: 10.1038/365664a0. [DOI] [PubMed] [Google Scholar]
- Rudiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO (Eur Mol Biol Organ) J. 1997;16:1501–1507. doi: 10.1093/emboj/16.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JF, Ferro-Novick S, Rose MD, Helenius A. BiP/Kar2p serves as a molecular chaperone during carboxypeptidase Y folding in yeast. J Cell Biol. 1995;130:41–49. doi: 10.1083/jcb.130.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronek MH, Hendershot LM, Haas IG. The variable domain of non-assembled Ig light chains determines both their half-life and binding to BiP. Proc Natl Acad Sci USA. 1998;95:1574–1578. doi: 10.1073/pnas.95.4.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka IM, Leung SM, McAndrew SJ, Brown JP, Hightower LE. Hsc70-binding peptides selected from a phage display peptide library that resemble organellar targeting sequences. J Biol Chem. 1995;270:19839–19844. doi: 10.1074/jbc.270.34.19839. [DOI] [PubMed] [Google Scholar]
- Vanhove M, Houba S, Lamotte-Brasseur J, Frere JM. Probing the determinants of protein stability: comparison of class A beta-lactamases. Biochem J. 1995;308:859–864. doi: 10.1042/bj3080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Misra LM, Rose MD. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol. 1990;110:1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Johnson AE. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1994;10:87–119. doi: 10.1146/annurev.cb.10.110194.000511. [DOI] [PubMed] [Google Scholar]
- Wei J-Y, Hendershot LM. Characterization of the nucleotide binding properties and ATPase activity of recombinant hamster BiP purified from bacteria. J Biol Chem. 1995;270:26670–26676. doi: 10.1074/jbc.270.44.26670. [DOI] [PubMed] [Google Scholar]
- Wei J-Y, Gaut JR, Hendershot LM. In vitro dissociation of BiP peptide complexes requires a conformational change in BiP after ATP binding but does not require ATP hydrolysis. J Biol Chem. 1995;270:26677–26682. doi: 10.1074/jbc.270.44.26677. [DOI] [PubMed] [Google Scholar]